Abstract
We have isolated a novel actin filament–binding protein, named afadin, localized at cadherin-based cell–cell adherens junctions (AJs) in various tissues and cell lines. Afadin has one PDZ domain, three proline-rich regions, and one actin filament–binding domain. We found here that afadin directly interacted with a family of the immunoglobulin superfamily, which was isolated originally as the poliovirus receptor–related protein (PRR) family consisting of PRR1 and -2, and has been identified recently to be the alphaherpes virus receptor. PRR has a COOH-terminal consensus motif to which the PDZ domain of afadin binds. PRR and afadin were colocalized at cadherin-based cell–cell AJs in various tissues and cell lines. In E-cadherin–expressing EL cells, PRR was recruited to cadherin-based cell–cell AJs through interaction with afadin. PRR showed Ca2+-independent cell–cell adhesion activity. These results indicate that PRR is a cell–cell adhesion molecule of the immunoglobulin superfamily which is recruited to cadherin-based cell–cell AJs through interaction with afadin. We rename PRR as nectin (taken from the Latin word “necto” meaning “to connect”).
Keywords: afadin, cadherin, poliovirus receptor– related protein, immunoglobulin superfamily, zonula adherens
Cell–cell adhesion plays essential roles in various functions, including the maintenance of the integrity of organized tissues, the control of cell growth, and tissue morphogenesis (Edelman, 1986; Takeichi, 1988, 1991, 1993; Albelda and Buck, 1990; Buck, 1992; Gumbiner, 1996; Barth et al., 1997). The functional units of cell–cell adhesion are typically composed of cell adhesion molecules (CAMs)1 and cytoplasmic peripheral membrane proteins (Gumbiner, 1996). CAMs mediate cell–cell adhesion at the extracellular surface by a homophilic or heterophilic interaction and determine the specificity of cell–cell recognition. At the intracellular surface, CAMs interact with cytoplasmic peripheral membrane proteins, which are linked to the cytoskeleton, to regulate the functions of CAMs and to transduce signals initiated by CAMs.
CAMs are classified into groups, including the cadherin family (cadherin), the Ig superfamily (IgCAM), the integrin family, and the selectin family. Of the CAM families, cadherin plays crucial roles in cell–cell adhesion of a majority of cell types. In polarized epithelial cells, cell–cell adhesion forms a specialized membrane structure, comprised of zonula occludens (ZO, tight junctions), zonula adherens (ZA; cell–cell adherens junctions [AJs]), and desmosome, which is known as the junctional complex. These junctional structures are typically aligned from the apical side to the basal side, although desmosome is independently distributed in other areas.
At ZA, classic cadherins interact with each other at the extracellular surface in a Ca2+-dependent manner (Takeichi, 1988, 1991; Geiger and Ginsberg, 1991; Tsukita et al., 1992). The cytoplasmic region of cadherin interacts with β- and γ-catenins, and β-catenin interacts with α-catenin (Ozawa et al., 1989; Nagafuchi et al., 1991; Takeichi, 1991; Tsukita et al., 1992). α-Catenin interacts directly with actin filament (F-actin) (Rimm et al., 1995) and indirectly with it through α-actinin and vinculin, other F-actin–binding proteins (Knudsen et al., 1995; Weiss et al., 1998). ZA is distinguished from cadherin-based cell–cell AJs in nonepithelial cells by having a thick F-actin belt, called the circumferential filament band. Furthermore, while ZA is defined as a junctional structure observed by electron microscopy, the cadherin-catenin system is not confined to ZA, but is distributed along the entire lateral membrane (Tsukita et al., 1992; Gumbiner, 1996). α-Actinin is also broadly distributed (Geiger et al., 1981). In this study, therefore, we use “cell–cell AJs” as cell–cell adhesion sites where cadherin is present, and distinguish “cell–cell AJs” from “ZA.” Vinculin is more highly concentrated at ZA than the cadherin-catenin system (Geiger et al., 1981; Yonemura et al., 1995).
Thus, many cytoplasmic peripheral membrane proteins, which directly or indirectly link CAMs to the actin cytoskeleton, have been identified, but little is still known about the mechanism of how these F-actin–binding proteins are involved in the establishment of the polarized junctional alignment in epithelial cells. It has neither been fully understood how these F-actin–binding proteins regulate the function of the adhesion molecules, nor how the adhesion molecule–initiated signals are transduced to the F-actin–binding proteins.
Recently, we have isolated a novel ZA component, named l-afadin (Mandai et al., 1997). l-Afadin has been isolated as an F-actin–binding protein from rat brain. l-Afadin has one PDZ domain at the middle region, three proline-rich regions following the PDZ domain, and one F-actin–binding domain at the COOH-terminal region. l-Afadin is ubiquitously expressed and specifically localized at ZA in epithelial cells and at cell–cell AJs in nonepithelial cells. l-Afadin has a splicing variant, named s-afadin, which has one PDZ domain but lacks the third proline-rich region and the F-actin–binding domain (Mandai et al., 1997). s-Afadin is abundantly expressed in neural tissue. s-Afadin is the protein encoded by the AF-6 gene, which is originally found to be fused to the ALL-1 gene (Prasad et al., 1993), known to be involved in acute leukemia (Cimino et al., 1991).
The result that l-afadin is specifically localized at ZA in epithelial cells and at cell–cell AJs in nonepithelial cells suggests the presence of an integral membrane protein(s) which interacts with l-afadin. On this assumption, we have attempted here to isolate an l-afadin–binding integral membrane protein(s) specifically localized at ZA in epithelial cells and at cell–cell AJs in nonepithelial cells. We have identified it to be the poliovirus receptor–related protein (PRR) family members (Morrison and Racaniello, 1992; Eberlé et al., 1995; Lopez et al., 1995), identified recently as the alphaherpes virus receptor (Geraghty et al., 1998; Warner et al., 1998). The PRR family belongs to the Ig superfamily and consists of PRR1 and -2, which are ubiquitously expressed (Morrison and Racaniello, 1992; Aoki et al., 1994; Eberlé et al., 1995; Lopez et al., 1995). PRR2 has two splicing variants, PRR2α and -2δ, shown to serve as homophilic CAMs (Aoki et al., 1997; Lopez et al., 1998). We describe here that PRR is a cell–cell adhesion molecule of the Ig superfamily which is specifically localized at ZA in epithelial cells and at cell–cell AJs in nonepithelial cells and recruited to cadherin-based cell–cell AJs through interaction with l-afadin. Therefore, we rename PRR as nectin (taken from the Latin word “necto” meaning “to connect”).
