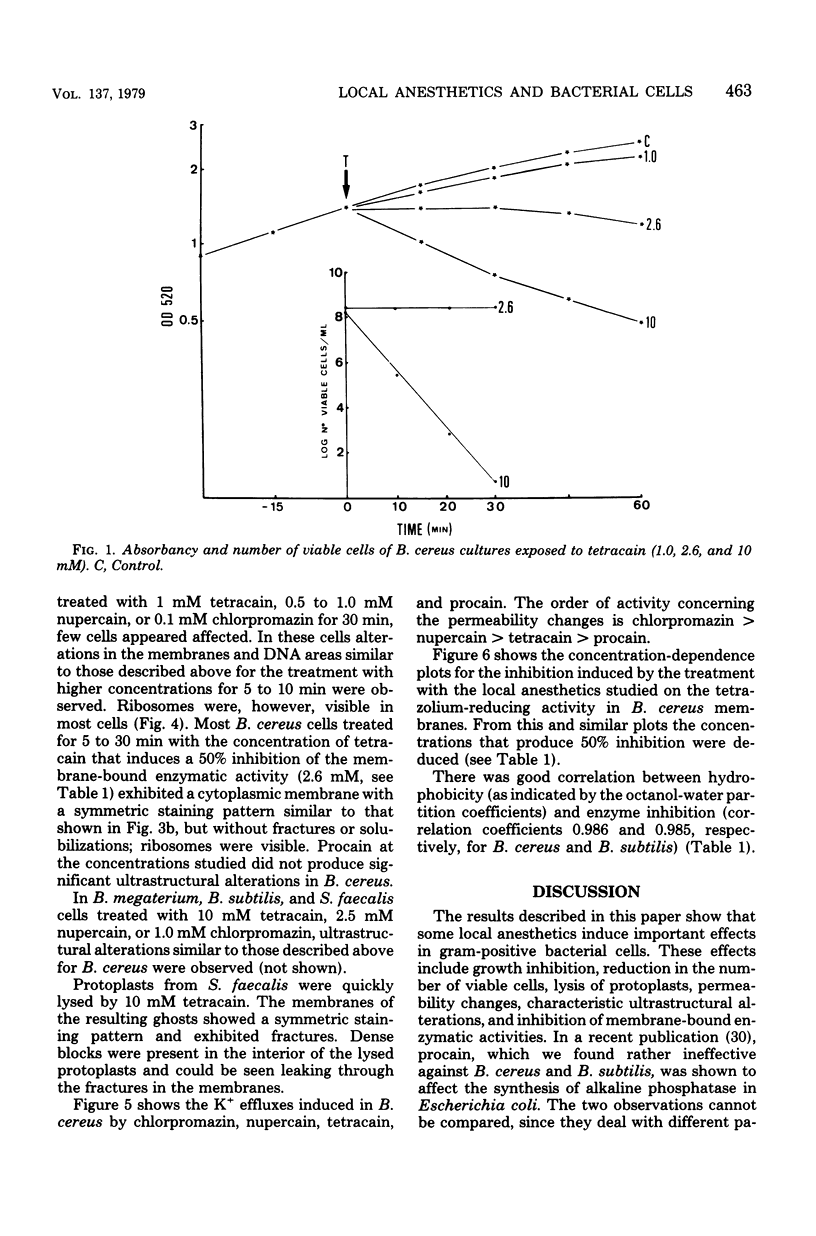
Abstract
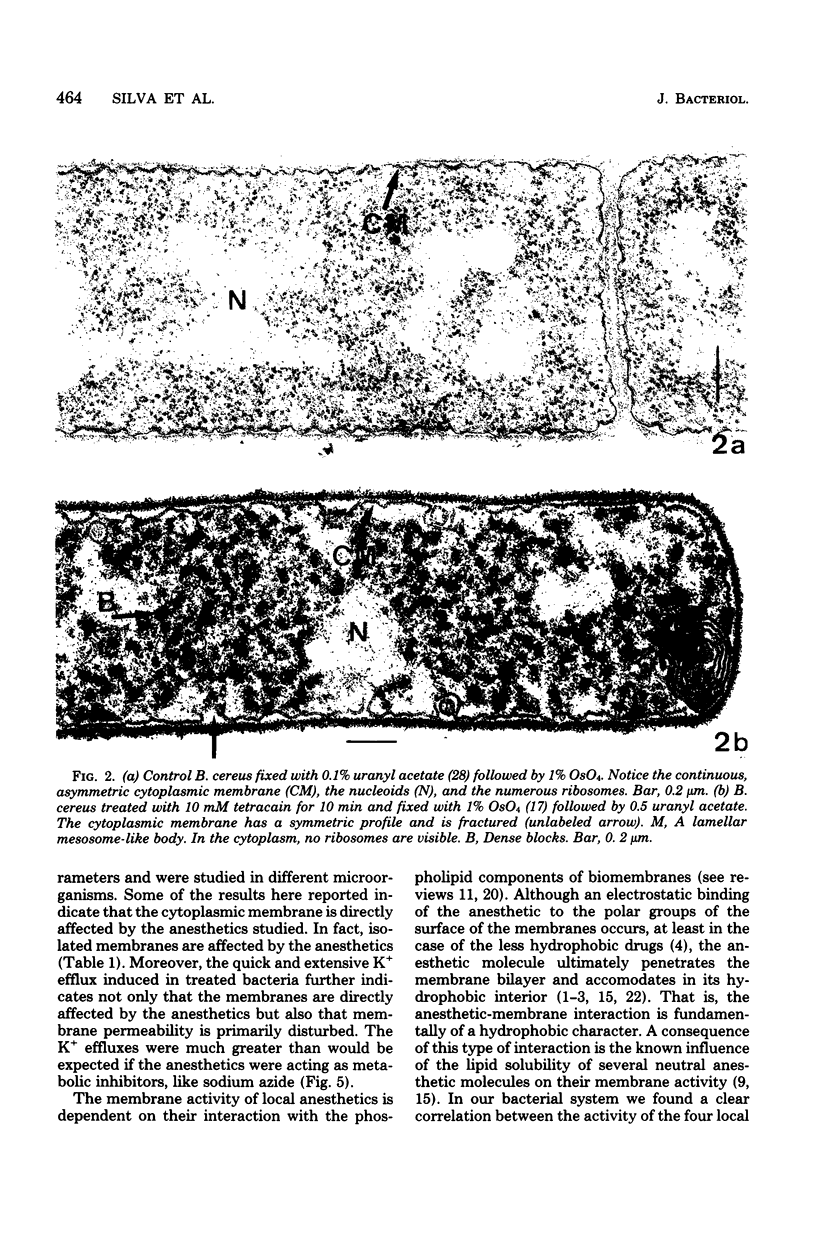
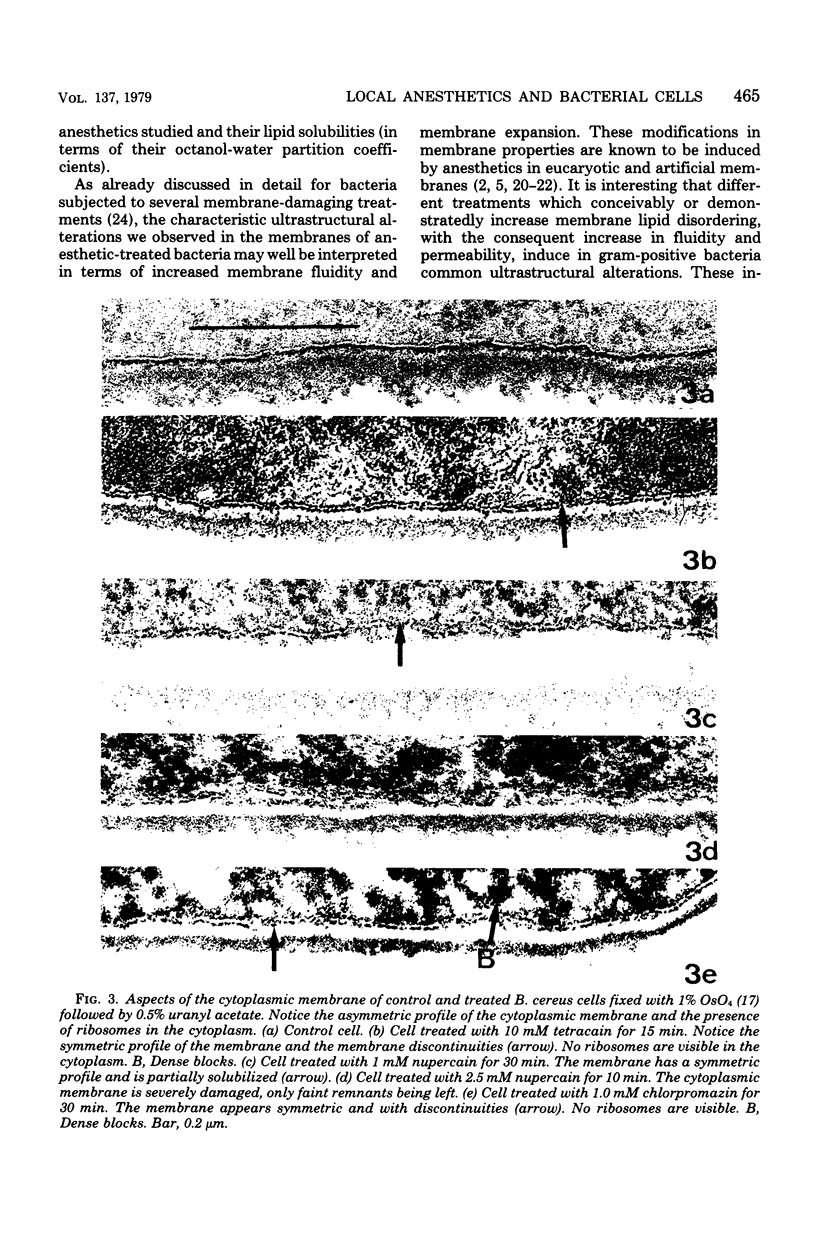
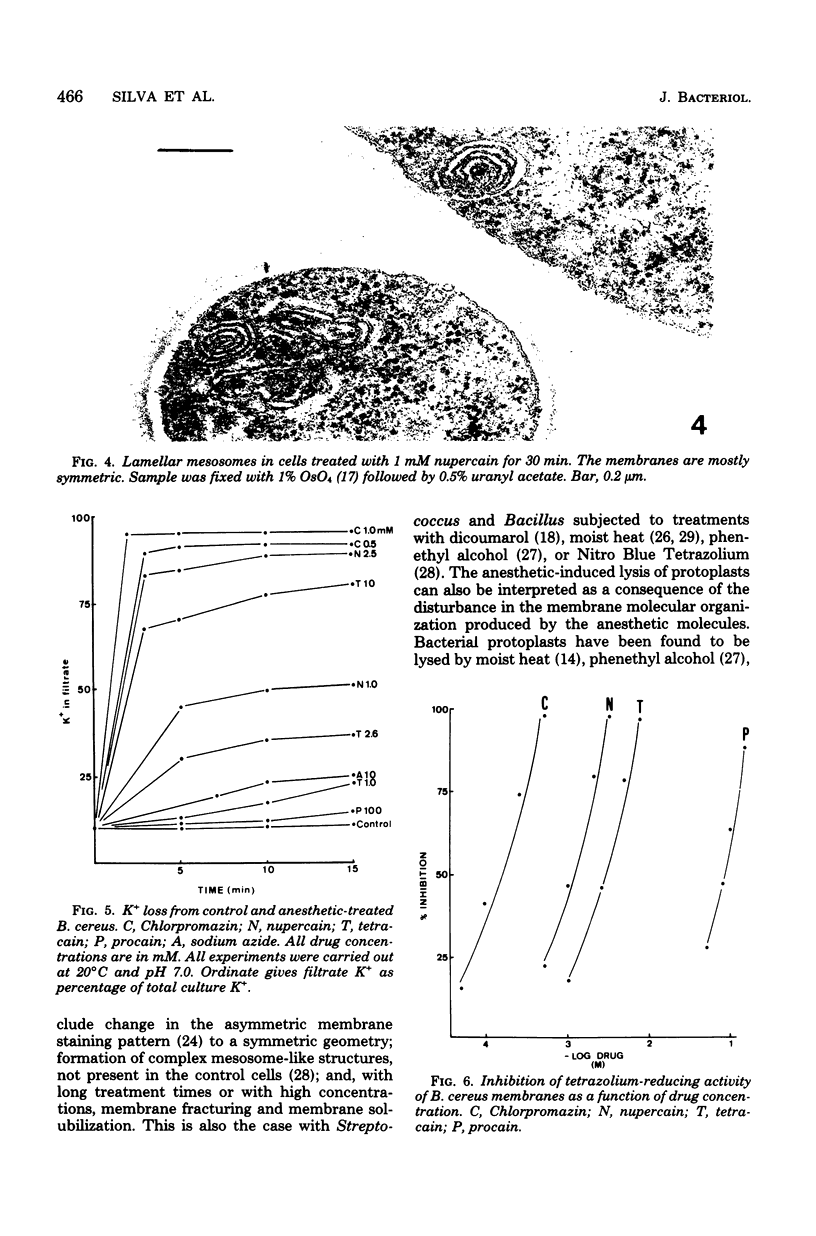
The membrane effects of chlorpromazine, nupercain, tetracain, and procain were studied using Bacillus cereus, B. megaterium, B. subtilis, and Streptococcus faecalis, protoplasts from S. faecalis, and isolated membranes from B. subtilis. Chlorpromazin, nupercain, and tetracain produced characteristic micromorphological alterations after treatment for 5 to 30 min at pH 7.0 and 20 degrees C; the membrane staining pattern changed from asymmetric to symmetric, complex mesosome-like structures appeared, and membrane fractures and solubilization occurred. Procain at concentrations up to 100 mM did not induce detectable alterations. Protoplasts were quickly lysed by 10 mM tetracain. A rapid and extensive leakage of K+ was induced by chlorpromazin, nupercain, and tetracain. Procain (100 mM) induced a slight K+ leakage. The membrane respiratory activity of intact B. cereus cells (as measured by the triphenyl tetrazolium reduction) and the succinic dehydrogenase activity of B. subtilis isolated membranes were found to be inhibited by the four local anesthetics. The concentrations that produced 50% inhibition of those activities are correlated with the hydrophobicities of the anesthetic molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feinstein M. B., Fernandez S. M., Sha'afi R. I. