Summary
For mammalian somatic cells the importance of microtubule cytoskeleton integrity in interphase cell cycle progression is uncertain. The loss, diminishment, or stabilization of the microtubule cytoskeleton has been widely reported to cause a G1 arrest in a variable, and often high, proportion of cell populations suggesting the existence of a “microtubule damage”, “microtubule integrity”, or “post-mitotic” checkpoint in G1 or G2 [1-7]. We find that when normal human cells (hTERT RPE1 and primary fibroblasts) are continuously exposed to nocodazole, they remain in mitosis for 10-48 hours before they slip out of mitosis and arrest in G1, consistent with previous reports [2, 4 and 6]. To eliminate the persistent effects of prolonged mitosis, we isolate anaphase-telophase cells just finishing a mitosis of normal duration and then rapidly/completely disassemble microtubules with a pulse of cold followed by continuous nocodazole or Colcemid treatment to ensure that the cells enter G1 without a microtubule cytoskeleton. Without microtubules, cells progress from anaphase to a subsequent mitosis with essentially normal kinetics. Similar results are obtained for cells in which the microtubule cytoskeleton is partially diminished by lower nocodazole doses or augmented/stabilized with Taxol. Thus, after a preceding mitosis of normal duration, the integrity of the microtubule cytoskeleton is not subject to checkpoint surveillance nor is it required for the normal human cell to progress through G1 and the remainder of interphase.
Keywords: cell cycle, cytoskeleton, G1, microtubule
Results and Discussion
The mammalian somatic cell in early G1 is sensitive to a number of intracellular and extracellular stimuli; the integration of growth promoting and growth inhibiting inputs determines whether the cell will commit to enter the cell cycle or not [8-12]. The integrity of the actin cytoskeleton is important for the cell to enter S phase. Even a slight perturbation of the actin cytoskeleton with cytochalasin leads to a durable G1 arrest [13-20]. Since the interphase array of microtubules, focused on the centrosome, is necessary for a variety of important cellular processes, its integrity could be necessary for the cell to progress through G1 as is the case for the actin cytoskeleton. In this regard, at least 27 studies (tabulated in Supplemental Materials Table S1, A-C) contain data bearing on the impact of altering the microtubule cytoskeleton on G1 progression for a variety of mammalian cell lines. Almost all report that alterations of the microtubule cytoskeleton lead to a G1 arrest in a variable and often high proportion of the cell populations, particularly for cell lines expected to have an intact p53 pathway. However, the response of cells within a population to microtubule perturbation is typically not uniform, with a variable portion of the cells progressing past G1 in the absence of microtubules. Of note are three studies which report substantial G1 progression in relatively normal cells with a partially or completely disassembled microtubule array (Table S1, A, lines highlighted in gray). The varied results of these studies prompted us to directly examine if and how perturbation of the microtubule cytoskeleton influences G1 progression in normal human cells. This issue is of interest, because a number of microtubule targeting drugs are currently used as chemotherapeutic agents for human cancer patients (reviewed in [21, 22]).
We used primary human fibroblasts and hTERT RPE1 cells which are normal human cells immortalized by the expression of the reverse-transcriptase subunit of telomerase. These cells have an intact p53 pathway as evidenced by cell cycle arrest with elevated levels of p21 in response to DNA damage (data not shown). We shook off mitotic cells from asynchronous populations (Figure 1, A, F) to obtain cells in mitotic stages ranging from prometaphase to telophase (Figure 1, B, G). Within 3 minutes the cells were exposed to 1.6-3.2 μM nocodazole or 1μM Colcemid and then chilled to 0°C. This caused the immediate and complete disassembly of spindle microtubules (Figure 1, C). For anaphase-telophase cells no stable microtubules were seen in the region between the separated chromosomes after cold/nocodazole treatment (Figure 1, C and Figure S1, A). This rapid and persistent disassembly of microtubules was important because it ensured that the cells did not later enter G1 with partial but declining microtubule cytoskeleton that could in principle support some measure of G1 progression. After 10 minutes of cold, the population was plated out on coverslips and warmed to 37°C in the continued presence of microtubule inhibitor. Those cells in prometaphase remained arrested in mitosis due to the activity of the spindle assembly checkpoint while those in anaphase-telophase completed mitosis without completion of cleavage in ∼2/3 cases, flattened out, and attached to the coverslip (Figure 1, D). ∼60 minutes after the cells were re-plated, the coverslips were washed to remove the round, non-adherent prometaphase cells and BrdU was added to the medium. This protocol allowed us to obtain a population of initially anaphase-telophase cells that completed mitosis and later entered G1 in the complete absence of microtubules. Three hours after shake off these cells contained no microtubules (Figure 1E) indicating a persistent microtubule knockdown. Some coverslips were later fixed to assay for BrdU incorporation and individual cells were continuously followed on other coverslips by video time lapse microscopy to determine if and when they entered the next mitosis.
