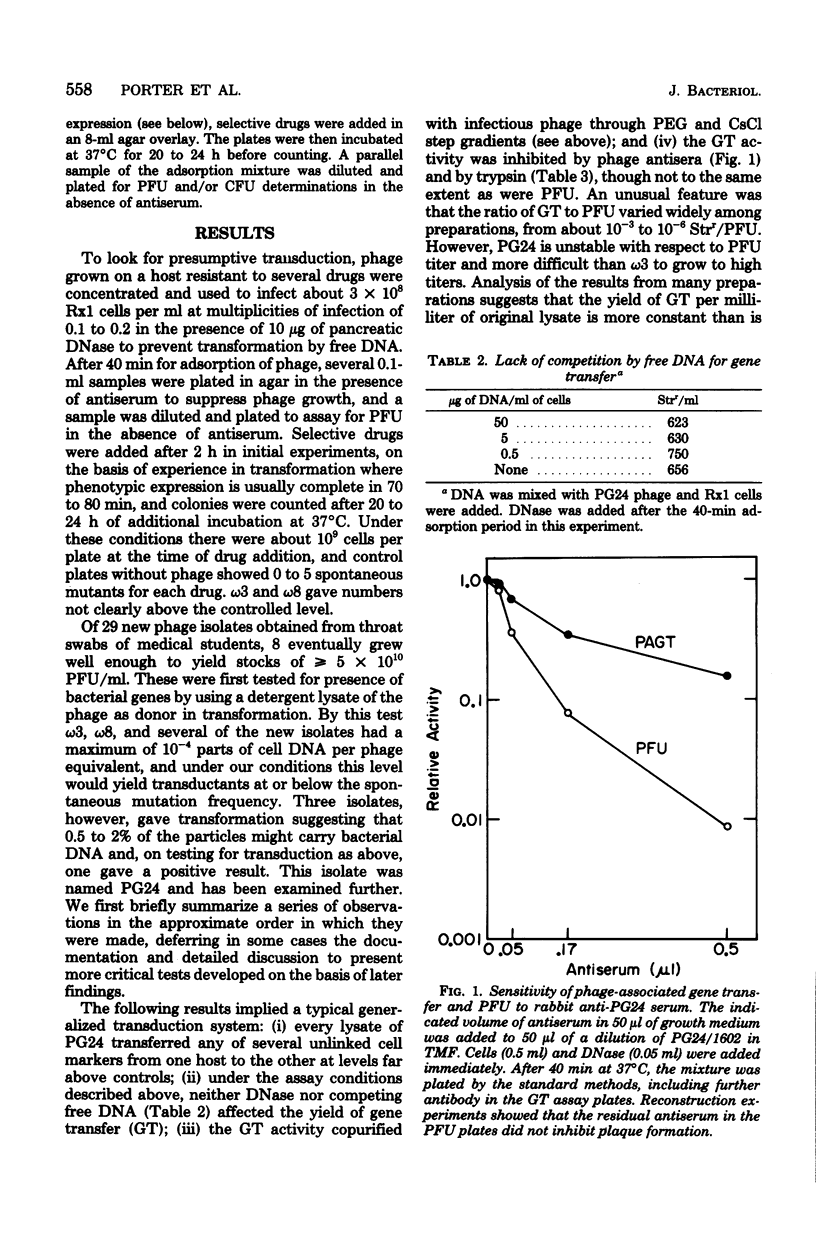
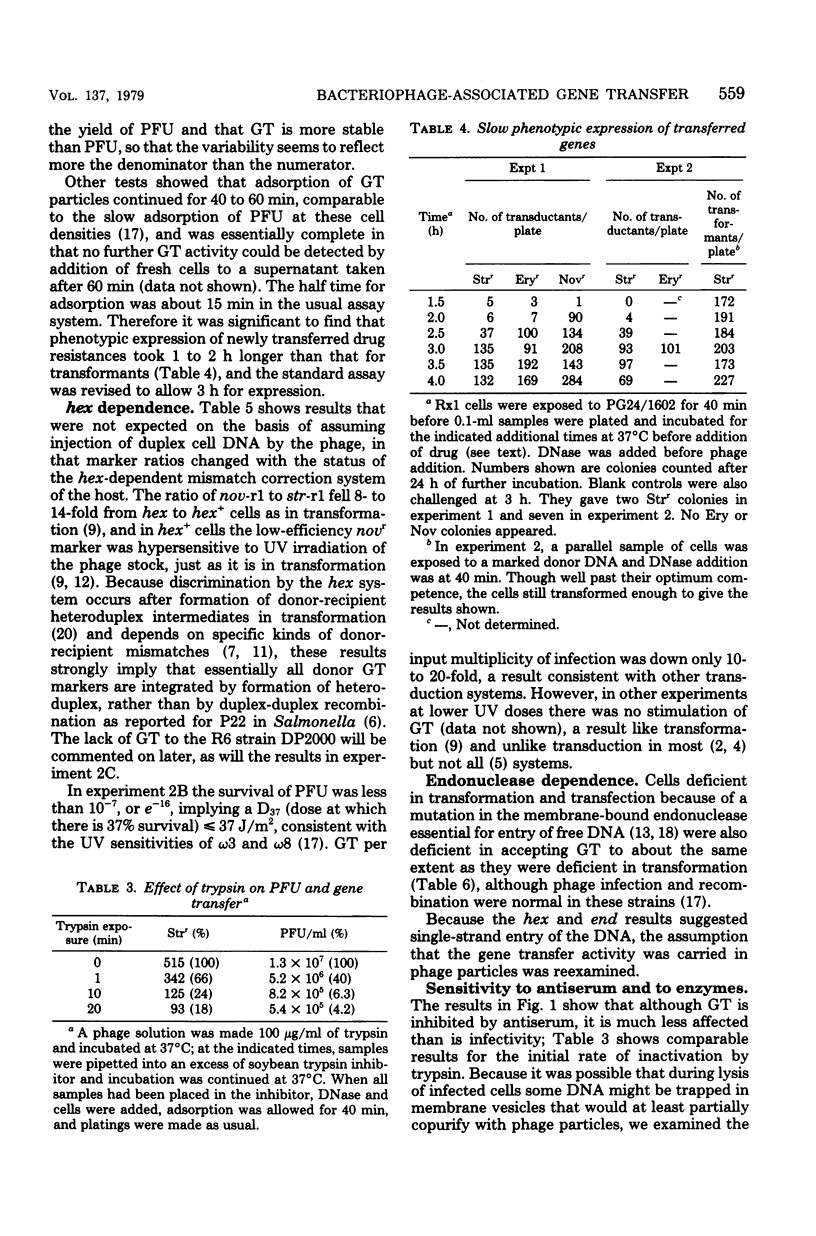
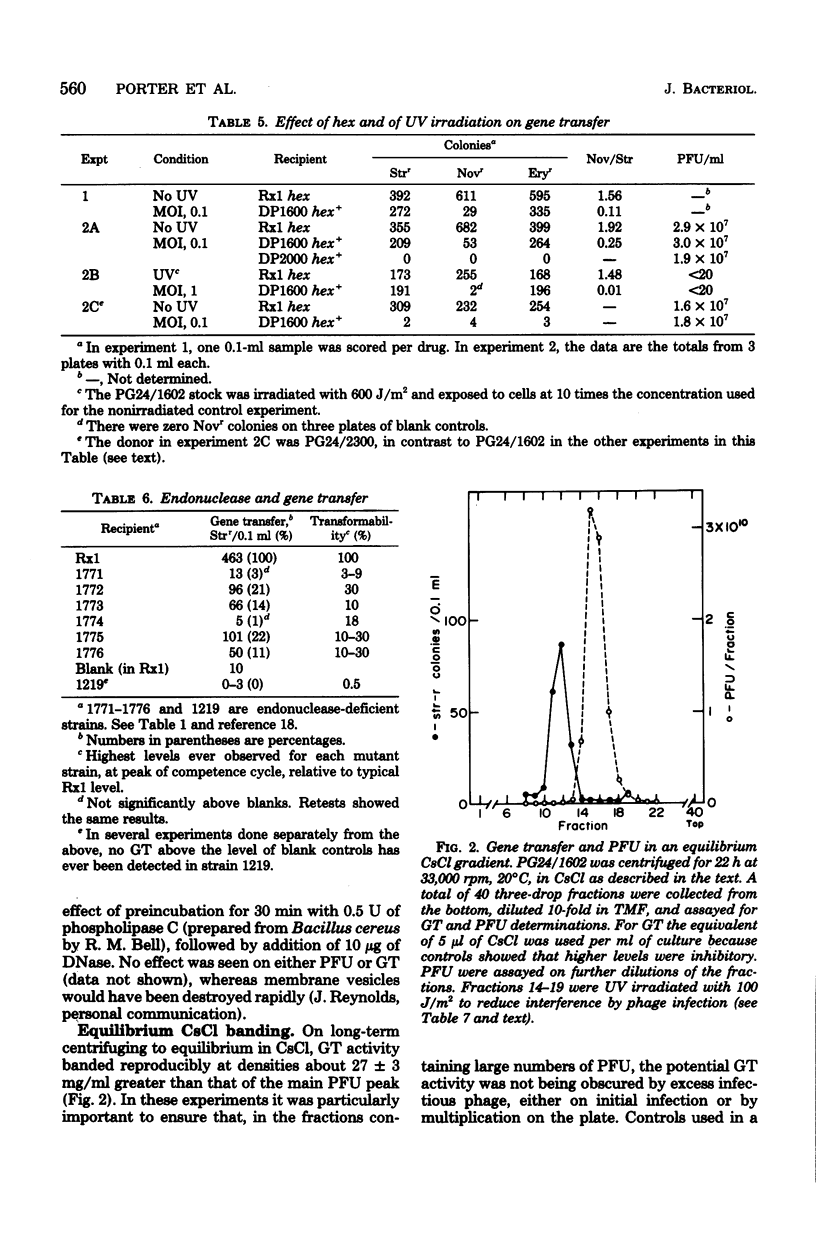
Abstract
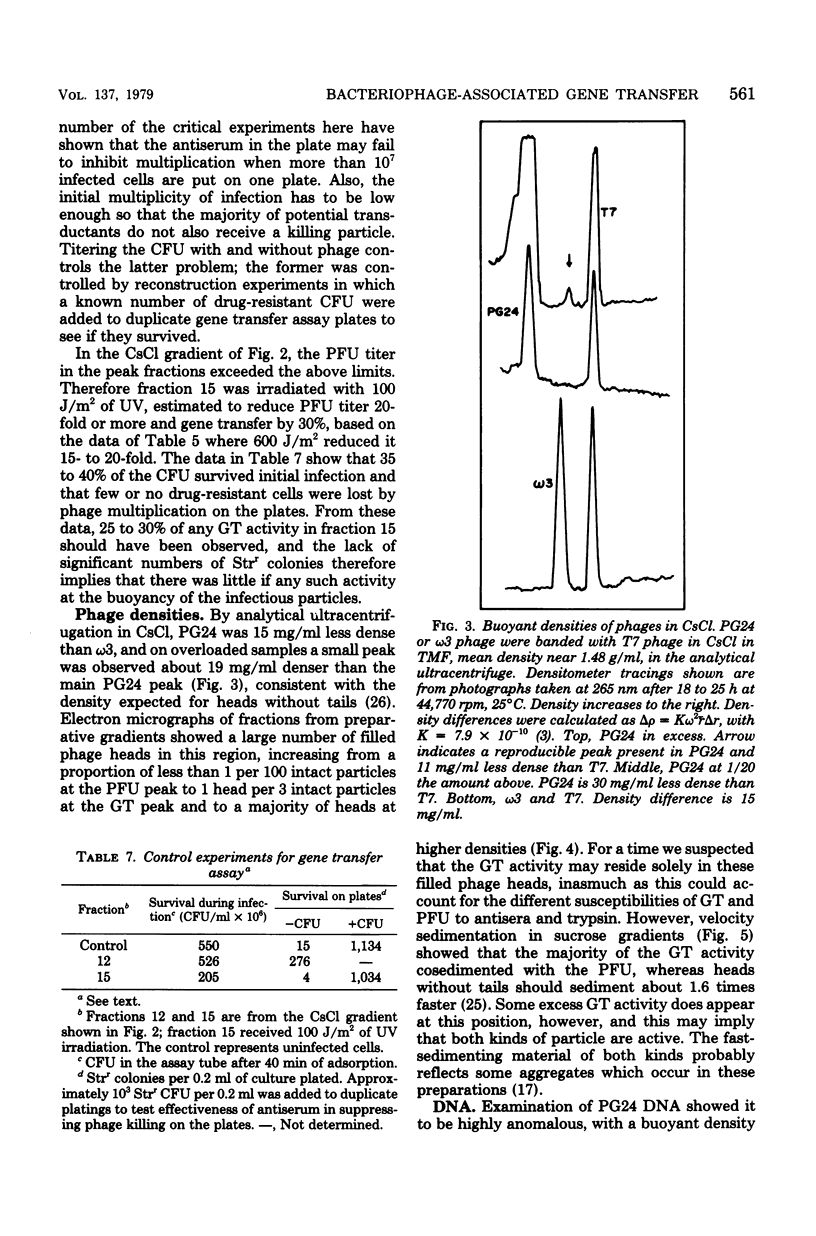
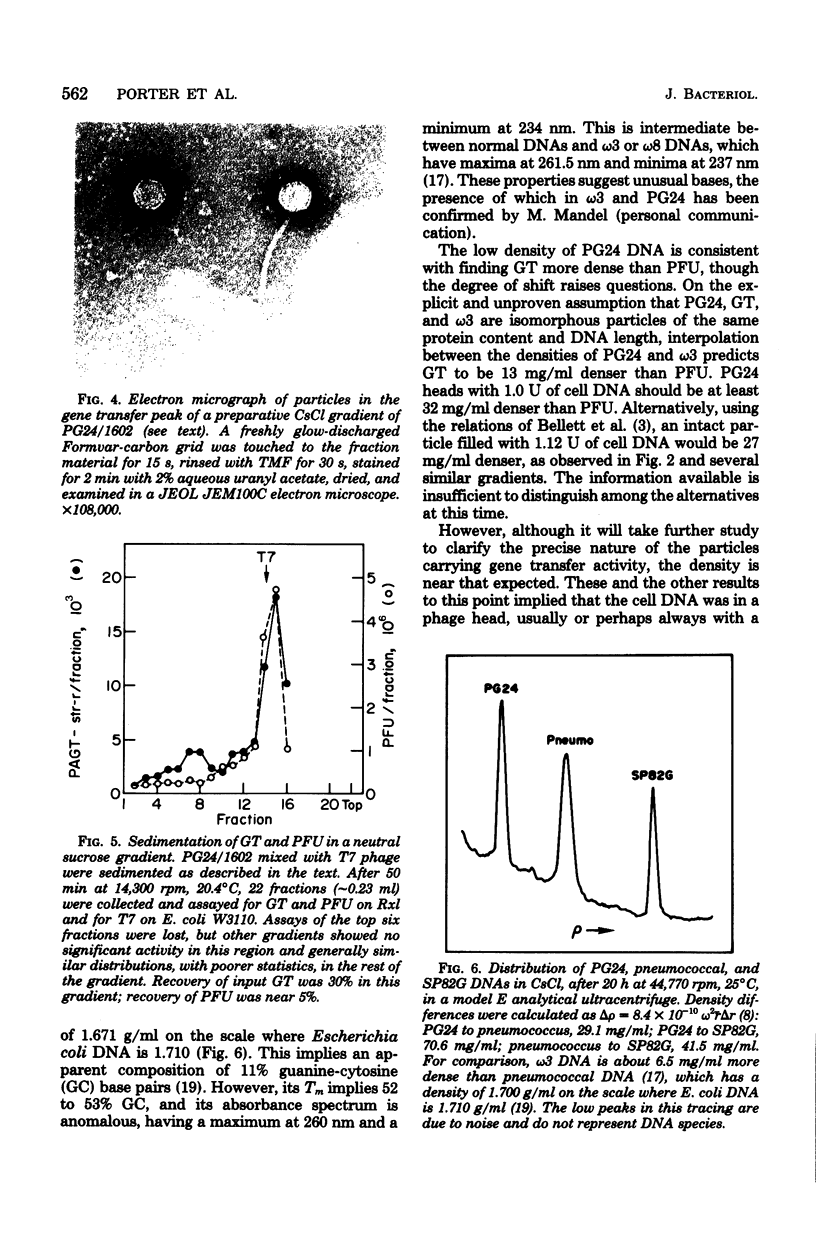
Lysates of pneumococcal phage PG24 transferred genes from one host to another in a process with many of the properties of generalized transduction, in that the host genes were packaged in DNase-resistant particles that closely resembled infectious phage in physical properties, adsorbed to the recipient cells like phage, and were inhibited by antisera to the phage and by trypsin. However, phage processes did not complete the transfer of host DNA as they did phage DNA. Instead, gene transfer required development of competence and entry of the host DNA by the endonuclease-dependent pathway used for transforming and transfecting DNA. This