Abstract
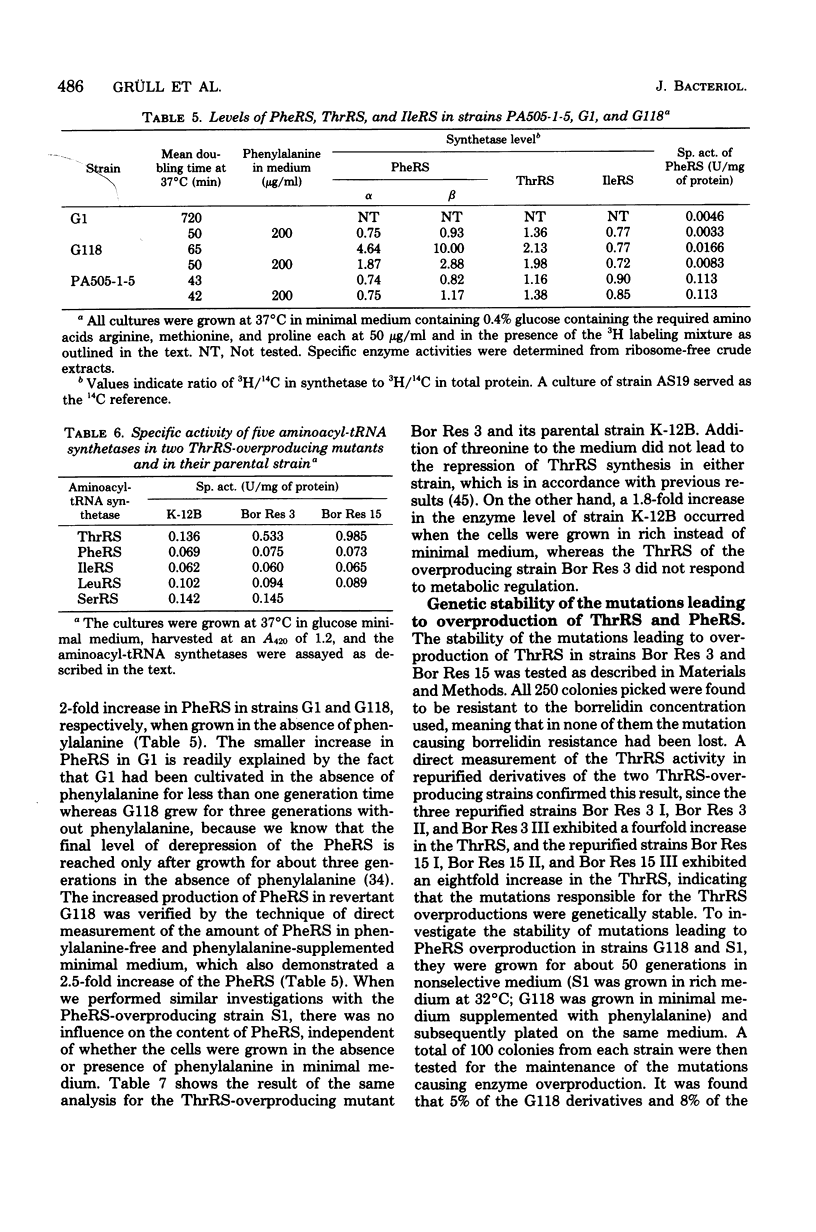
The structural genes for threonyl-tRNA synthetase (ThrRS) and phenylalanyl-tRNA synthetase (PheRS) are closely linked on the Escherichia coli chromosome. To study whether these enzymes share a common regulatory element, we have investigated their synthesis in mutants which were selected for overproduction of either ThrRS or PheRS. It was found that mutants isolated previously for overproduction of ThrRS as strains resistant to the antibiotic borrelidin (strains Bor Res 3 and Bor Res 15) did not show an elevated level of PheRS. PheRS-overproducing strains were then isolated as revertants of strains with structurally altered enzymes. Strain S1 is a temperature-resistant derivative of a temperature-sensitive PheRS mutant, and strain G118 is a prototrophic derivative of a PheRS mutant which shows phenylalanine auxotrophy as a consequence of an altered Km of this enzyme for the amino acid. In both kinds of revertants, S1 and G118, the concentration of PheRS and ThrRS was increased by factors of about 2.5 and 1.8, respectively, whereas the level of other aminoacyl-tRNA synthetases was not affected by the mutations. Genetic studies showed that the simultaneous overproduction of PheRS and ThrRS in revertants G118 and S1 is based upon gene amplification, since this property was easily lost after growing the cells in the absence of the selective stimulus, and since this loss could be prevented by the presence of the recA allele. By similar criteria, the four- and eightfold overproduction of ThrRS in strains Bor Res 3 and Bor Res 15, respectively, was very stable genetically, indicating that it is caused by a mutational event other than gene amplification. From these results, we conclude that the concomitant increase of PheRS and ThrRS in strains G118 and S1 is an expression of gene duplication and not of a joint regulation of these two aminoacyl-tRNA synthetases. This conclusion is further supported by the result that, in mutant G118 as well as in its parental strain G1, growth in minimal medium lacking phenylalanine led to an additional twofold increase of their PheRS concentration. This increase was restricted to the PheRS, since the level of other aminoacyl-tRNA synthetases, including the ThrRS, stayed unchanged.