Figure 1.
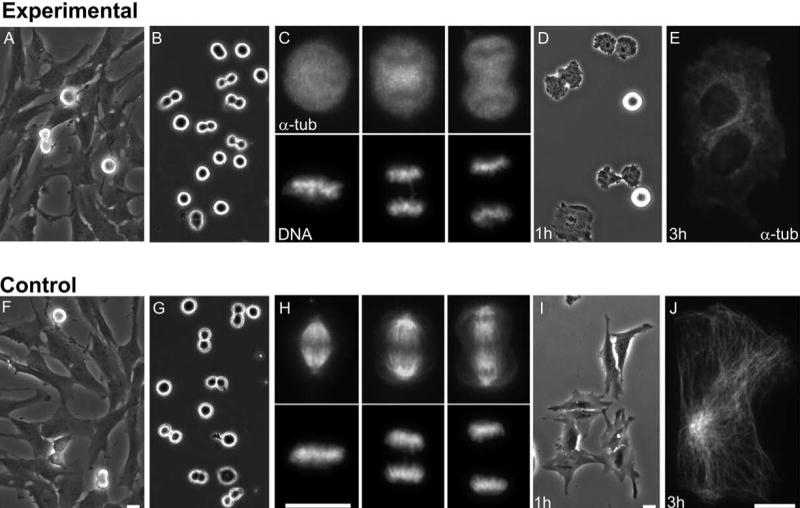
Collecting anaphase-telophase cells and microtubule disassembly. A-E. Experimental cells. A. Culture of freely cycling RPE1 cells. B. Cells shaken off such cultures, chilled to 0 °C for 10 minutes, and treated with microtubule inhibitor. C. Representative mitotic cells fixed immediately after rewarming and introduction of 1.6-3.2μM Nocodazole (or 1μM Colcemid). Upper panels: immuno-staining for alpha tubulin reveals that no microtubules are present. Lower panels: chromosome distribution in the same cells (Hoechst label). D. One hour after shake off. Anaphase-telophase cells finish mitosis and begin to spread out on the coverslip. Cells arrested in prometaphase are still round and non-adherent. E. A cell fixed for alpha tubulin immunofluorescence 3 hours after shake off. No microtubules are present. This cell failed cleavage and is thus binucleate. F-J. Control cells. F. Culture before shake off of mitotic cells. G. Cells shaken off such cultures, chilled to 0 °C for 10 minutes. H. Representative mitotic cells fixed 10 minutes after rewarming. Upper panels: immuno-staining for alpha tubulin reveals reassembly of normal spindles. Lower panels: chromosome distribution in the same cells (Hoechst label). I. Cells 1 hour after shake off. All cells finish mitosis and begin to spread out on the coverslip. J. Normal interphase microtubule cytoskeleton in a cell fixed for alpha tubulin immunofluorescence 3 hours after shake off. Phase contrast and fluorescence microscopy. Bars = 20μm
Control experiments were conducted in the same fashion with the exception that no microtubule inhibitor was added. We found that control cells rapidly reassembled spindles upon re-warming after the cold treatment, divided in a normal fashion within 1 hour, and flattened out as they entered G1 (Figure 1, H, I). Three hours after shake off all cells contained a normal interphase array of microtubules (Figure 1, J). By 18 hours, 94% had entered S phase as determined by BrdU incorporation and all 34 cells individually followed entered mitosis by 28 hours. Interphase duration was on average 21 hours (Figure 2, top line).