Materials and Methods
Yeast Two-Hybrid Screening and β-Galactosidase Assay
The bait vectors, pBTM116-l-afadin-1 (amino acid [aa] 1–1015) and -2 (aa 706–1425), were constructed by subcloning the inserts encoding the respective aa residues of l-afadin into pBTM116 (Vojtek et al., 1993). The yeast two-hybrid library constructed from an adult rat brain cDNA was screened using a mixture of pBTM116-l-afadin-1 and -2 as baits as described (Hata et al., 1996). β-Galactosidase assay was performed as described (Hata et al., 1996).
Construction of Expression Vectors
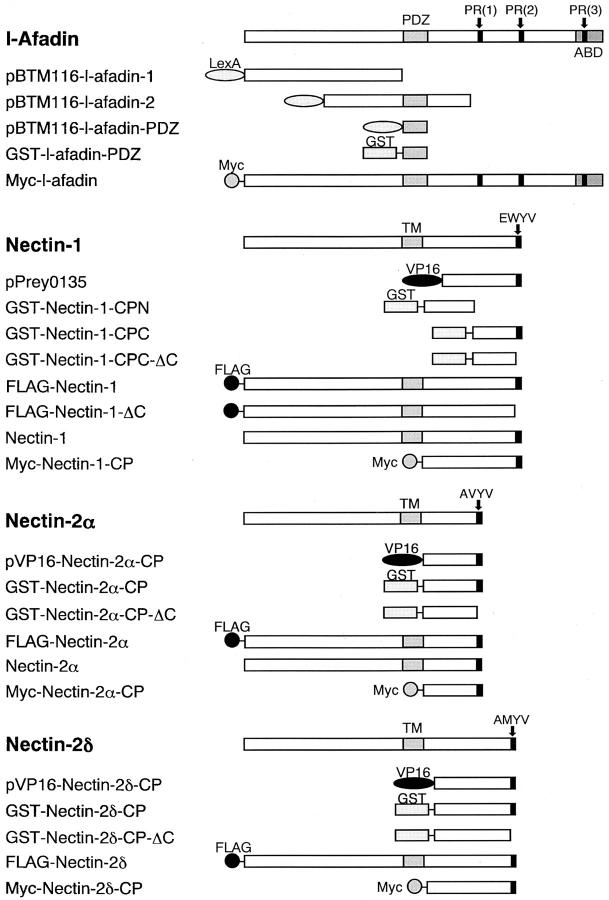
The cDNAs of human nectin-1 and mouse nectin-2α and -2δ were obtained by PCR using human and mouse brain cDNAs as templates, respectively. Nucleotide sequence analysis was performed by the dideoxynucleotide termination method using a DNA sequencer (model 373; Applied Biosystems Inc.). The nucleotide sequence of our isolated human nectin-1 cDNA was different from the originally published sequence (Lopez et al., 1995), but identical to the recently published sequence (Geraghty et al., 1998). Prokaryote and eukaryote expression vectors, bait vectors, and prey vectors were constructed in pGEX-KG (Guan and Dixon, 1991), pCMV-Myc (Nakanishi et al., 1997), pFLAG-CMV1 (Eastman Kodak Co.), pCAGGS (Niwa et al., 1991), pCAGGS-FLAG, pBTM116, and pVP16-3 (Hata and Südhof, 1995) using standard molecular biology methods (Sambrook et al., 1989). pCAGGS-FLAG was constructed by subcloning the insert encoding the preprotrypsin-signal peptide and FLAG epitope of pFLAG-CMV1 into pCAGGS. The glutathione S-transferase (GST)-fusion vectors, containing α-, β-catenins, and the cytoplasmic region of E-cadherin (aa 734–884) (Itoh et al., 1997), were kindly supplied by Drs. M. Itoh and S. Tsukita (Kyoto University, Kyoto, Japan). Various constructs of l-afadin and nectin shown in Fig. 1 contained the following aa: pBTM116-l-afadin-PDZ, aa 1007–1125; pVP16-nectin-2α-CP, aa 387–467; pVP16-nectin-2δ-CP, aa 403–530; GST-nectin-1-CPN, aa 379–438; GST-nectin-1-CPC, aa 449–518; GST-nectin-1-CPC-ΔC, aa 449–514; GST-nectin-2α-CP, aa 387–467; GST-nectin-2α-CP-ΔC, aa 387–463; GST-nectin-2δ-CP, aa 403–530; GST-nectin-2δ-CP-ΔC, aa 403–526; GST-l-afadin-PDZ, aa 1007–1125; pFLAG-CMV1-nectin-1, aa 27–518; pFLAG-CMV1-nectin-2α, aa 28–467; pFLAG-CMV1-nectin-2δ, aa 28–530; pCMV-Myc-l-afadin, aa 1–1829 (full-length); pCMV-Myc-nectin-1-CP, aa 379–518; pCMV-Myc-nectin-2α-CP, 387–467; pCMV-Myc-nectin-2δ-CP, aa 403–530; pCAGGS-FLAG-nectin-1, aa 27–518; pCAGGS-FLAG-nectin-1-ΔC, aa 27–514; pCAGGS-nectin-1, aa 1–518; and pCAGGS-nectin-2α, aa 1–467. Other constructs contained the following aa: pBTM116-neurabin-II, aa 145–600; and pVP16-neurexin-2α, aa 1658–1715. The GST-fusion proteins were purified by use of glutathione-Sepharose beads (Amersham-Pharmacia Biotech Ltd.).
Figure 1.
Structures of the full length and various fragments of l-afadin and nectin. PR, proline-rich region; ABD, F-actin–binding domain; TM, transmembrane region.
In Vitro Binding of l-Afadin to Nectin
Affinity chromatographies were done as follows. Anti-Myc epitope Ab-coupled beads were prepared by cross-linking of a mouse anti-Myc mAb with protein A–Sepharose beads (Amersham-Pharmacia Biotech Ltd.) via dimethylpimelimidate (Harlow and Lane, 1988). COS7 cells transfected with pCMV-Myc-nectin-1-CP, pCMV-Myc-nectin-2α-CP, or pCMV-Myc-nectin-2δ-CP were sonicated in a lysis buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 1 mM EDTA, 10 μg/ml of leupeptin, 1 mM PMSF, and 1 μg/ml of pepstatin A). The lysate was subjected to centrifugation, and the supernatant was incubated with the anti-Myc Ab-coupled beads. After the beads were washed extensively, the beads were used as an affinity column. The purified GST-fusion protein to be tested was applied to the affinity column. After the column was washed with PBS containing 0.1% Triton X-100, the bound proteins were eluted by boiling the beads in an SDS sample buffer [60 mM Tris-Cl, pH 6.7, 3% SDS, 2% (vol/vol) 2-mercaptoethanol, and 5% glycerol]. The sample was then subjected to SDS-PAGE, followed by staining with Coomassie brilliant blue.