Full text
PDF


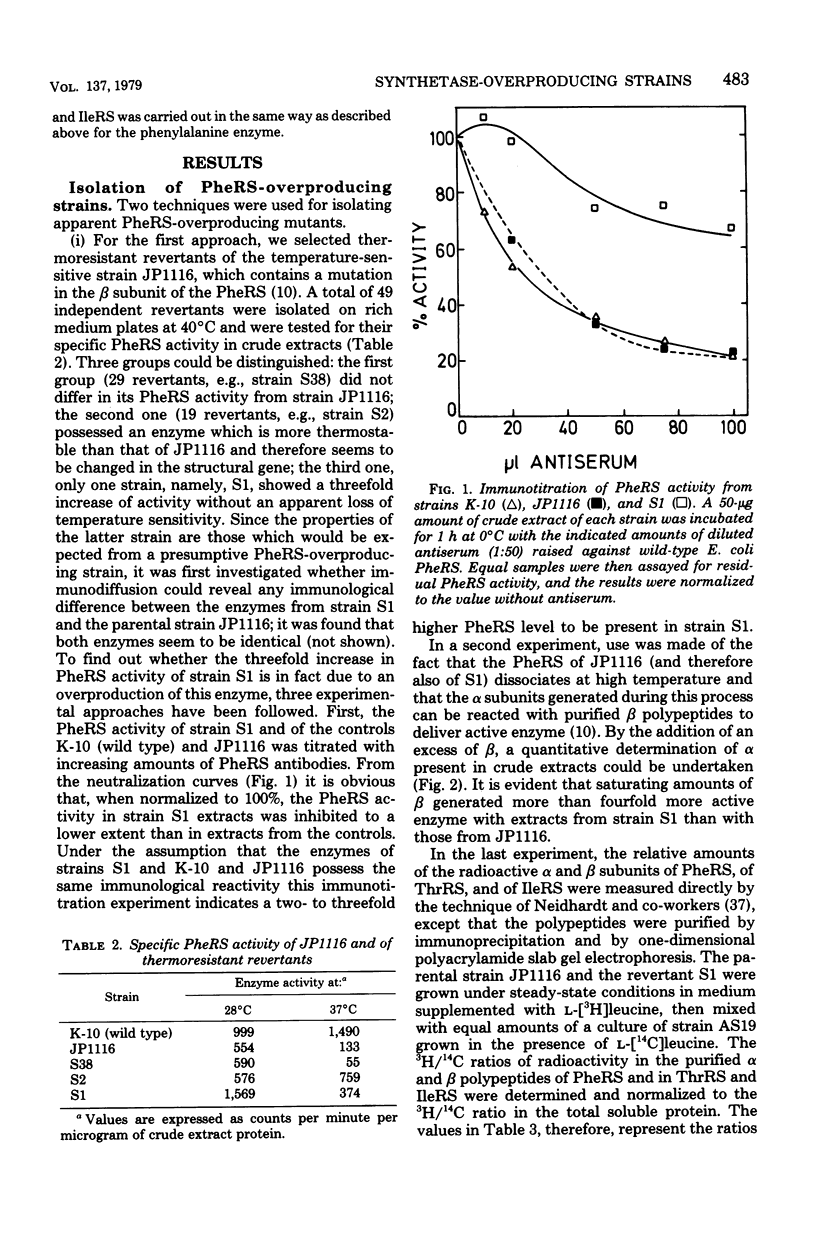
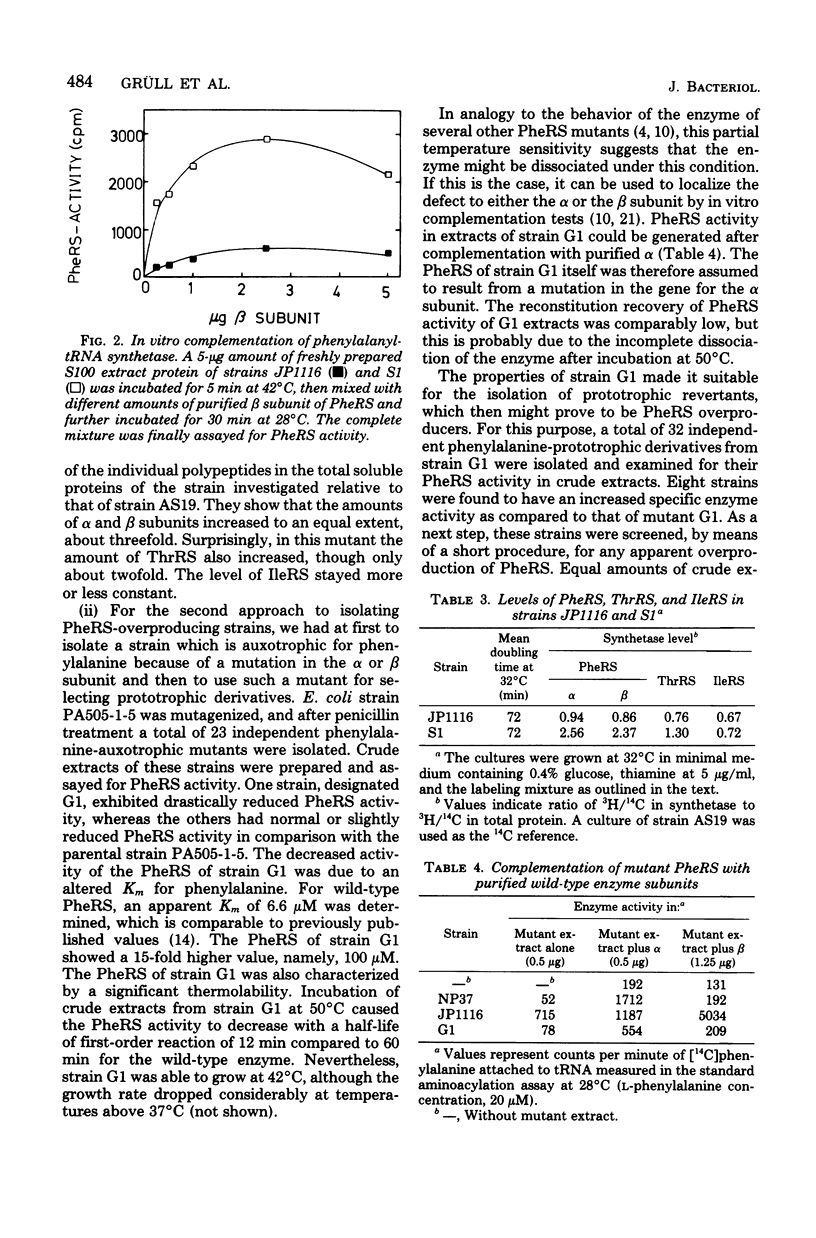
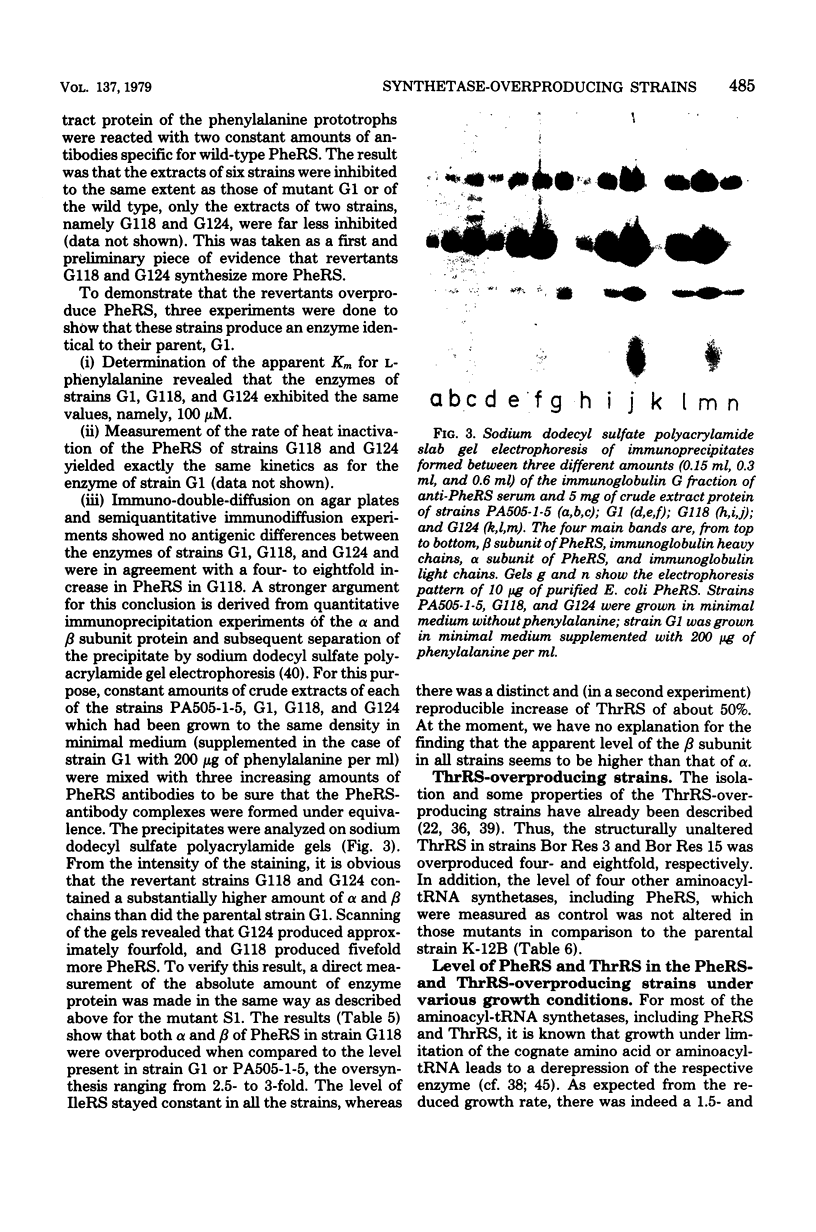



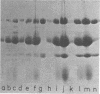
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A. Relation between subunit structure and temperature-sensitivity of mutant phenylalanyl RNA synthetases of Escherichia coli. Eur J Biochem. 1968 Apr;4(3):395–400. doi: 10.1111/j.1432-1033.1968.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem. 1971 May 28;20(2):283–300. doi: 10.1111/j.1432-1033.1971.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. Isolation and characterization of a regulatory mutant of an aminoacyl-transfer ribonucleic acid synthetase in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1096–1103. doi: 10.1128/jb.113.3.1096-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Nass G., Neidhardt F. C. Immunological and chemical studies of phenylalanyl sRNA synthetase from Escherichia coli. J Mol Biol. 1965 Aug;13(1):202–219. doi: 10.1016/s0022-2836(65)80090-0. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Duplication of the structural gene for glycyl-transfer RNA synthetase in Escherichia coli. J Mol Biol. 1971 Jun 14;58(2):595–610. doi: 10.1016/0022-2836(71)90374-3. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T., Bartmann P., Hennecke H., Kosakowski H. M., Jaenicke R., Holler E., Böck A. L-phenylalanyl-tRNA synthetase of Escherichia coli K-10. A reinvestigation of molecular weight and subunit structure. Eur J Biochem. 1974 Apr 16;43(3):601–607. doi: 10.1111/j.1432-1033.1974.