Figure 2.
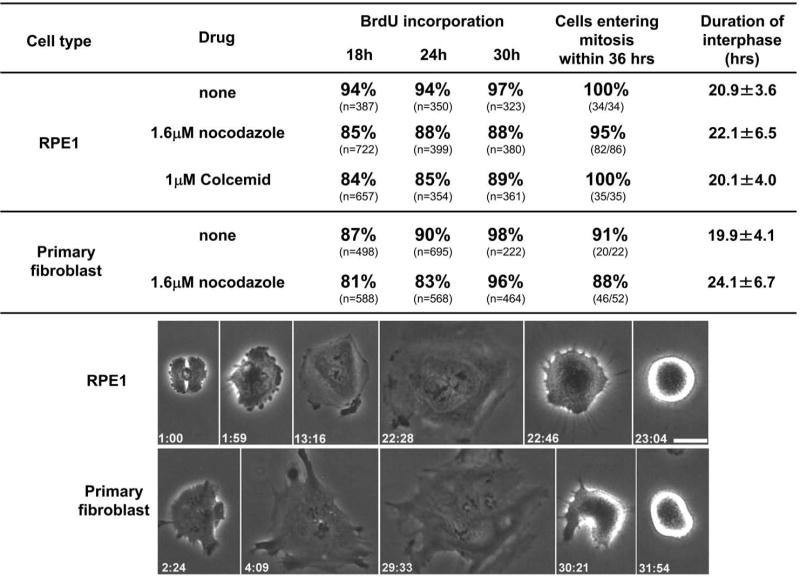
Interphase cell cycle progression of RPE1 and primary human fibroblast cells without a microtubule cytoskeleton. Upper portion shows the percent BrdU incorporation at the indicated times and the proportion of cells entering mitosis. The “duration of interphase” (mean, ± the standard deviation) is the time from shake off to the rounding up at the subsequent mitosis for cells continuously followed by time lapse video microscopy. First row of images: RPE1 cell progressing from telophase to mitosis without a microtubule cytoskeleton. This cell failed cleavage and was consequently binucleate. Images were taken from Movie 1. Lower row of images: Primary human fibroblast progressing from telophase to mitosis without a microtubule cytoskeleton. This cell failed cleavage and was consequently binucleate. Phase contrast microscopy; hours:minutes from shake off are shown in the lower corners of each frame. Bar = 20μm.
To set the stage for subsequent work, we first examined interphase progression after prolonged mitosis. Prometaphase cells washed off the coverslips after chilling were continuously followed by time lapse video microscopy in the presence of microtubule inhibitors and BrdU. We found that both RPE1 and primary fibroblasts remained in mitosis for 10-48 hours before slipping into G1 as undivided mononucleated cells (see [23]). Observations carried out to 100 hours revealed that such cells did not progress into mitosis. None showed incorporation of BrdU at 24, 48 or 72 hours (n>200 at each time point) indicating a G1 arrest, consistent with previous reports [2, 4, 6]. This arrest is not due to the lack of cytokinesis because normal human cells do not have a tetraploidy checkpoint [33]. In the continuous presence of microtubule inhibitors, cells arrest in G1 after prolonged mitosis.
We next examined interphase progression without a microtubule cytoskeleton this time after a mitosis of normal duration. Nocodazole or Colcemid treated RPE1cells that were initially in anaphase-telophase were assayed for BrdU incorporation at 18, 24 and 30 hours after shake off (Figure 2, second and third lines). 85% showed BrdU incorporation by 18 hours and slightly higher rates at 24 and 30 hours. Long term video time lapse observations (Figure 2, top line of images and Movie 1) revealed that such cells became extensively flattened during interphase and later entered a subsequent mitosis. By 36 hours after the shake off, 95% (82/86) of the nocodazole treated cells and 100% (35/35) of the Colcemid treated cells entered mitosis where they arrested due to the spindle assembly checkpoint. Ten to 48 hours later the cells slipped out of this mitosis and entered interphase; a few cells died during prolonged mitosis. Progression through interphase for cells without microtubules proceeded on average with normal kinetics (Figure 2, “Duration of interphase”). The time from shake off to the subsequent mitosis was on average 21 hours for the control cells, 22 hours for the nocodazole treated populations, and 20 hours for the Colcemid treated cells.
