Abstract
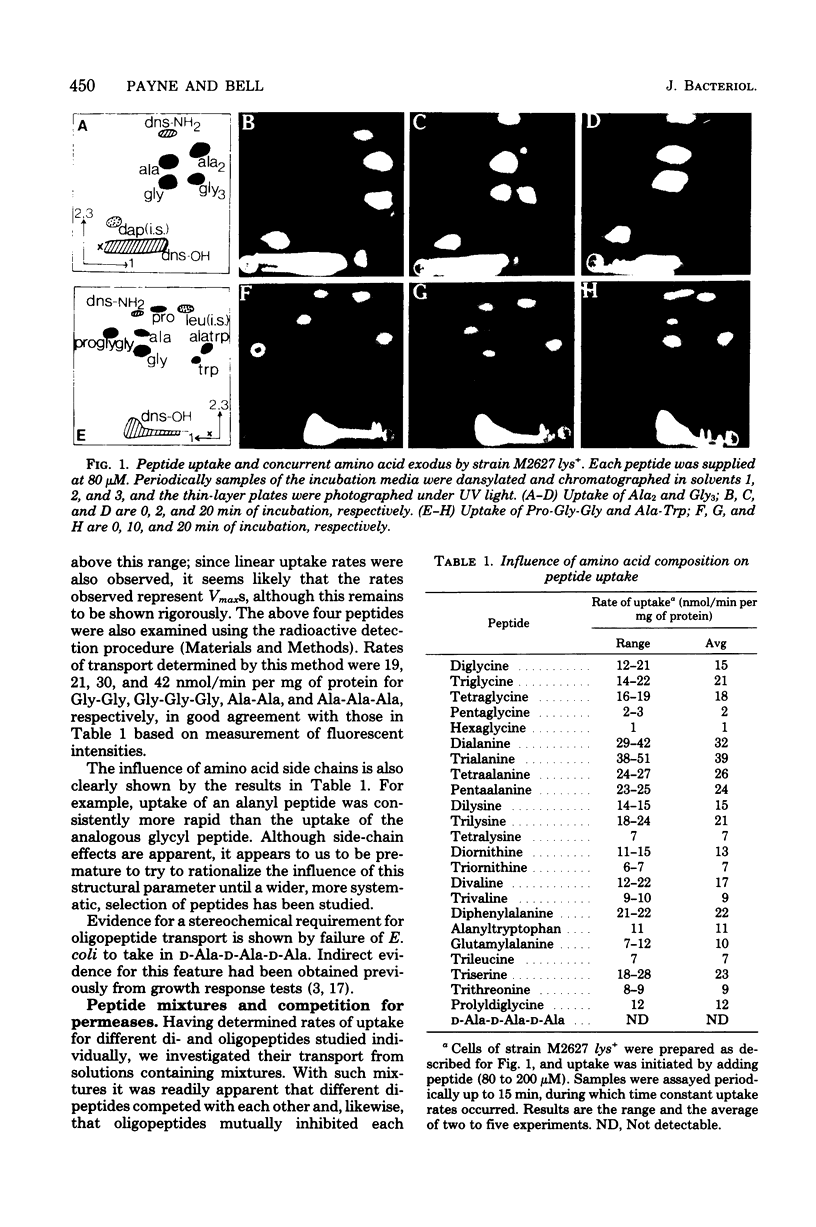
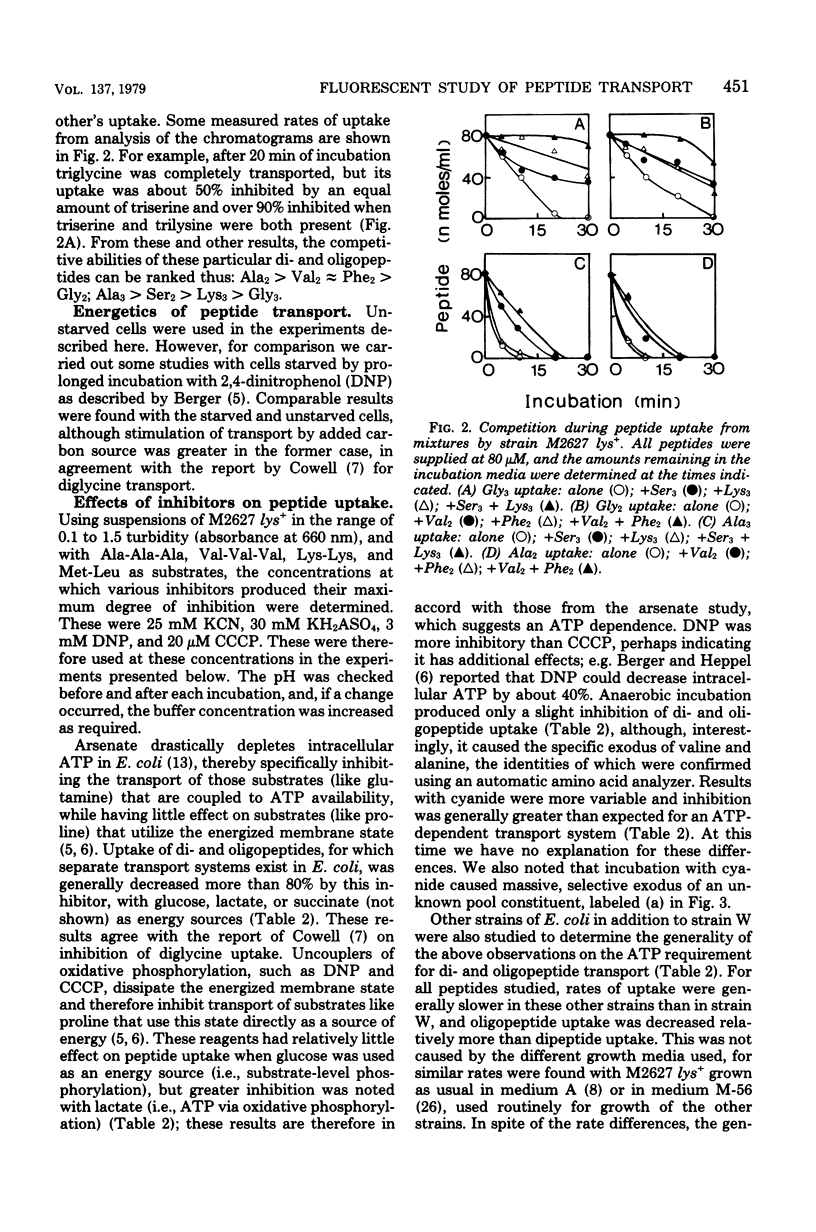
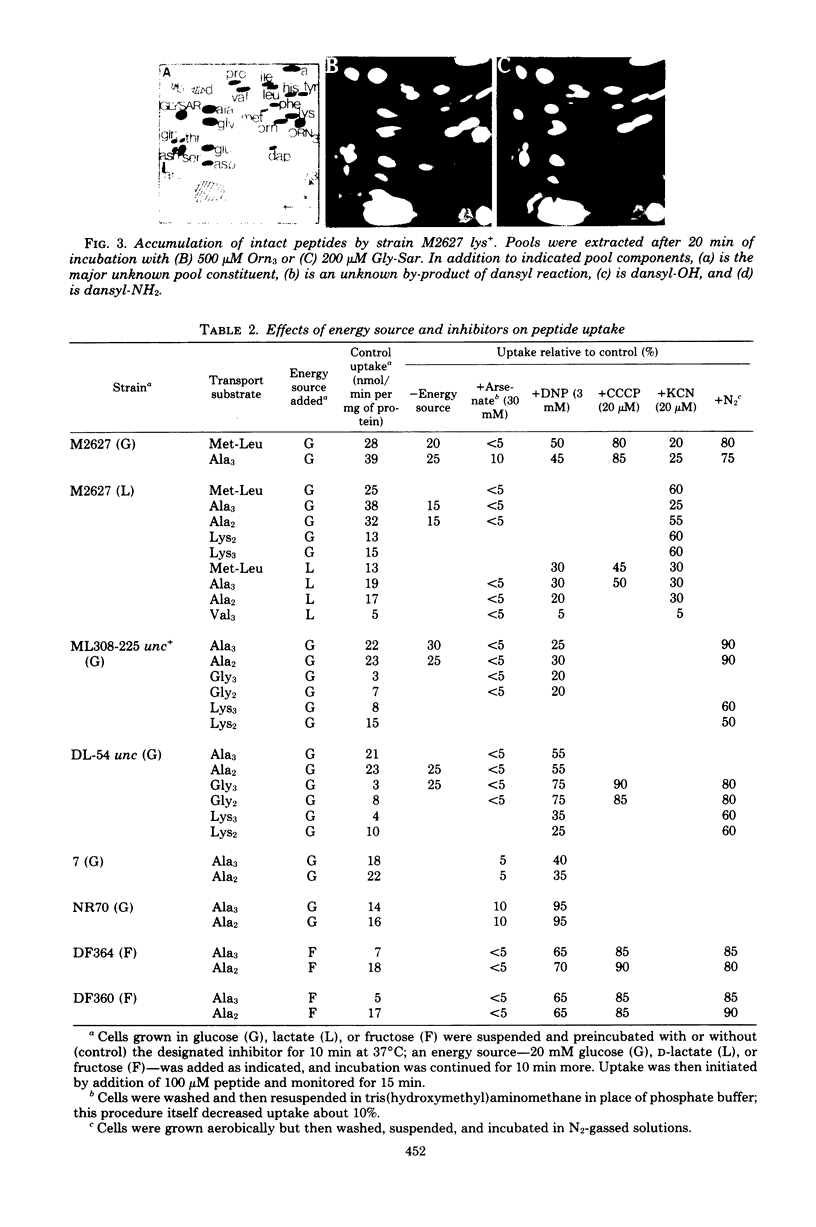
A direct study of peptide uptake by Escherichia coli was made using a fluorescent procedure. After incubation with the bacteria, peptides remaining in the medium were dansylated, separated chromatographically, and quantitated from their fluorescent intensities and/or from their incorporated radioactivity when tritiated dansyl derivatives were prepared. Peptide uptake was apparently not regulated and proceeded continuously until complete, with the absorbed peptides undergoing rapid intracellular hydrolysis and the excess amino acid residues leaving the cell. Thus, peptide uptake and amino acid exodus occur concurrently. However, peptidase-resistant substrates, e.g. triornithine and glycylsarcosine, which can be similarly estimated in cell extracts, were accumulated about 1,000-fold. The influence of amino acid composition and chain length on rates of transport was assessed. Different strains of E. coli showed variability in their rates of di- and oligopeptide transport. With respect to energy coupling, both the di- and oligopeptide permeases behaved like shock-sensitive transport systems.