process often occurred on the assay plate hours after adsorption of the particles to the cells, and the transfer was DNase sensitive if challenged at this time. Phenotypic expression was therefore also delayed. The product of entry was like that in transformation, a single strand of DNA that integrates by formation of a hex-sensitive donor-recipient heteroduplex. Whether this gene transfer process is unique to this system or is only the first one described is not clear. The term "pseudotransduction" may be useful in calling attention to its unexpected features. The DNA of PG24 phage has anomalous physical properties reflecting unusual bases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Adams A. Transformation and transduction of a large deletion mutation in Bacillus subtilis. Mol Gen Genet. 1972;118(4):311–320. doi: 10.1007/BF00333566. [DOI] [PubMed] [Google Scholar]
- BENZINGER R., HARTMAN P. E. Effects of ultraviolet light on transducing phage P22. Virology. 1962 Dec;18:614–626. doi: 10.1016/0042-6822(62)90064-8. [DOI] [PubMed] [Google Scholar]
- Drexler H., Kylberg K. J. Effect of UV irradiation on transduction by coliphage T1. J Virol. 1975 Aug;16(2):263–266. doi: 10.1128/jvi.16.2.263-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Fox M. S., Botstein D. Generalized transduction by bacteriophage P22 in Salmonella typhimurium. II. Mechanism of integration of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):449–469. doi: 10.1016/0022-2836(72)90362-2. [DOI] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILD W. R. Evidence for intramolecular heterogeneity in pneumococcal DNA. J Mol Biol. 1963 Mar;6:214–229. doi: 10.1016/s0022-2836(63)80071-6. [DOI] [PubMed] [Google Scholar]