To test if these results reflect an unexpected peculiarity of hTERT RPE1 cells, we conducted the same experiments with primary human fibroblasts. As shown in Figure 2 fifth line, 81% of these cells showed BrdU incorporation by 18 hours and 96% by 30 hours. 88% entered mitosis by 36 hours and interphase duration was on average 4 hours longer than the controls (Figure 2 forth versus fifth line). The bottom line of images in Figure 2 shows a primary fibroblast flattening out at the end of mitosis and progressing to the next mitosis in the complete absence of a microtubule cytoskeleton.
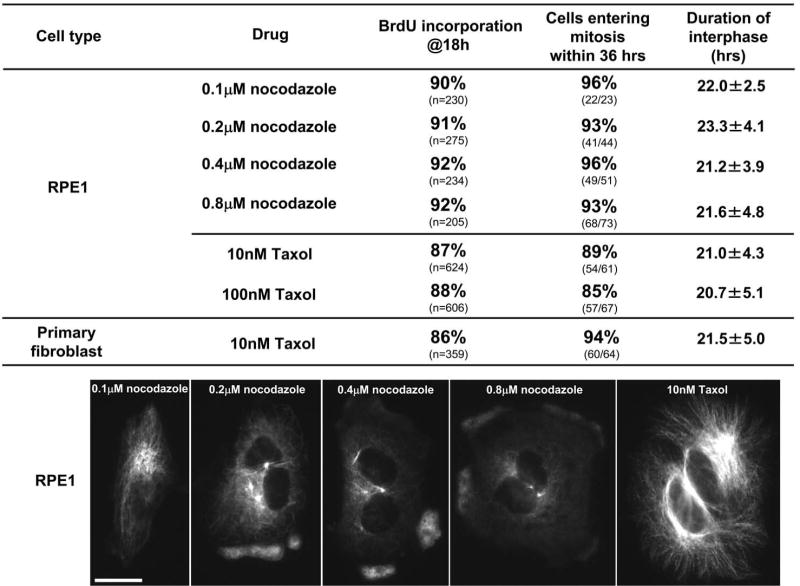
We next used RPE1 cells to test if partial disruption of microtubules influences cell cycle progression through G1. These experiments were motivated by the possibility that cells might be able to sense the presence of a dysfunctional microtubule array even though they cannot sense its complete absence. We conducted the same experiments using 0.1 to 0.8 μM nocodazole. As shown in Figure 3 (first four images), these cells completed mitosis and assembled a partial interphase microtubule cytoskeleton whose size was a function of nocodazole concentration. We found that 90-92% of the cells incorporated BrdU by 18 hours irrespective of the nocodazole concentration used (Figure 3, top four lines). 93-96% of the cells entered mitosis by 36 hours, values comparable to those observed for cells without a microtubule cytoskeleton (Figure 3, top four lines). Average interphase durations ranged from 21 to 23 hours with no dependence on nocodazole concentration.
Figure 3.
Cell cycle progression of RPE1 and primary human fibroblasts in which the microtubule cytoskeleton has been diminished or augmented by 0.1-0.8μM nocodazole or 10-100 nM Taxol respectively. Upper portion shows the percent BrdU incorporation at 18 hours and the proportion of RPE1 cells or primary human fibroblasts entering a subsequent mitosis. The “duration of interphase” (mean, ± the standard deviation) is the time from shake off to the rounding up at the subsequent mitosis for cells continuously followed by time lapse video microscopy. The images show the extent of the diminishment/augmentation of the microtubule cytoskeleton in RPE1 cells continuously exposed to the indicated drugs and drug concentrations. The cells were fixed at 3 hours after shake off and immunostained for alpha tubulin. Fluorescence microscopy; Bar = 20μm.