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effect of local anesthetics, cholesterol and protein. Biochim Biophys Acta. 1975 Dec 16;413(3):354–370. doi: 10.1016/0005-2736(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Fernández M. S., Cerbón J. The importance of the hydrophobic interactions of local anesthetics in the displacement of polyvalent cations from artificial lipid membranes. Biochim Biophys Acta. 1973 Feb 27;298(1):8–14. doi: 10.1016/0005-2736(73)90003-5. [DOI] [PubMed] [Google Scholar]
- Giotta G. J., Chan D. S., Wang H. H. Binding of spin-labeled local anesthetics to phosphatidylcholine and phosphatidylserine liposomes. Arch Biochem Biophys. 1974 Aug;163(2):453–458. doi: 10.1016/0003-9861(74)90501-3. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Metcalfe J. C., Metcalfe S. M., McConnell H. M. The interaction of small molecules with spin-labelled erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):415–427. doi: 10.1016/0005-2736(70)90219-1. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Machleidt H., Roth S., Seeman P. The hydrophobic expansion of erythrocyte membranes by the phenol anesthetics. Biochim Biophys Acta. 1972 Jan 17;255(1):178–189. doi: 10.1016/0005-2736(72)90020-x. [DOI] [PubMed] [Google Scholar]
- Mota J. S., Silva M. T., Guerra F. C. Variations in the membranes of Streptococcus faecalis related to different cultural conditions. Arch Mikrobiol. 1972;83(4):293–302. doi: 10.1007/BF00425241. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., MARGULIES S. I., SELIGMAN A. M. Sites of electron transfer to tetrazolium salts in the succinoxidase system. J Biol Chem. 1960 Sep;235:2739–2743. [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Poste G., Shepherd G. Effects of local anesthetics on membrane properties. I. Changes in the fluidity of phospholipid bilayers. Biochim Biophys Acta. 1975 Jul 18;394(4):504–519. doi: 10.1016/0005-2736(75)90137-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Studies on the mechanism of action of local anesthetics with phospholipid model membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):169–186. doi: 10.1016/0304-4157(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Ray P. H., Brock T. D. Thermal lysis of bacterial membranes and its prevention by polyamines. J Gen Microbiol. 1971 May;66(2):133–135. doi: 10.1099/00221287-66-2-133. [DOI] [PubMed] [Google Scholar]