35S-Labeled l-afadin blot overlay was done as described (Mandai et al., 1999). In brief, 35S-labeled l-afadin was generated using the TNT T7 quick coupled transcription/translation system (Promega Corp.) and used as a probe. The sample to be tested was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked in PBS containing 5% defatted powder milk and 1% Triton X-100. The membrane was then incubated at 4°C for 16 h with 40 μl of the 35S-labeled l-afadin probe in 1 ml of PBS containing 5% defatted powder milk and 1% Triton X-100. After the incubation, the membrane was washed with PBS containing 5% defatted powder milk and 1% Triton X-100, followed by autoradiography using an image analyzer (Fujix BAS-2000II; Fuji Photo Film Co.).
Antibodies
Rabbit antisera against nectin-1, -2α, and -2δ were raised against GST-nectin-1-CPN, GST-nectin-2α-CP, and GST-nectin-2δ-CP, respectively. These antisera were separately affinity-purified by use of the respective GST-fusion proteins covalently coupled to NHS-activated Sepharose beads (Amersham-Pharmacia Biotech Ltd.), and used as pAbs. A rat anti–nectin-2 mAb was prepared as described (Aoki et al., 1997). The specificity of the anti–nectin-2 mAb used here was confirmed as follows. Immunofluorescence microscopic analysis showed that an HeLa cell line stably expressing mouse nectin-2α reacted with this mAb but wild-type HeLa cells did not (Aoki et al., 1997). In addition, when a cell lysate from mouse mammary tumor MTD-1A cells, which were metabolically radiolabeled with [35S]methionine, was subjected to immunoprecipitation with this mAb, followed by autoradiography as described (Yoshida and Takeichi, 1982), a radioactive band with a molecular mass of ∼70–80 kD was specifically immunoprecipitated (data not shown). This band was recognized by another anti–nectin-2 pAb described above (data not shown). A rabbit anti–l-afadin pAb and a mouse anti–l-afadin mAb were prepared as described (Mandai et al., 1997; Sakisaka et al., 1999). Mouse and rat (ECCD2) anti–E-cadherin mAbs were purchased from Transduction Laboratories and TAKARA Shuzo, respectively. Mouse anti-Myc and anti-FLAG mAbs were from American Type Culture Collection and Eastman Kodak Co., respectively.
Immunoprecipitation
The bile canaliculi-rich fraction was prepared from mouse liver (Tsukita and Tsukita, 1989). This fraction was sonicated in the lysis buffer described above and subjected to ultracentrifugation. After the supernatant was diluted with buffer A (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 μg/ml of leupeptin, 1 mM PMSF, and 1 μg/ml of pepstatin A) to make a final concentration of 0.2% deoxycholate, this sample was incubated with the anti–nectin-2 mAb at 4°C for 3 h. Protein G–Sepharose beads (Amersham-Pharmacia Biotech Ltd.) were added to this diluted sample, and incubation was further performed at 4°C for 1 h. After the beads were extensively washed with buffer A, the bound proteins were eluted by boiling the beads in the SDS sample buffer, and subjected to SDS-PAGE, followed by Western blot analysis.
Immunoprecipitation experiments using cultured cells were done as follows. Mammary tumor MTD-1A cells were sonicated in buffer A. The lysate was then subjected to ultracentrifugation. The supernatant was subjected to immunoprecipitation with the anti–nectin-2 mAb as described above. For COS7 cells expressing the FLAG-tagged protein and/or the Myc-tagged protein, the cells were similarly subjected to immunoprecipitation with the anti-FLAG or anti-Myc mAb. For EL cells expressing the FLAG-tagged protein, the cells were also similarly subjected to immunoprecipitation with the anti-FLAG mAb.
Cell Culture and DNA Transfection
MDCK cells were kindly supplied by Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany). EL and L cells were kindly supplied by Drs. A. Nagafuchi and S. Tsukita (Kyoto University, Kyoto, Japan). EL cells were cloned by introduction of the exogenous E-cadherin cDNA to cadherin-deficient L cells (Nagafuchi et al., 1987). These cells were maintained in DME containing 10% FCS.
To prepare COS7 cells transiently expressing the FLAG-tagged protein and/or the Myc-tagged protein, COS7 cells were transfected with the pFLAG-CMV1 construct and/or the pCMV-Myc construct, respectively, using the DEAE-dextran method and cultured for 2 d (Hata and Südhof, 1995). To prepare EL cells transiently expressing the FLAG-tagged protein, EL cells were transfected with the pCAGGS-FLAG construct using Lipofectamine reagent (GIBCO BRL) according to the manufacturer's protocol. The cells were then cultured for 1 d, replated, and cultured for 4 d. An MDCK cell line stably expressing FLAG-nectin-1 was prepared as described (Furuse et al., 1998). In brief, MDCK cells were transfected with pCAGGS-FLAG-nectin-1 using Lipofectamine reagent (GIBCO BRL). The cells were then cultured for 1 d, replated, and selected by culturing in the presence of 300 μg/ml of Geneticin (GIBCO BRL). An L cell line stably expressing full-length nectin-1 (nectin-1-L cells) or nectin-2α (nectin-2α-L cells) was similarly prepared with pCAGGS-nectin-1 or pCAGGS-nectin-2α, respectively, except that the concentration of Geneticin was increased to 500 μg/ml.
Immunofluorescence and Immunoelectron Microscopy
Immunofluorescence microscopy of cultured cells and frozen sections of various mouse tissues was done as described (Mandai et al., 1997). Immunoelectron microscopy of mouse intestine absorptive epithelial cells was done using the silver-enhancement technique as described (Mizoguchi et al., 1994; Mandai et al., 1997).
Cell Aggregation Assay
Cell aggregation assay was done according to the method described by Takeichi (1977) with slight modifications. To obtain a single-cell suspension, cells were washed with PBS, incubated with 0.2% trypsin and 1 mM EDTA at 37°C for 5 min, and dispersed by gentle pipetting. Cells were then suspended in HBSS in the presence of 1 mM CaCl2 or 1 mM EDTA (Miura et al., 1992) (106 cells/ml), placed in 12-well plates precoated with BSA, and rotated on a gyratory shaker at 37°C for indicated periods of time. Aggregation was stopped with the addition of 2% glutaraldehyde. The extent of aggregation of cells was represented by the ratio of the total particle number at time t of incubation (Nt) to the initial particle number (No).
Other Procedures
Vinculin was purified from chicken gizzard as described (O'Halloran et al., 1986). Protein concentrations were determined with BSA as a reference protein (Bradford, 1976). SDS-PAGE was done as described (Laemmli, 1970). The protein markers used were myosin (197 kD), BSA (78 kD), ovalbumin (50 kD), carbonic anhydrase (33 kD), and soybean trypsin inhibitor (28 kD).