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Barak Z., Sarid S., Katchalski E. Inhibition of protein biosynthesis in Escherichia coli B by tri-L-ornithine. Eur J Biochem. 1973 Apr;34(2):317–324. doi: 10.1111/j.1432-1033.1973.tb02761.x. [DOI] [PubMed] [Google Scholar]
- Becker J. M., Naider F. Stereospecificity of tripeptide utilization in a methionine auxotroph of Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):191–196. doi: 10.1128/jb.120.1.191-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G., Payne G. M., Payne J. W. Monitoring enzyme synthesis as a means of studying peptide transport and utilization in Escherichia coli. J Gen Microbiol. 1977 Feb;98(2):485–491. doi: 10.1099/00221287-98-2-485. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILVARG C., KATCHALSKI E. PEPTIDE UTILIZATION IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:3093–3098. [PubMed] [Google Scholar]
- Gilvarg C., Levin Y. Response of Escherichia coli to ornithyl peptides. J Biol Chem. 1972 Jan 25;247(2):543–549. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Latil M., Murgier M., Lazdunski A., Lazdunski C. Isolation and genetic mapping of Escherichia coli aminopeptidase mutants. Mol Gen Genet. 1976 Oct 18;148(1):43–47. doi: 10.1007/BF00268544. [DOI] [PubMed] [Google Scholar]
