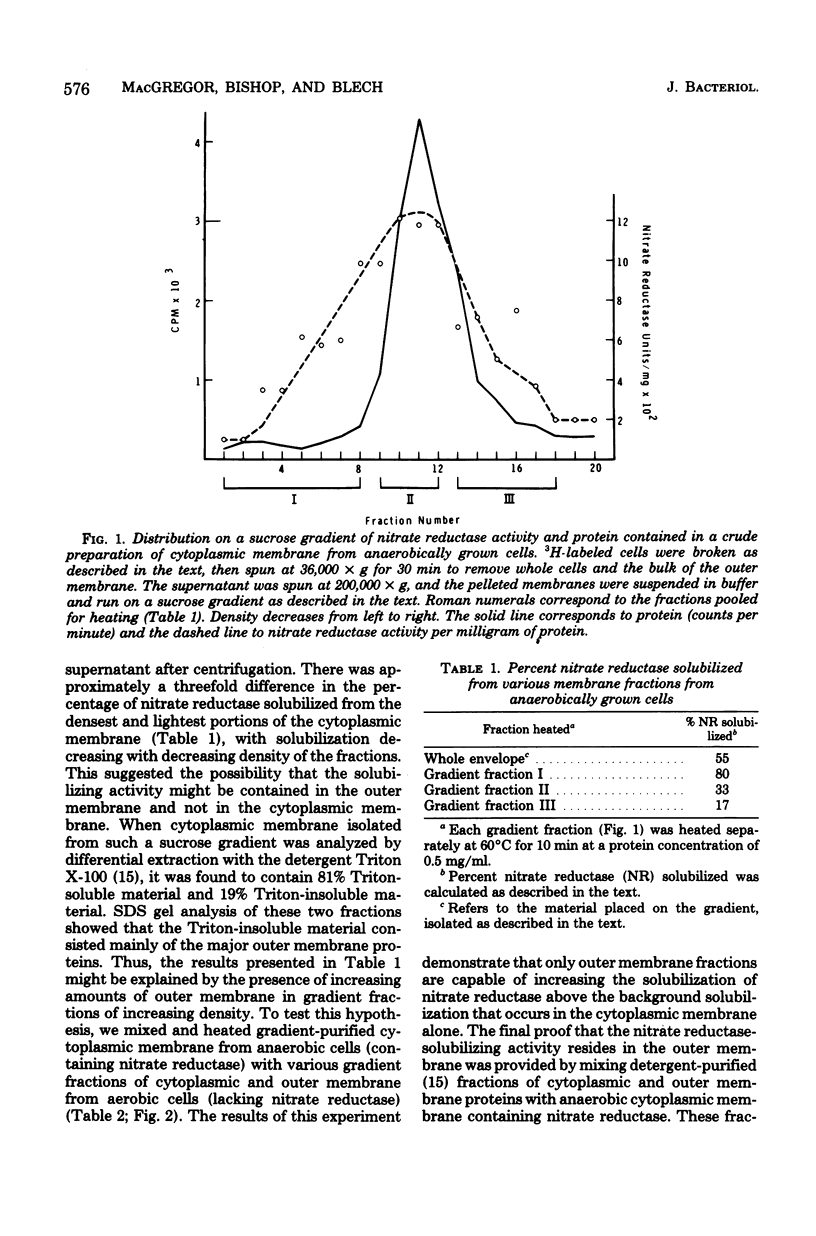
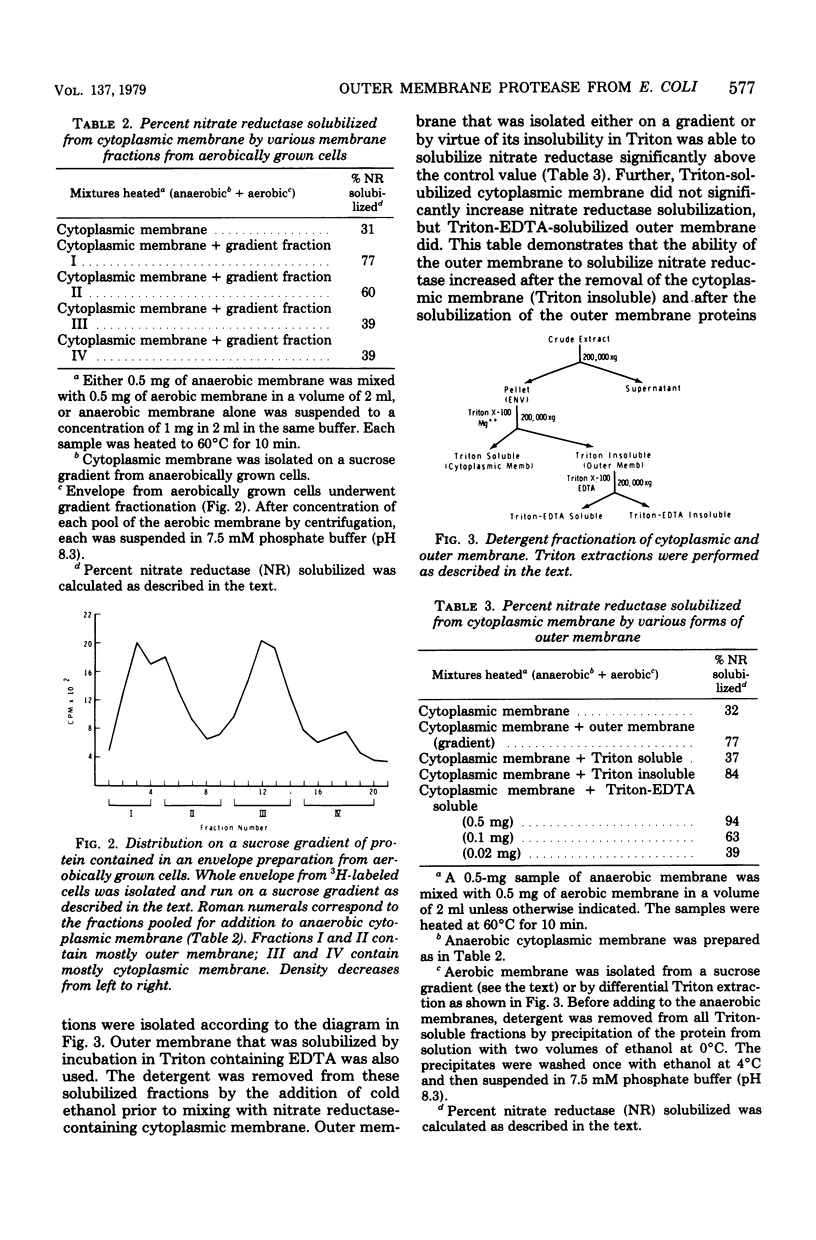
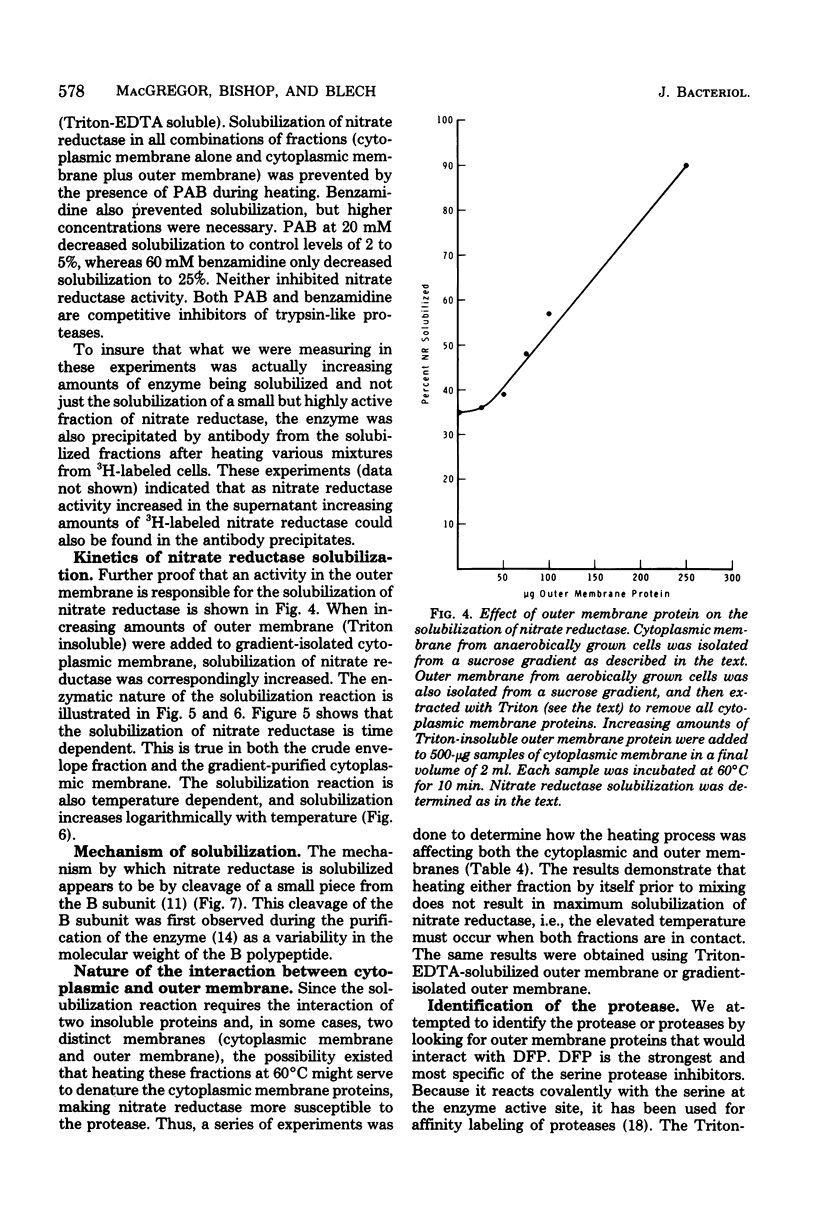
Abstract
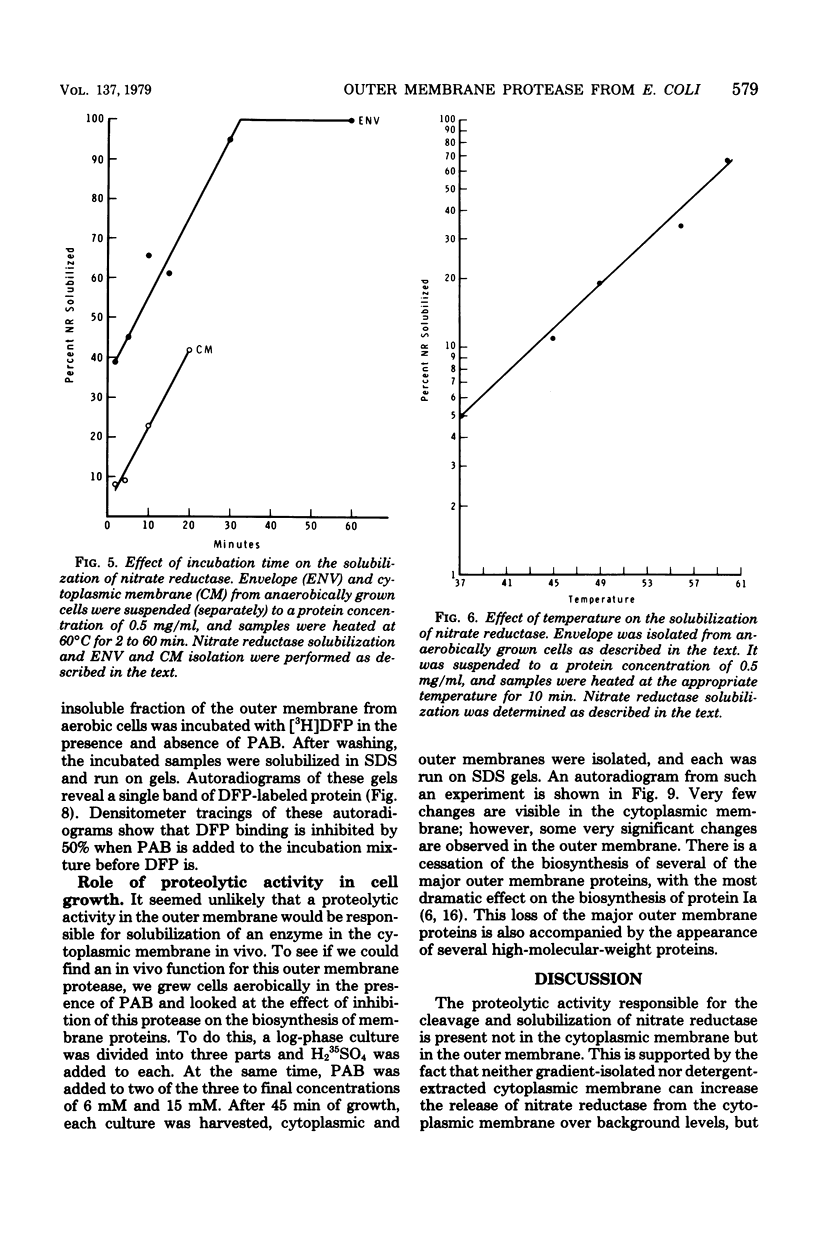
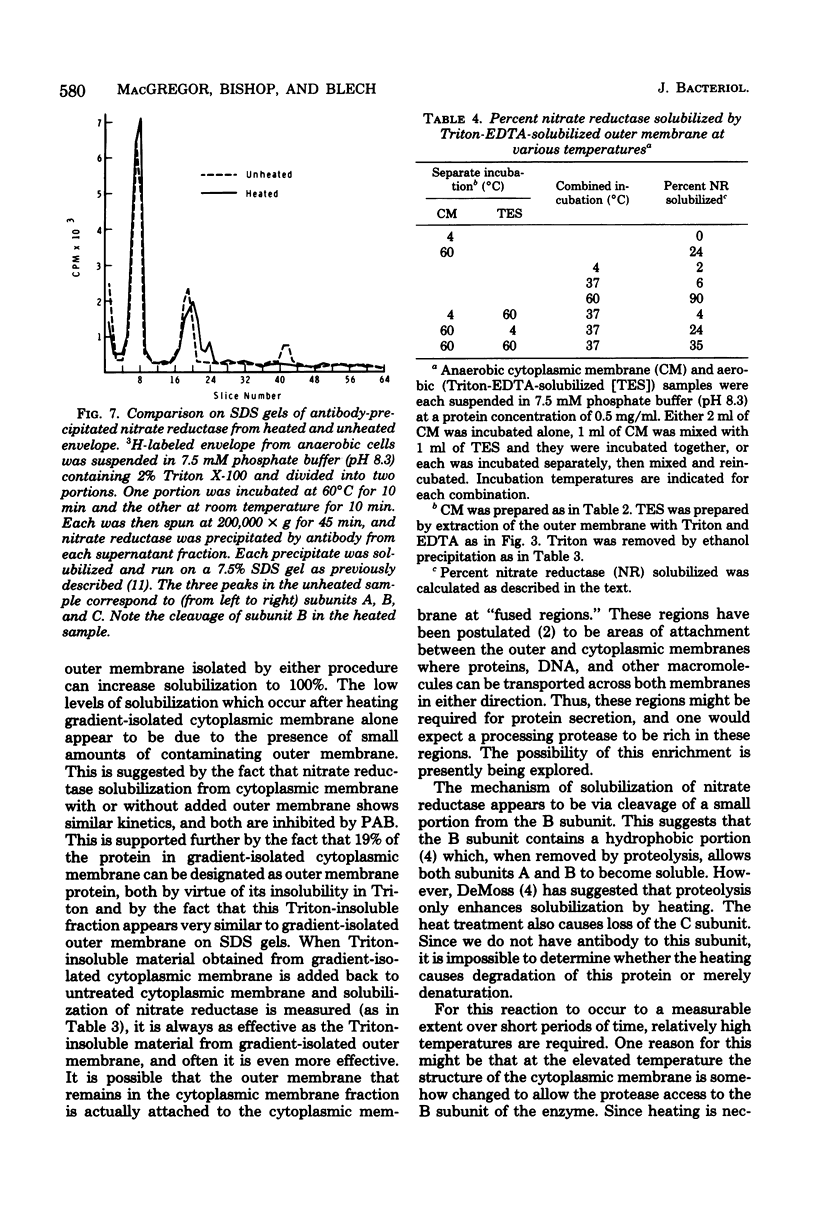
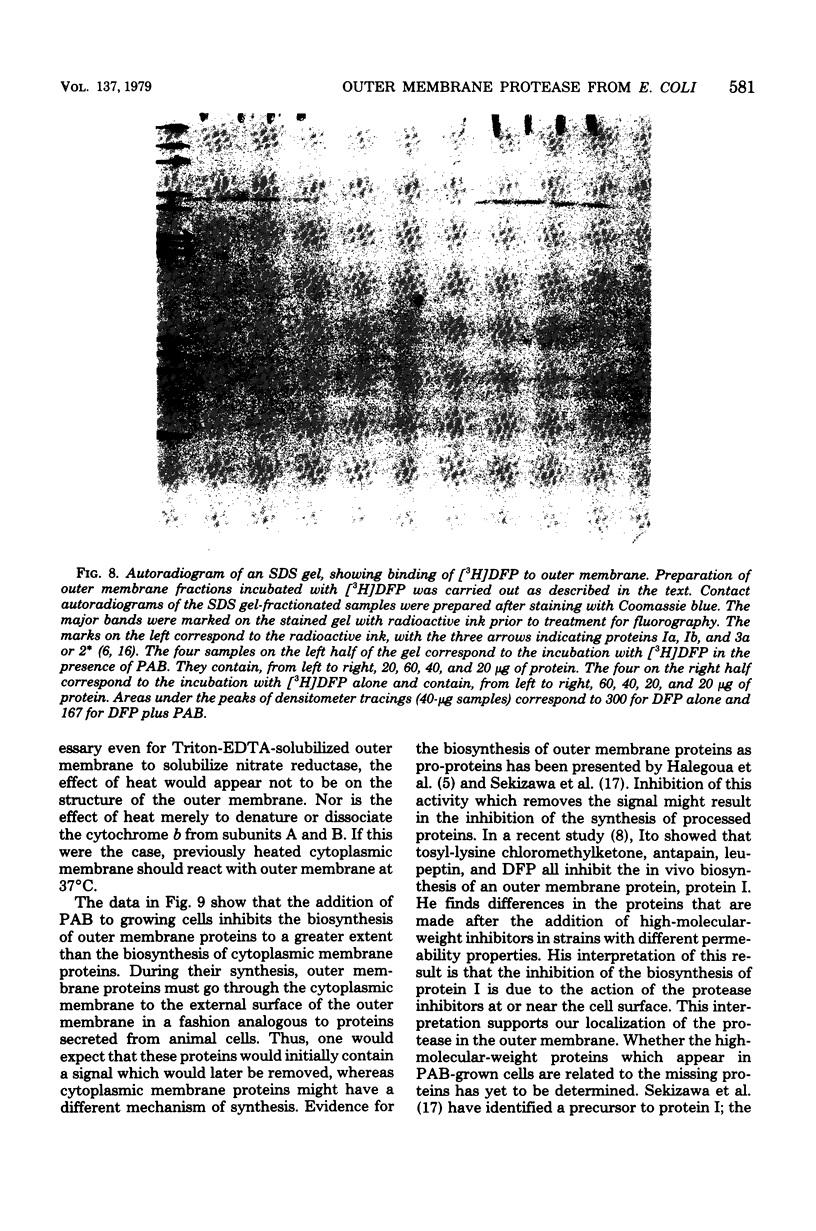
An enzyme in the cytoplasmic membrane, nitrate reductase, can be solubilized by heating membranes to 60 degrees C for 10 min at alkaline pH. A protease in the cell envelope has been shown to be responsible for this solubilization. The localization of this protease in the outer membrane was demonstrated by separating the