To test if the stabilization of the microtubule cytoskeleton influences the ability of cells to progress through G1, we conducted the same experiments using 10 or 100 nM paclitaxel (Taxol) without exposing the cells to cold. These Taxol doses led to a pronounced augmentation of the interphase microtubule array (Figure 3, last image). These doses should also damp microtubule tip dynamics because even 1nM Taxol is sufficient to activate the spindle assembly checkpoint in RPE1 cells (data not shown). At 18 hours after shake off 87% of the cells treated with 10nM Taxol and 88% of those treated with 100nM Taxol showed BrdU incorporation (Figure 3 fifth and sixth line). 85% - 89% of the cells entered mitosis with normal kinetics. Closely similar results were obtained with primary human fibroblasts (Figure 3, lowest line).
Together, our results reveal that the ability of normal human cells to progress through G1 and the remainder of interphase with an altered or disassembled microtubule cytoskeleton critically depends upon whether the preceding mitosis was prolonged or of normal duration. When mitosis is prolonged by >10 hours, cells arrest in the following G1 in a p53 dependent fashion whether or not the microtubule inhibitor is washed out before slippage into interphase [this study and 2, 4, 6, 27, 28]. This G1 arrest may be the consequence of the formation of DNA breaks during or just after prolonged mitosis [29] and/or the accumulation of p53 during an extended mitosis [30, 31]. However, when the microtubule cytoskeleton is completely disassembled starting in early anaphase of a normal mitosis, the cells proceed through G1 and on to the subsequent mitosis with essentially normal kinetics. Thus, the presence of a microtubule cytoskeleton is not required for a cell to commit during G1 to enter the cell cycle and there is no indication of a “microtubule damage checkpoint' or microtubule dependent “postmitotic checkpoint” operating during G1 or G2 as previously proposed [1-7]. We note, however, that a small percentage of both RPE1 and primary human fibroblasts, whose microtubule cytoskeleton is disassembled, are slower than the controls and the majority of their cohorts to come into S phase. We speculate this could indicate that loss of microtubules is a stress for cells that acts additively with stresses found under culture conditions to slow but not stop G1 in a minority of the experimental cells. For the vast majority of the cells in our study the stress of microtubule loss is not one of sufficient strength to slow the cell cycle. In principle, consideration of stress could provide an explanation for why many investigators observe a G1 arrest in a proportion of the cells when the microtubule cytoskeleton is disassembled (Table S1). Stresses inherent in synchronization protocols, such as serum starvation or side effects of high microtubule inhibitor doses could in principle work additively with microtubule loss to cause a p53 dependent G1 arrest. The notion that the disassembly of the microtubule cytoskeleton stresses the cell is consistent with the finding that microtubule disassembly in early prophase causes transient return to interphase [24] in a p38 stress activated kinase dependent fashion [25]. That said, we note that we did not observe a systematic prolongation of interphase as might be predicted by these studies. We speculate that cells already progressing through interphase without a microtubule cytoskeleton have accommodated to the stress of microtubule loss and thus do not delay entry into mitosis. Another indication that microtubule loss can be a stress comes from our observations that centrosome removal from RPE1 cells does not impede G1 progression [26], but exposure of such acentrosomal cells to 1.6 μM nocodazole to disassemble microtubules causes a G1 arrest in all 6 cells examined (Uetake and Sluder, unpublished).
We also found that normal human cells do not detect the presence of a diminished or stabilized microtubule cytoskeleton after mitosis of normal duration, as indicated by most previous studies (Table S1). This argues against the formal possibility that complete loss of the microtubule cytoskeleton eliminates structural components or microtubule dependent interactions that are necessary for the cell to sense a dysfunctional microtubule cytoskeleton. In addition, our results indicate that the loss or alteration of the microtubule cytoskeleton does not functionally impact the actin cytoskeleton in a way that will trigger a G1 arrest, such as that observed for low doses of cytochalasin [13-20].
In summary, our results demonstrate that the normal human cell does not have a checkpoint mechanism that detects the loss, diminishment, or augmentation of the interphase microtubule cytoskeleton during G1 as long as the preceding mitosis was of normal duration. Under our experimental conditions, cells can proceed through one entire cell cycle without a microtubule cytoskeleton; they progress from the end of mitosis, through interphase, enter mitosis, and eventually slip out of mitosis into G1. Drugs that destabilize or stabilize microtubules are used for chemotherapy in the treatment for a number of human tumors (reviewed in [21, 22]). Although the way in which these drugs lead to killing of cells is not fully understood, work on cancer cell lines indicates that the drugs promote apoptosis during prolonged mitosis and/or during the subsequent G1 arrest (reviewed in [32]). Our results suggest that the G1 killing of cancer cells by drugs that stabilize or destabilize microtubules is not due to dysfunction of the microtubule cytoskeleton per se during G1. Rather, killing may be linked to the G1 arrest following slippage through a grossly prolonged mitosis.