- Maeda M., Futai M., Anraku Y. Biochemical characterization of the uncA phenotype of Escherichia coli. Biochem Biophys Res Commun. 1976 May 23;76(2):331–338. doi: 10.1016/0006-291x(77)90729-x. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Payne J. W. Peptide transport in Escherichia coli: permease specificity towards terminal amino group substituents. J Gen Microbiol. 1974 Jan;80(1):269–276. doi: 10.1099/00221287-80-1-269. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Peptides and micro-organisms. Adv Microb Physiol. 1976;13:55–113. doi: 10.1016/s0065-2911(08)60038-7. [DOI] [PubMed] [Google Scholar]
- Ramshaw J. A., Thompson E. W., Boulter D. The amino acid sequence of Helianthus annuus L. (sunflower) cyrochrome c deduced from chymotryptic peptides. Biochem J. 1970 Sep;119(3):535–539. doi: 10.1042/bj1190535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Archer E. G., Dunn F. W. Uptake of [14C]-labeled tri-, tetra-, and pentapeptides of phenylalanine and glycine by Escherichia coli. J Biol Chem. 1970 Jun 10;245(11):2967–2971. [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. I. Purification and physical chemical properties of the enzyme amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:417–426. doi: 10.1016/0006-3002(60)90194-3. [DOI] [PubMed] [Google Scholar]