outer membrane from the cytoplasmic membrane, adding back various forms of outer membrane protein to the cytoplasmic membrane, and following the increase in nitrate reductase solubilization with increasing amounts of outer membrane proteins. This solubilization is accompanied by the cleavage of one of the subunits of nitrate reductase and is inhibited by the protease inhibitor p-aminobenzamidine. Analysis of membrane proteins synthesized by cells grown in the presence of various amounts of p-aminobenzamidine revealed that p-aminobenzamidine affects the synthesis of the major outer membrane proteins but has little effect on the synthesis of cytoplasmic membrane proteins. When outer membrane is reacted with the protease inhibitor [3H]diisopropylfluorophosphate, a single protein in the outer membrane is labeled. Since the interaction with diisopropylfluorophosphate is inhibited by p-aminobenzamidine, it is suggested that this single outer membrane protein is responsible for the in vitro solubilization of nitrate reductase and the in vivo processing of the major outer membrane proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyappa P. S., Traficante L. J., Lampen J. O. Penicillinase-releasing protease of Bacillus licheniformis: purification and general properties. J Bacteriol. 1977 Jan;129(1):191–197. doi: 10.1128/jb.129.1.191-197.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A. Limited proteolysis of nitrate reductase purified from membranes of Escherichia coli. J Biol Chem. 1977 Mar 10;252(5):1696–1701. [PubMed] [Google Scholar]
- Halegoua S., Sekizawa J., Inouye M. A new form of structural lipoprotein of outer membrane of Escherichia coli. J Biol Chem. 1977 Apr 10;252(7):2324–2330. [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Protease inhibitors inhibit production of protein I of the outer membrane in Escherichia coli. Biochem Biophys Res Commun. 1978 May 15;82(1):99–107. doi: 10.1016/0006-291x(78)90582-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Tökés Z. A., Chambers S. M. Proteolytic activity associated with human erythrocyte membranes. Self-digestion of isolated human erythrocyte membranes. Biochim Biophys Acta. 1975 May 6;389(2):325–338. doi: 10.1016/0005-2736(75)90325-9. [DOI] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]