Results
Identification of PRR as an l-Afadin–Binding Protein
We first attempted to identify an l-afadin–binding protein(s) by use of the yeast two-hybrid method. We screened 2 × 107 clones of a prey cDNA library from rat brain with a mixture of two bait constructs, pBTM116-l-afadin-1 (aa 1–1015) and -2 (aa 706–1425). Three independent clones, pPrey 0135, pPrey 0139, and pPrey 0140, were obtained. We focused on pPrey 0135 because this clone encoded the cytoplasmic COOH-terminal region of PRR1. pPrey 0135 encoded a 97-aa sequence which was identical to the originally identified human PRR1 (aa 421– 518) except for the absence of a single aa, glutamate, at position 438 (AF091111; GenBank/EMBL/DDBJ). The clone is likely to encode a rat counterpart of human PRR1. PRR has been identified recently as the alphaherpes virus receptor, and PRR1 and -2 have been designed as HveC and HveB, respectively (Geraghty et al., 1998; Warner et al., 1998) (Table I). PRR was here renamed nectin. Retransformation of fresh yeast cells confirmed that pPrey 0135 bound to pBTM116-l-afadin-2 containing the PDZ domain. Analysis of other clones will be described elsewhere.
Table I.
COOH-terminal Sequences of the Nectin and Neurexin Family Members
| Nectin family | Nectin-1 | Human PRR1/HveC | SFISKKEWYV | |||
| Rat PRR1 | SFISKKEWYV | |||||
| Nectin-2α | Human PRR2α/HveB | SLISRRAVYV | ||||
| Mouse PRR2α/MPHα | SLISRRAVYV | |||||
| Nectin-2δ | Human PRR2δ | GFVMSRAMYV | ||||
| Mouse PRR2δ/MPHβ | DFFVSRAMYV | |||||
| Consensus | E/AXYV | |||||
| Neurexin family | Neurexin-2α | KKNKDKEYYV |
l-Afadin has a PDZ domain and nectin has cytoplasmic regions with a COOH-terminal motif of 4 aa residues, E/A-X-Y-V (X indicates W, V, and M for nectin-1, -2α, and -2δ, respectively) (Table I). It has been shown that PDZ domains bind to unique COOH-terminal motifs of 4-aa residues of integral membrane proteins (Saras and Heldin, 1996; Songyang et al., 1997; Hata et al., 1998). We next examined whether l-afadin specifically binds to nectin through the PDZ domain and the COOH-terminal motif. For this purpose, we constructed pBTM116-l-afadin-PDZ containing only the PDZ domain, and pVP16-nectin-2α-CP and pVP16-nectin-2δ-CP, both of which contained the cytoplasmic regions with the COOH-terminal motif (Fig. 1). We also prepared pVP16-neurexin-2α (containing the cytoplasmic COOH-terminal region of neurexin-2α) and pBTM116-neurabin-II (containing the PDZ domain of neurabin-II) as controls. Neurexin-2α has a COOH-terminal motif similar to that of nectin, E-Y-Y-V, which has been shown to bind to the PDZ domain of CASK (Hata et al., 1996). Neurabin-II is an F-actin–binding protein with one PDZ domain localized at cadherin-based cell– cell AJs (Satoh et al., 1998; Sakisaka et al., 1999), although its PDZ domain–binding protein(s) has not yet been identified. We quantified the yeast two-hybrid interactions by measuring β-galactosidase transactivation. The PDZ domain of l-afadin bound to the cytoplasmic regions of nectin, but not to that of neurexin-2α (Table II). The PDZ domain of neurabin-II did not bind to any of these proteins.
Table II.
Interactions between the Cytoplasmic Regions of the Nectin Family Members and the PDZ Domain of l-Afadin
| Control (pVP16-3) | Nectin-1 (pPrey 0135) | Nectin-2α (pVP16-Nectin-2α-CP) | Nectin-2δ (pVP16-Nectin-2δ-CP) | Neurexin-2α (pVP16-Neurexin-2α) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (pBTM116) | ND | ND | ND | ND | ND | |||||
| l-Afadin (pBTM116-l-Afadin-PDZ) | ND | 22,810 ± 2,300 | 3,420 ± 40 | 2,650 ± 70 | ND | |||||
| Neurabin-II (pBTM116-Neurabin-II) | ND | ND | ND | ND | ND |
The data list β-galactosidase activities of yeast strains harboring the respective bait and prey plasmids. The data shown are nanomoles of substrate hydrolyzed per minute per milligram of protein ± SD after background subtraction. The β-galactosidase activities were determined by three independent experiments. ND, no detectable activity.
Direct Binding of l-Afadin to Nectin In Vitro and In Vivo
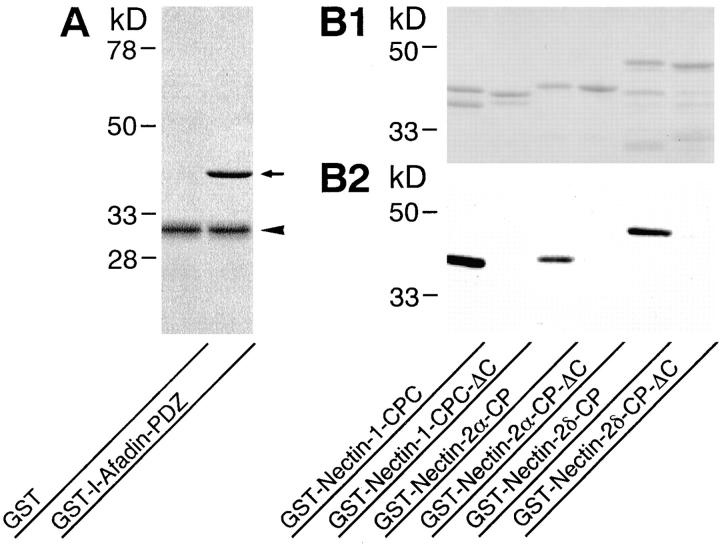
We first examined the in vitro direct binding of l-afadin to nectin by affinity chromatography. A GST-fusion protein of the PDZ domain of l-afadin (GST-l-afadin-PDZ) bound to a Myc-tagged protein of the cytoplasmic region of nectin-1 (Myc-nectin-1-CP) immobilized on protein A–Sepharose beads through the anti-Myc mAb (Fig. 2 A). The stoichiometry of the binding of l-afadin to nectin-1 was ∼1:1. Similar results were obtained with nectin-2α and -2δ (data not shown). To further examine the direct binding of l-afadin to nectin in vitro, GST-fusion proteins of the cytoplasmic regions of nectin were subjected to SDS-PAGE, followed by a blot overlay with 35S-labeled l-afadin. 35S-Labeled l-afadin bound to the GST-fusion proteins (Fig. 2, B1 and B2). However, when the COOH-terminal motif of 4-aa residues of each GST-fusion protein, which were shown in bold characters in Table I, were deleted, 35S-labeled l-afadin did not bind to the GST-fusion proteins. Consistent with the earlier observation that PDZ domains bind to unique COOH-terminal motifs of 4-aa residues of integral membrane proteins (Saras and Heldin, 1996; Songyang et al., 1997; Hata et al., 1998), these results indicate that the PDZ domain of l-afadin directly binds to nectin and that the COOH-terminal motif of 4-aa residues of nectin is essential for this binding.