Experimental Procedures
Cell culture, drug treatments, and immunofluorescence
HTERT-RPE1 cells were obtained from CLONTECH Laboratories and human primary foreskin fibroblasts (BJ strain) were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured as described in [33]. Nocodazole, Colcemid and paclitaxel (Taxol) were purchased from Sigma-Aldrich and used at the indicated concentrations by 1:2000 dilutions of DMSO stocks into medium. Mitotic cells were collected from freely cycling populations by shaking plates and gentle pipetting of medium across the surface of the culture dish. Within 3 minutes the cells were exposed to nocodazole or Colcemid in test tubes and the tubes were inserted into wet ice for 10 minutes. For the Taxol experiments, the cells were exposed to the drug in test tubes without chilling.
Cells were plated on 22-mm coverslips and warmed up to 37°C in a CO2 incubator. One hour after the cells were re-plated, the round, non-adherent prometaphase cells were washed off, and the cells that spread out on the coverslips were cultured with media containing microtubule inhibitors and BrdU (5 μg/ml). The round prometaphase cells in the media were placed in a new culture dish with new coverslips and cultured with media containing the microtubule inhibitors and BrdU (5 μg/ml). Coverslip bearing cells were cultured in CO2 incubator and later fixed for BrdU analysis; other coverslips were mounted in observation chambers for continuous time-lapse video analysis. To assay the efficacy of the microtubule inhibitors, cells on some coverslips were fixed in cold methanol and incubated with monoclonal anti alpha-tubulin antibody (Sigma-Aldrich) followed by incubation with Alexa Fluor 488 goat anti-mouse antibody (Molecular Probes, Inc.) and Hoechst 33258 [34]. BrdU incorporation was determined as previously described [33]. Observations were made with a Leica DMR series microscope equipped for phase contrast and fluorescence.
Time-lapse video analysis
Coverslips bearing cells were assembled into chambers [35] containing nocodazole, Colcemid or Taxol at the indicated concentrations. Individual cells were followed at 37°C with Zeiss Universal (Carl Zeiss MicroImaging, Inc.) or Olympus BH-2 (Olympus) microscopes equipped with phase contrast optics. Images were recorded with Orca ER, Orca 100 (Hamamatsu Corporation), Retiga EX and or Retiga EXi cameras (Qimaging Corp.); sequences were written to the hard drives of PC computers using C-imaging software (Compix, Inc.) and were exported as QuickTime movies.
Supplemental materials
Time-lapse sequences of three RPE1 cells (Movie 1) treated with 1.6μM nocodazole are shown progressing from the end of mitosis to the subsequent mitosis. In this 1 minute long movie, the three cells enter mitosis at 23, 24, 36 hours after shake off. Two cells slipped from mitosis into G1 phase at 41 and 43 hours; the cell in lower right portion of the frame is an example of a cell that dies during prolonged mitosis.
Table S1 is a tabulation of studies investigating the consequences of microtubule cytoskeleton disassembly or augmentation on the ability of various cell lines to progress through G1.
Figure S1 shows mitotic cells fixed 10 minutes after chilling in the presence of 1.6μM nocodazole (A) or without drug (B).