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Heinonen J., Artz S. W., Zalkin H. Regulation of the tyrosine biosynthetic enzymes in Salmonella typhimurium: analysis of the involvement of tyrosyl-transfer ribonucleic acid and tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1972 Dec;112(3):1254–1263. doi: 10.1128/jb.112.3.1254-1263.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Böck A. Altered alpha subunits in phenylalanyl-tRNA synthetases from p-fluorophenylalanine-resistant strains of Escherichis coli. Eur J Biochem. 1975 Jul 1;55(2):431–437. doi: 10.1111/j.1432-1033.1975.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Böck A., Thomale J., Nass G. Threonyl-transfer ribonucleic acid synthetase from Escherichia coli: subunit structure and genetic analysis of the structural gene by means of a mutated enzyme and of a specialized transducing lambda bacteriophage. J Bacteriol. 1977 Sep;131(3):943–950. doi: 10.1128/jb.131.3.943-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Springer M., Böck A. A specialized transducing lambda phage carrying the Escherichia coli genes for phenylalanyl-tRNA synthetase. Mol Gen Genet. 1977 Apr 29;152(3):205–210. doi: 10.1007/BF00268819. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C., Konigsberg W., Katze J. R. A structural gene for seryl-tRNA synthetase in Escherichia coli K12. J Mol Biol. 1970 Feb 14;47(3):619–625. doi: 10.1016/0022-2836(70)90332-3. [DOI] [PubMed] [Google Scholar]