Figure 2.
Direct binding of l-afadin to nectin in vitro. (A) Affinity chromatography. GST-l-afadin-PDZ (aa 1007–1125) or GST alone (20 μg of protein each) was applied to protein A–Sepharose beads on which Myc-nectin-1-CP (aa 379–518) was immobilized through the anti-Myc mAb. After the beads were extensively washed, the bound proteins were subjected to SDS-PAGE (12% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue. Arrow, GST-l-afadin-PDZ; arrowhead, Myc-nectin-1-CP. (B) 35S-Labeled l-afadin blot overlay. GST-fusion proteins of the cytoplasmic regions of nectin (0.3 μg of protein each) were subjected to SDS-PAGE (12% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue or by 35S-labeled l-afadin blot overlay. (B1) Protein staining. (B2) 35S-Labeled l-afadin blot overlay. GST-Nectin-1-CPC, aa 449–518; GST-Nectin-1-CPC-ΔC, aa 449–514; GST-Nectin-2α-CP, aa 387–467; GST-Nectin-2α-CP-ΔC, aa 387–463; GST-Nectin-2δ-CP, aa 403–530; GST-Nectin-2δ-CP-ΔC, aa 403–526.
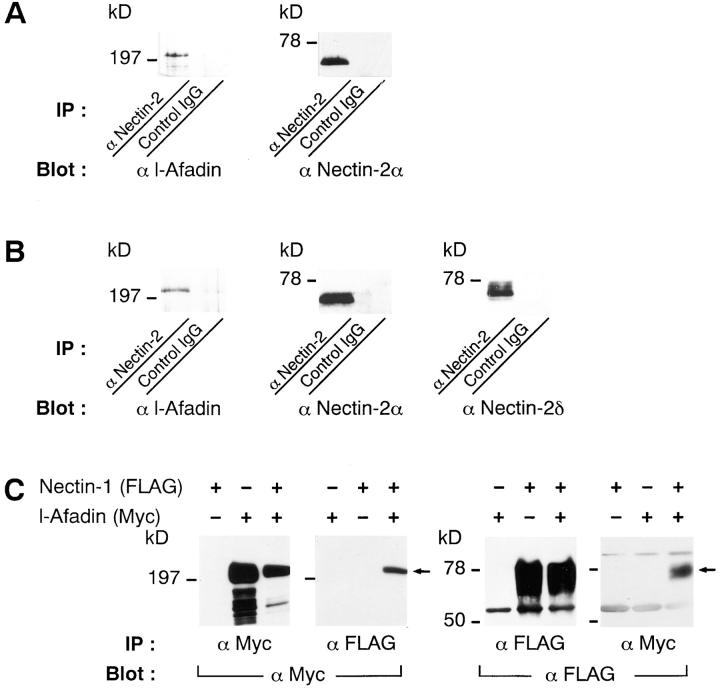
We next examined the binding of l-afadin to nectin in vivo. Western blot analysis indicated that nectin-2α, but not nectin-2δ, was detected in mouse liver (data not shown). An extract from the bile canaliculi-rich fraction of mouse liver was subjected to immunoprecipitation with the anti–nectin-2 mAb, which recognizes the extracellular domains of both nectin-2α and -2δ (Aoki et al., 1997), followed by Western blot analysis with the anti–l-afadin mAb and the anti–nectin-2α pAb. l-Afadin was coimmunoprecipitated with nectin-2α (Fig. 3 A). When a cell extract from mouse mammary tumor MTD-1A cells was similarly subjected to immunoprecipitation with the anti–nectin-2 mAb, l-afadin was coimmunoprecipitated with nectin-2α and -2δ (Fig. 3 B). Myc-l-afadin and FLAG-nectin-1, -2α, or -2δ were overexpressed in various combinations in COS7 cells and the cell extracts were subjected to immunoprecipitation with the anti-Myc or anti-FLAG mAb, followed by Western blot analysis with these mAbs. Myc-l-Afadin and FLAG-nectin-1 were coimmunoprecipitated (Fig. 3 C). Myc-l-Afadin and FLAG-nectin-2α or -2δ were also coimmunoprecipitated (data not shown). These results indicate that l-afadin binds to nectin in vivo.
Figure 3.
Coimmunoprecipitation of l-afadin and nectin. (A) Immunoprecipitation from the bile canaliculi-rich fraction of mouse liver. An extract from the bile canaliculi-rich fraction was subjected to immunoprecipitation with the anti–nectin-2 mAb or control IgG. The immunoprecipitate was then subjected to SDS-PAGE (8 or 12% polyacrylamide gel), followed by Western blot analysis with the anti–l-afadin mAb or the anti–nectin-2α pAb. (B) Immunoprecipitation from mouse mammary tumor MTD-1A cells. A cell extract from MTD-1A cells was subjected to immunoprecipitation with the anti–nectin-2 mAb or control IgG. The immunoprecipitate was then subjected to SDS-PAGE (8 or 12% polyacrylamide gel), followed by Western blot analysis with the anti–l-afadin mAb, the anti–nectin-2α pAb, or the anti– nectin-2δ pAb. (C) Immunoprecipitation from COS7 cells expressing FLAG-nectin-1 and/or Myc-l-afadin. FLAG-Nectin-1 alone, Myc-l-afadin alone, or both the proteins were transiently expressed in COS7 cells. Each cell extract was subjected to immunoprecipitation with the anti-FLAG or anti-Myc mAb. The immunoprecipitate was then subjected to SDS-PAGE (8 or 12% polyacrylamide gel), followed by Western blot analysis with the anti-FLAG or anti-Myc mAb. IP, immunoprecipitation.