- Roufogalis B. D. Comparative studies on the membrane actions of depressant drugs: the role of lipophilicity in inhibition of brain sodium and potassium-stimulated ATPase. J Neurochem. 1975 Jan;24(1):51–61. doi: 10.1111/j.1471-4159.1975.tb07627.x. [DOI] [PubMed] [Google Scholar]
- Santos Mota J. M., Silva M. T., Carvalho Guerra F. Ultrastructural and chemical alterations induced by dicumarol in Streptococcus faecalis. Biochim Biophys Acta. 1971 Oct 12;249(1):114–121. doi: 10.1016/0005-2736(71)90088-5. [DOI] [PubMed] [Google Scholar]
- Seeman P., Roth S. General anesthetics expand cell membranes at surgical concentrations. Biochim Biophys Acta. 1972 Jan 17;255(1):171–177. doi: 10.1016/0005-2736(72)90019-3. [DOI] [PubMed] [Google Scholar]
- Seeman P., Roth S., Schneider H. The membrane concentrations of alcohol anesthetics. Biochim Biophys Acta. 1971 Feb 2;225(2):171–184. doi: 10.1016/0005-2736(71)90210-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic aspects of membrane alterations during bacterial cell lysis. Exp Cell Res. 1967 May;46(2):245–251. doi: 10.1016/0014-4827(67)90062-6. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Santos Mota J. M., Melo J. V., Guerra F. C. Uranyl salts as fixatives for electron microscopy. Study of the membrane ultrastructure and phospholipid loss in bacilli. Biochim Biophys Acta. 1971 Jun 1;233(3):513–520. doi: 10.1016/0005-2736(71)90151-9. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C., Macedo M. A., Polónia J., Parente A. M. Effects of phenethyl alcohol on Bacillus and Streptococcus. J Bacteriol. 1976 Sep;127(3):1359–1369. doi: 10.1128/jb.127.3.1359-1369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C., Polónia J. J., Macedo M. A., Parente A. M. Bacterial mesosomes. Real structures or artifacts? Biochim Biophys Acta. 1976 Aug 4;443(1):92–105. doi: 10.1016/0005-2736(76)90493-4. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructural alterations induced by moist heat in Bacillus cereus. Appl Microbiol. 1972 Sep;24(3):463–476. doi: 10.1128/am.24.3.463-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribhuwan R. C., Pradhan D. S. Induction of alkaline phosphatase in Escherichia coli: effect of procaine hydrochloride. J Bacteriol. 1977 Aug;131(2):431–437. doi: 10.1128/jb.131.2.431-437.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell J. R., Hubbell W. L., Cohen E. N. Electron spin resonance studies with the volatile anesthetics on phospholipid model membranes. Ann N Y Acad Sci. 1973 Dec 31;222:530–538. doi: 10.1111/j.1749-6632.1973.tb15285.x. [DOI] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]