Acknowledgments
We thank Joshua Nordberg for helpful discussions and assistance with technical matters. We also thank Drs. Dannel McCollum and Anna Krzywicka-Racka for comments on the manuscript. This work was supported by NIH GM030758 to GS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blajeski AL, Phan VA, Kottke TJ, Kaufmann SH. G(1) and G(2) cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Invest. 2002;110:91–99. doi: 10.1172/JCI13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 3.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–3813. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 4.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantel C, Braun SE, Reid S, Henegariu O, Liu L, Hangoc G, Broxmeyer HE. p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood. 1999;93:1390–1398. [PubMed] [Google Scholar]
- 6.Minn AJ, Boise LH, Thompson CB. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 7.Sablina AA, Agapova LS, Chumakov PM, Kopnin BP. p53 does not control the spindle assembly cell cycle checkpoint but mediates G1 arrest in response to disruption of microtubule system. Cell Biol Int. 1999;23:323–334. doi: 10.1006/cbir.1999.0362. [DOI] [PubMed] [Google Scholar]
- 8.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 9.Hulleman E, Boonstra J. Regulation of G1 phase progression by growth factors and the extracellular matrix. Cell Mol Life Sci. 2001;58:80–93. doi: 10.1007/PL00000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 11.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 12.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 13.Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell. 2001;12:1315–1328. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohmer RM, Scharf E, Assoian RK. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell. 1996;7:101–111. doi: 10.1091/mbc.7.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter SB. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- 16.Hansen LK, Mooney DJ, Vacanti JP, Ingber DE. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994;5:967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano A, Kurimura T. Virally transformed cells and cytochalasin B. I. The effect of cytochalasin B on cytokinesis, karyokinesis and DNA synthesis in cells. Exp Cell Res. 1974;89:111–120. doi: 10.1016/0014-4827(74)90193-1. [DOI] [PubMed] [Google Scholar]
- 18.Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 19.Lohez OD, Reynaud C, Borel F, Andreassen PR, Margolis RL. Arrest of mammalian fibroblasts in G1 in response to actin inhibition is dependent on retinoblastoma pocket proteins but not on p53. J Cell Biol. 2003;161:67–77. doi: 10.1083/jcb.200208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright WE, Hayflick L. Formation of anucleate and multinucleate cells in normal and SV 40 transformed WI-38 by cytochalasin B. Exp Cell Res. 1972;74:187–194. doi: 10.1016/0014-4827(72)90496-x. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini F, Budman DR. Review: tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest. 2005;23:264–273. doi: 10.1081/cnv-200055970. [DOI] [PubMed] [Google Scholar]
- 22.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieder CL, Cole R. Microtubule disassembly delays the G2-M transition in vertebrates. Curr Biol. 2000;10:1067–1070. doi: 10.1016/s0960-9822(00)00678-3. [DOI] [PubMed] [Google Scholar]
- 25.Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J Cell Biol. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciciarello M, Mangiacasale R, Casenghi M, Zaira Limongi M, D'Angelo M, Soddu S, Lavia P, Cundari E. p53 displacement from centrosomes and p53-mediated G1 arrest following transient inhibition of the mitotic spindle. J Biol Chem. 2001;276:19205–19213. doi: 10.1074/jbc.M009528200. [DOI] [PubMed] [Google Scholar]
- 28.Vogel C, Kienitz A, Hofmann I, Muller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23:6845–6853. doi: 10.1038/sj.onc.1207860. [DOI] [PubMed] [Google Scholar]
- 29.Quignon F, Rozier L, Lachages AM, Bieth A, Simili M, Debatisse M. Sustained mitotic block elicits DNA breaks: one-step alteration of ploidy and chromosome integrity in mammalian cells. Oncogene. 2007;26:165–172. doi: 10.1038/sj.onc.1209787. [DOI] [PubMed] [Google Scholar]
- 30.Blagosklonny MV. Prolonged mitosis versus tetraploid checkpoint: how p53 measures the duration of mitosis. Cell Cycle. 2006;5:971–975. doi: 10.4161/cc.5.9.2711. [DOI] [PubMed] [Google Scholar]
- 31.Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Uetake Y, Sluder G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J Cell Biol. 2004;165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uetake Y, Terada Y, Matuliene J, Kuriyama R. Interaction of Cep135 with a p50 dynactin subunit in mammalian centrosomes. Cell Motil Cytoskeleton. 2004;58:53–66. doi: 10.1002/cm.10175. [DOI] [PubMed] [Google Scholar]
- 35.Sluder G, Nordberg JJ, Miller FJ, Hinchcliffe EH. A sealed preparation for long-term observations of cultured cells. In: Goldman RD, Spector DL, editors. Live Cell Imaging: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2005. pp. 345–349. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.