Colocalization of Nectin with l-Afadin at ZA in Epithelial Cells
We examined by immunofluorescence microscopy of frozen sections of small intestine and MDCK cells whether nectin and l-afadin are colocalized. When the frozen sections of small intestine were triply stained with the rat anti–nectin-2 mAb, the rabbit anti–l-afadin pAb, and the mouse anti–E-cadherin mAb, nectin-2 and l-afadin were concentrated with E-cadherin at the junctional complex region of intestine absorptive epithelia, but they were more highly concentrated at the junctional complex region than E-cadherin (Fig. 4, A1–A3). In MDCK cells, nectin was detected with the anti–nectin-1, anti–nectin-2α, and anti–nectin-2δ pAbs by Western blot analysis, but not with these pAbs by immunofluorescence microscopy (data not shown). Canine nectin-2 in MDCK cells was not detected with the anti–nectin-2 mAb by immunofluorescence microscopy either (data not shown). Therefore, we prepared a MDCK cell line stably expressing FLAG-nectin-1. In this MDCK cell line, FLAG-nectin-1 was colocalized with l-afadin at the junctional complex region (Fig. 4, B1–B6). They were more highly concentrated at the junctional complex region than E-cadherin. These results indicate that nectin is colocalized with l-afadin at the junctional complex region in epithelial cells.
Figure 4.
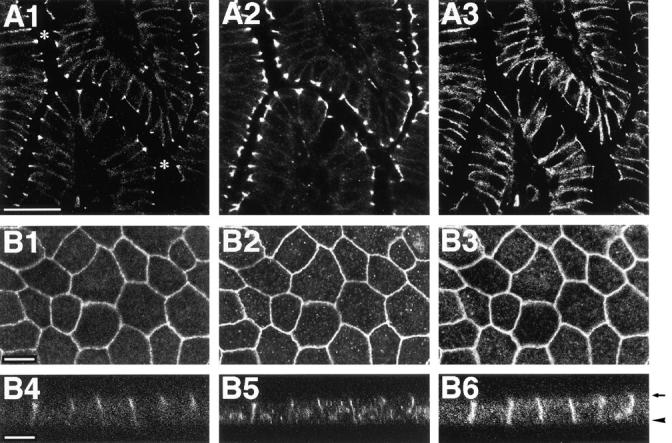
Localization of nectin, l-afadin, and E-cadherin in epithelial cells. (A) Localization sites of nectin-2, l-afadin, and E-cadherin in mouse small intestine. The frozen sections of mouse small intestine were triply stained with the rat anti–nectin-2 mAb, the rabbit anti–l-afadin pAb, and the mouse anti–E-cadherin mAb. They were visualized with rhodamine-conjugated anti– rat IgG, FITC-conjugated anti– rabbit IgG, and Cy5-conjugated anti–mouse IgG Abs. (A1) Nectin-2; (A2) l-afadin; (A3) E-cadherin. Asterisks mark the inner space of small intestine. Bar, 50 μm. (B) Localization sites of nectin-1, l-afadin, and E-cadherin in MDCK cells. MDCK cells stably expressing FLAG-nectin-1 were triply stained with the mouse anti-FLAG mAb, the rabbit anti–l-afadin pAb, and the rat anti–E-cadherin mAb. They were visualized with FITC-conjugated anti–mouse IgG, rhodamine-conjugated anti–rabbit IgG, and Cy5-conjugated anti–rat IgG Abs. (B1 and B4) FLAG-nectin-1; (B2 and B5) l-afadin; (B3 and B6) E-cadherin; (B1–B3) junction-level view; (B4–B6) cross-sectional view. The cross-sectional view was generated by confocal microscopy. Arrow, apical level; arrowhead, basal level. Bars, 10 μm.
To examine the precise localization sites of nectin-2 at the junctional complex region of intestine absorptive epithelia, immunoelectron microscopy was performed. Nectin-2 was localized at ZA, but not at ZO or desmosome (Fig. 5). This result is consistent with our previous observation that l-afadin is localized at ZA (Mandai et al., 1997), and indicates that nectin is colocalized with l-afadin at ZA in epithelial cells.
Figure 5.
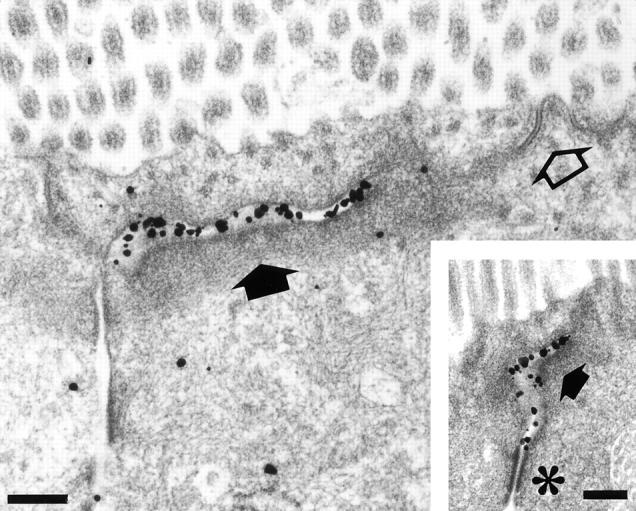
Ultrastructural localization sites of nectin-2 in mouse small intestine absorptive epithelial cells. Intestine absorptive epithelial cells were labeled with the anti–nectin-2 mAb using the silver-enhancement technique. Open arrow, ZO; closed arrow, ZA; asterisk, desmosome. Bars, 0.2 μm.
Colocalization of Nectin with l-Afadin at Cell–Cell AJs in Nonepithelial Cells
We next examined whether nectin and l-afadin are colocalized in nonepithelial cells. When the frozen sections of heart were doubly stained with the anti–nectin-2 mAb and the anti–l-afadin pAb, both of the proteins were colocalized at intercalated discs (cell–cell AJs) and not observed at costameres (cell–matrix AJs) (Fig. 6, A1 and A2). This result suggests that nectin and l-afadin are colocalized at cell–cell AJs in nonepithelial cells. To confirm this result, we examined their colocalization in EL cells expressing E-cadherin. EL cells were cloned by introduction of the exogenous E-cadherin cDNA to cadherin-deficient L cells (Nagafuchi et al., 1987). We have shown previously that l-afadin is colocalized with E-cadherin at cell–cell AJs in cultured EL cells (Mandai et al., 1997; Sakisaka et al., 1999). In this cell line, nectin-2 was also colocalized with l-afadin at cell–cell AJs (Fig. 6, B1 and B2). These results indicate that nectin is colocalized with l-afadin at cadherin-based cell–cell AJs in nonepithelial cells.
Figure 6.