- Kosakowski M. H., Böck A. The subunit structure of phenylalanyl-tRNA synethetase of Escherichia coli. Eur J Biochem. 1970 Jan;12(1):67–73. doi: 10.1111/j.1432-1033.1970.tb00821.x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRossa R., Vögell G., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. II. Isolation of regulatory mutants affecting leucyl-tRNA synthetase levels. J Mol Biol. 1977 Dec 25;117(4):1033–1048. doi: 10.1016/s0022-2836(77)80011-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matzura H., Molin S., Maaloe O. Sequential biosynthesis of the and ' subunits of the DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):17–25. doi: 10.1016/0022-2836(71)90410-4. [DOI] [PubMed] [Google Scholar]
- McKeever W. G., Neidhardt F. C. Growth rate modulation of four aminoacyl-transfer ribonucleic acid synthetases in enteric bacteria. J Bacteriol. 1976 May;126(2):634–645. doi: 10.1128/jb.126.2.634-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G., Poralla K., Zähner H. Effect of the antibiotic Borrelidin on the regulation of threonine biosynthetic enzymes in E. coli. Biochem Biophys Res Commun. 1969 Jan 6;34(1):84–91. doi: 10.1016/0006-291x(69)90532-4. [DOI] [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Nass G., Thomale J. Alteration of structure of level of threonyl-tRNA-synthetase in Borrelidin resistant mutants of E. coli. FEBS Lett. 1974 Feb 15;39(2):182–186. doi: 10.1016/0014-5793(74)80046-3. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Paetz W., Nass G. Biochemical and immunological characterization of threonyl-tRNA synthetase of two borrelidin-resistant mutants of Escherichia coli K12. Eur J Biochem. 1973 Jun;35(2):331–337. doi: 10.1111/j.1432-1033.1973.