Abstract
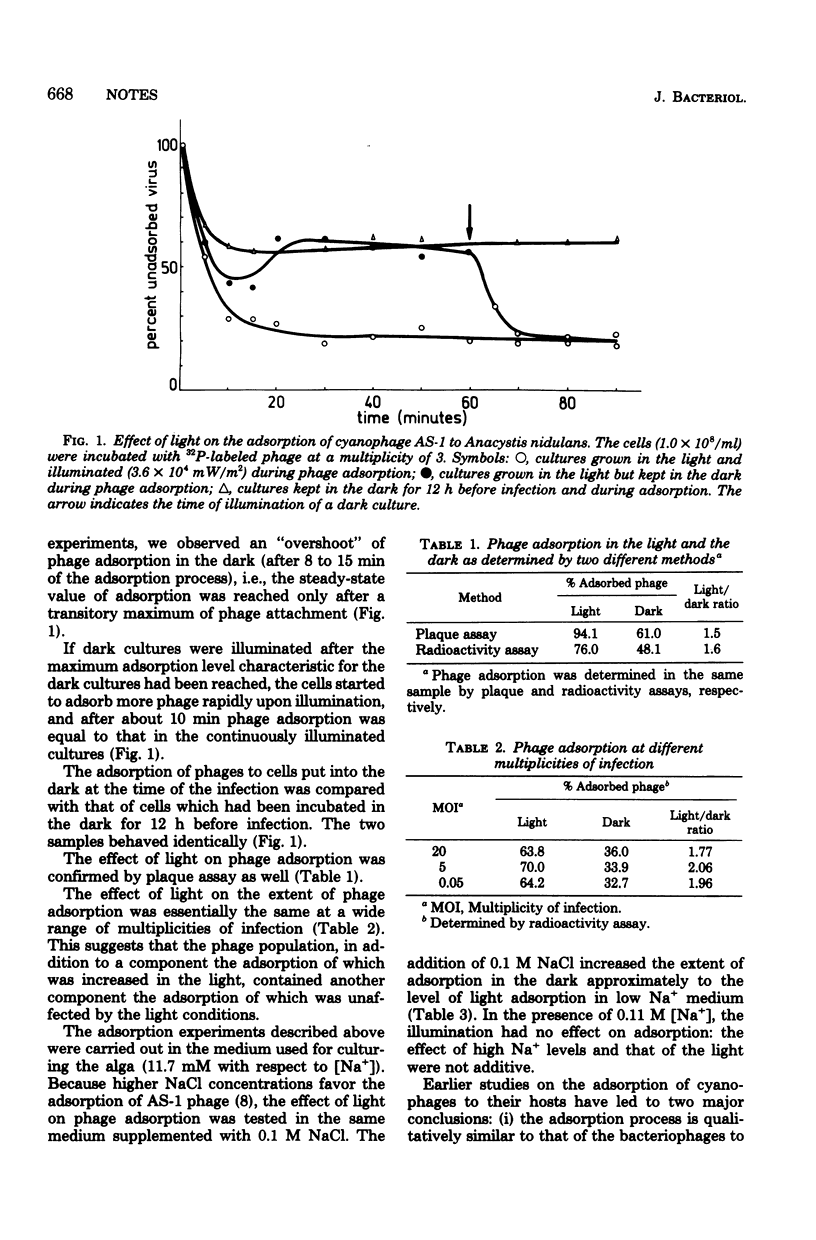
The effect of illumination on the extent and kinetics of the adsorption of cyanophage AS-1 to the blue-green alga (cyanobacterium) Anacystis nidulans was studied by using 32P-labeled phage. The initial rate of adsorption was not significantly affected by light. However, at Na+ levels used ordinarily to culture the alga ([Na+] = 11.7 mM), the total amount of phage adsorbed was doubled in the illuminated cultures, as compared with the dark-grown ones, over a wide range of multiplicities of infection (0.05 to 20). Upon a 10-fold increase in Na+ concentration in the medium ([Na+] = 0.11 M), the dark adsorption of the phage increased to the level of light adsorption found in low Na+ medium. The effects on phage adsorption of high Na+ concentration and light were not additive.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W., Haselkorn R. Photosynthesis and the development of blue-green algal virus N-1. Virology. 1972 Feb;47(2):370–374. doi: 10.1016/0042-6822(72)90272-3. [DOI] [PubMed] [Google Scholar]
- Batterton J. C., Jr, Van Baalen C. Growth responses of blue-green algae to sodium chloride concentration. Arch Mikrobiol. 1971;76(2):151–165. doi: 10.1007/BF00411789. [DOI] [PubMed] [Google Scholar]
- Padan E., Ginzburg D., Shilo M. The reproductive cycle of cyanophage LPP1-G in Plectonema boryanum and its dependence on photosynthetic and respiratory systems. Virology. 1970 Mar;40(3):514–521. doi: 10.1016/0042-6822(70)90194-7. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Diener T. O., Desjardins P. R., Morris M. E. Isolation and characterization of AS-1, a phycovirus infecting the blue-green algae, Anacystis nidulans and Synechococcus cedrorum. Virology. 1972 Jan;47(1):105–113. doi: 10.1016/0042-6822(72)90243-7. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Connelly M., Sherman D. M. Infection of Synechococcus cedrorum by the cyanophage AS-1M. I. Ultrastructure of infection and phage assembly. Virology. 1976 May;71(1):1–16. doi: 10.1016/0042-6822(76)90089-1. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. I. Electron microscopy. J Virol. 1970 Dec;6(6):820–833. doi: 10.1128/jvi.6.6.820-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A. Infection of Synechococcus cedrorum by the cyanophage AS-1M. III. Cellular metabolism and phage development. Virology. 1976 May;71(1):199–206. doi: 10.1016/0042-6822(76)90105-7. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Pauw P. Infection of Synechococcus cedrorum by the cyanophage AS-1M. II. Protein and DNA synthesis. Virology. 1976 May;71(1):17–27. doi: 10.1016/0042-6822(76)90090-8. [DOI] [PubMed] [Google Scholar]
- Udvardy J., Sivok B., Borbely G., Farkas G. L. Formation in the dark, of virus-induced deoxyribonuclease activity in Anacystis nidulans, an obligate photoautotroph. J Bacteriol. 1976 May;126(2):630–633. doi: 10.1128/jb.126.2.630-633.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