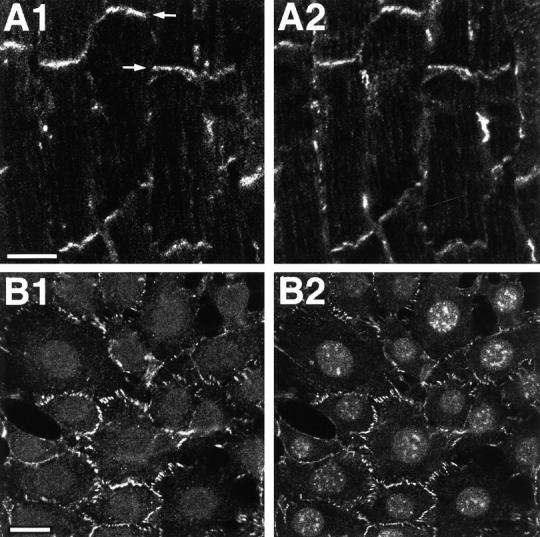
Localization of nectin and l-afadin in nonepithelial cells. (A) Localization sites of nectin-2 and l-afadin in mouse heart. The frozen sections of mouse heart were doubly stained with the rat anti–nectin-2 mAb and the rabbit anti–l-afadin pAb. They were visualized with rhodamine-conjugated anti–rat IgG and FITC-conjugated anti–rabbit IgG Abs. (A1) Nectin-2; (A2) l-afadin. Arrows, intercalated disc. Bar, 10 μm. (B) Localization sites of nectin-2 and l-afadin in cultured EL cells. EL cells were doubly stained with the rat anti–nectin-2 mAb and the mouse anti–l-afadin mAb. They were visualized with FITC-conjugated anti–rat IgG and rhodamine-conjugated anti–mouse IgG Abs. There was nuclear staining with this anti–l-afadin mAb, but its significance is not clear. (B1) Nectin-2; (B2) l-afadin. Bar, 10 μm.
Recruitment of Nectin to Cadherin-based Cell–Cell AJs through Interaction with l-Afadin in EL Cells
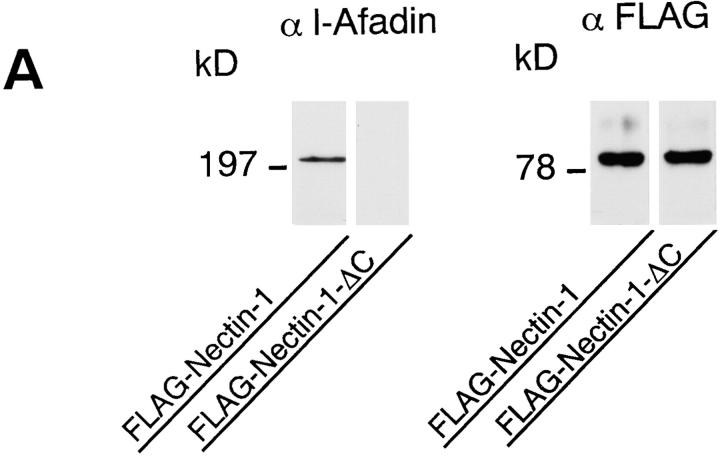
We then examined the function of the interaction of nectin with l-afadin. We prepared EL cells transiently expressing the FLAG-tagged full length of nectin-1 (FLAG-nectin-1-EL cells) or the FLAG-tagged, COOH-terminal 4 aa– deleted mutant of nectin-1 (FLAG-nectin-1-ΔC-EL cells). Immunoprecipitation analysis revealed that l-afadin was coimmunoprecipitated with FLAG-nectin-1, but not with FLAG-nectin-1-ΔC (Fig. 7 A). In FLAG-nectin-1-EL cells, FLAG-nectin-1 was colocalized with l-afadin and E-cadherin at cell–cell AJs (Fig. 7, B1–B6). In FLAG-nectin-1-ΔC-EL cells, however, nectin-1-ΔC was not recruited to cell–cell AJs where E-cadherin was localized (Fig. 7, B7–B12). Nectin-1-ΔC was not colocalized with l-afadin. These results indicate that nectin is recruited to cell–cell AJs through interaction with l-afadin in EL cells.
Figure 7.
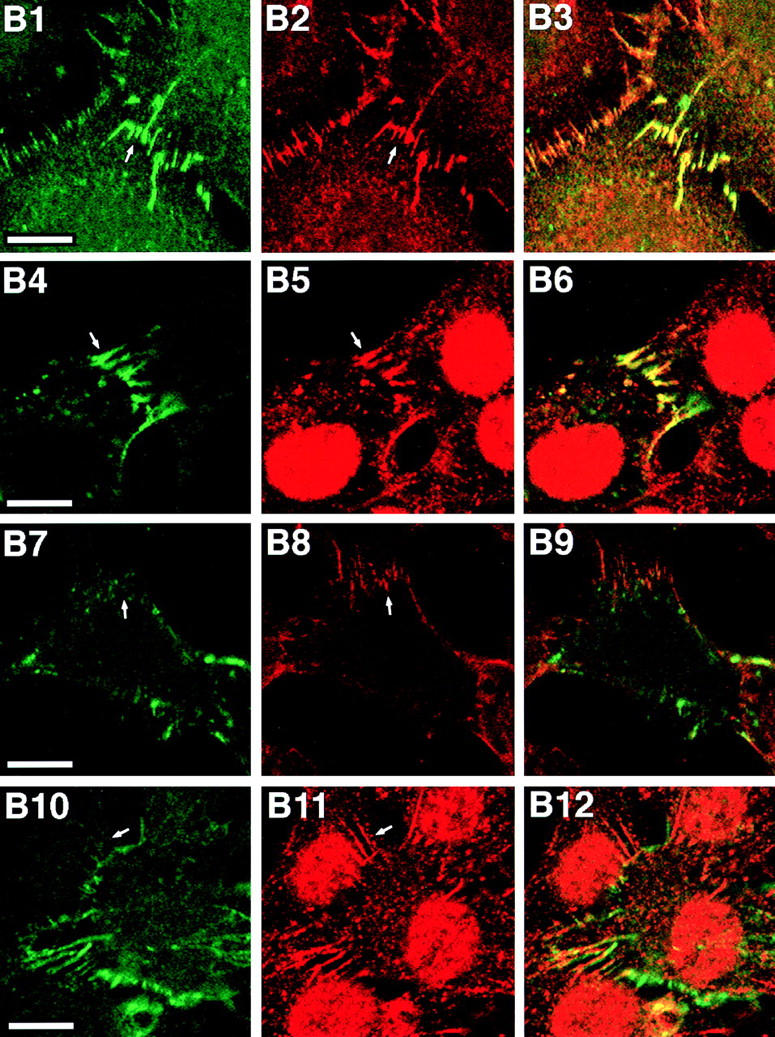
Recruitment of nectin to cadherin-based AJs through interaction with l-afadin. (A) Immunoprecipitation from EL cells expressing FLAG-nectin-1 or FLAG-nectin-1-ΔC. FLAG-Nectin-1 or FLAG-nectin-1-ΔC was transiently expressed in EL cells. Each cell extract was subjected to immunoprecipitation with the anti-FLAG mAb. The immunoprecipitate was then subjected to SDS-PAGE (8 or 12% polyacrylamide gel), followed by Western blot analysis with the anti-FLAG or anti–l-afadin mAb. (B) Immunofluorescence microscopy of FLAG-nectin-1-EL cells and FLAG-nectin-1-ΔC-EL cells. FLAG-nectin-1-EL cells and FLAG-nectin-1-ΔC-EL cells were doubly stained with the mouse anti-FLAG mAb and the rat anti–E-cadherin mAb or the rabbit anti–l-afadin pAb. They were visualized with FITC-conjugated anti–mouse IgG and rhodamine-conjugated anti–rat or anti–rabbit IgG Abs. There was nuclear staining with this anti–l-afadin pAb, but its significance is not clear. (B1–B6) FLAG-nectin-1-EL cells; (B7–B12) FLAG-nectin-1-ΔC-EL cells; (B1 and B4) FLAG-nectin-1; (B7 and B10) FLAG-nectin-1-ΔC; (B2 and B8) E-cadherin; (B5 and B11) l-afadin; (B3, B6, B9, and B12) merge. Arrows, cell–cell AJs. Bars, 10 μm.