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Piepersberg A., Hennecke H., Engelhard M., Nass G., Böck A. Cross-reactivity of phenylalanyl-transfer ribonucleic acid ligases from different microorganisms. J Bacteriol. 1975 Dec;124(3):1482–1488. doi: 10.1128/jb.124.3.1482-1488.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., McKitrick J., Tosa T. Characterization of a mutant of E. coli with elevated levels of seryl-tRNA synthetase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1351–1357. doi: 10.1016/0006-291x(72)90615-8. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. S., D'Ari Straus L. Large overlapping tandem genetic duplications in Salmonella typhimurium. J Mol Biol. 1976 May 5;103(1):143–153. doi: 10.1016/0022-2836(76)90056-5. [DOI] [PubMed] [Google Scholar]
- Thomale J., Nass G. Alteration of the intracellular concentration of aminoacyl-tRNA synthetases and isoaccepting tRNAs during amino-acid limited growth in Escherichia coli. Eur J Biochem. 1978 Apr 17;85(2):407–418. doi: 10.1111/j.1432-1033.1978.tb12253.x. [DOI] [PubMed] [Google Scholar]
- VON EHRENSTEIN G., LIPMANN F. Experiments on hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1961 Jul 15;47:941–950. doi: 10.1073/pnas.47.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]
- Wirth R., Buckel P., Böck A. DNA-dependent in vitro synthesis of Escherichia coli ribosomal protein S20 and isoleucyl-tRNA synthetase. Effect of guanosine-5'-diphosphate-3'-diphosphate. FEBS Lett. 1977 Nov 1;83(1):103–106. doi: 10.1016/0014-5793(77)80651-0. [DOI] [PubMed] [Google Scholar]