- Guild W. R., Shoemaker N. B. Intracellular competition for a mismatch recogition system and marker-specific rescue of transforming DNA from inactivation by ultraviolet irradiation. Mol Gen Genet. 1974;128(4):291–300. doi: 10.1007/BF00268517. [DOI] [PubMed] [Google Scholar]
- Guild W. R., Shoemaker N. B. Mismatch correction in pneumococcal transformation: donor length and hex-dependent marker efficiency. J Bacteriol. 1976 Jan;125(1):125–135. doi: 10.1128/jb.125.1.125-135.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Transfection in pneumococcus: single-strand intermediates in the formation of infective centers. J Virol. 1978 Jan;25(1):60–72. doi: 10.1128/jvi.25.1.60-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol Gen Genet. 1974;128(4):283–290. doi: 10.1007/BF00268516. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Guyer M. S., Timmis K., Cabello F., Cohen S. N., Davidson N., Clark A. J. Replication region fragments cloned from Flac+ are identical to EcoRI fragment f5 of F. J Bacteriol. 1976 Sep;127(3):1571–1575. doi: 10.1128/jb.127.3.1571-1575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M., Marrs B. The gene transfer agent of Rhodopseudomonas capsulata. Purification and characterization of its nucleic acid. Arch Biochem Biophys. 1977 May;181(1):300–307. doi: 10.1016/0003-9861(77)90508-2. [DOI] [PubMed] [Google Scholar]
- Tiraby J. G., Tiraby E., Fox M. S. Pneumococcal bacteriophages. Virology. 1975 Dec;68(2):566–569. doi: 10.1016/0042-6822(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. Studies on head-tail union in bacteriophage lambda. J Mol Biol. 1968 Apr 28;33(2):483–489. doi: 10.1016/0022-2836(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]