Cell Aggregation Activity of Nectin
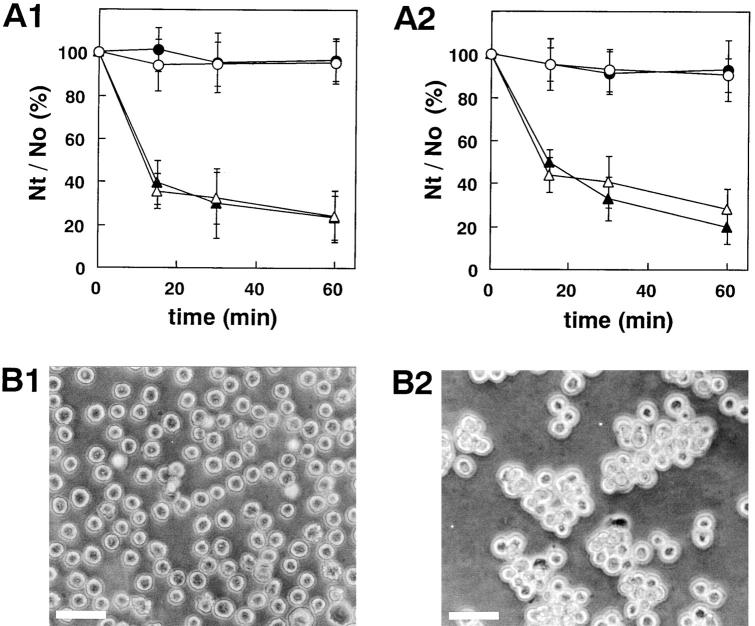
It has been shown previously that nectin-2α and -2δ show cell aggregation activity (Aoki et al., 1997; Lopez et al., 1998). To first confirm this result and to then examine cell aggregation activity of nectin-1, we prepared L cells stably expressing full-length nectin-1 (nectin-1-L cells) and -2α (nectin-2α-L cells). By use of these cell lines, we examined cell aggregation activity of nectin as described (Takeichi, 1977). Nectin-1 as well as nectin-2α showed cell aggregation activity in a time-dependent manner (Fig. 8). This activity was not affected by the presence or absence of Ca2+ in the medium, indicating that cell–cell adhesion activity of nectin-1 and -2α is Ca2+ independent.
Figure 8.
Cell aggregation activity of nectin. (A) Ca2+-independent aggregation activity of nectin-1 and -2α. L cells stably expressing nectin-1 or nectin-2α were treated with trypsin in the presence of EDTA and then dispersed by pipetting to obtain a single-cell suspension. Each single-cell suspension was rotated in HBSS in the presence of 1 mM CaCl2 or 1 mM EDTA for 15, 30, and 60 min. The extent of aggregation of cells was represented by the ratio of the total particle number at time t of incubation (Nt) to the initial particle number (No). (A1) Nectin-1-L cells. (Filled circles and open circles) Wild-type L cells; (filled triangles and open triangles) nectin-1-L cells; (filled circles and filled triangles) in the presence of 1 mM CaCl2; and (open circles and open triangles) in the presence of 1 mM EDTA. (A2) Nectin-2α-L cells. (Filled circles and open circles) Wild-type L cells; (filled triangles and open triangles) nectin-2α-L cells; (filled circles and filled triangles) in the presence of 1 mM CaCl2; and (open circles and open triangles) in the presence of 1 mM EDTA. The Nt/No values are the means ± SD of three independent experiments. (B) Cell aggregation of nectin-1-L cells. Single cells were rotated in HBSS in the presence of 1 mM EDTA for 60 min. (B1) Wild-type L cells. (B2) Nectin-1-L cells. Bars, 100 μm. These results are representative of three independent experiments.
No Direct Binding of Nectin to Known Components of Cell–Cell AJs
In the last set of experiments, to understand how nectin is recruited to cadherin-based cell–cell AJs through interaction with l-afadin, we examined the in vitro binding of nectin to the known components of cell–cell AJs, including α-, β-catenins, vinculin, and E-cadherin, by affinity chromatography. Under the conditions where Myc-nectin-1-CP bound to GST-l-afadin-PDZ, it did not bind to vinculin or any GST-fusion protein of α-, β-catenins, and the cytoplasmic region of E-cadherin (data not shown). Similar results were obtained with nectin-2α and -2δ (data not shown). Recently, we found that l-afadin does not bind directly to α-, β-catenin, or the cytoplasmic region of E-cadherin (Sakisaka et al., 1999). Thus, although nectin is recruited to cadherin-based cell–cell AJs through interaction with l-afadin, the mechanism of this recruitment remains unknown.
Discussion
Nectin constitutes a family consisting of three members, nectin-1, -2α, and -2δ, and belongs to the Ig superfamily (Morrison and Racaniello, 1992; Aoki et al., 1994; Eberlé et al., 1995; Lopez et al., 1995). The Ig superfamily encompasses diverse molecules that share a common structural homology (Edelman, 1986; Williams and Barclay, 1988; Buck, 1992; Walsh and Doherty, 1997). This superfamily includes CAMs and receptors for cytokines and growth factors. In contrast to cadherin, IgCAMs are far less well characterized with respect to their linkage to the actin cytoskeleton (Suter and Forscher, 1998). NCAM and L1, which are major IgCAMs expressed in neural tissue, regulate neurite outgrowth and guidance by the interaction with the actin cytoskeleton (Suter and Forscher, 1998). Other several IgCAMs redistribute or form “caps” on the surface of cells in an energy-dependent manner when cross-linked by divalent Abs (Pavalko and Otey, 1994). Capping requires the reorganization of the actin cytoskeleton, indicating that IgCAMs are linked to the actin cytoskeleton. However, no F-actin–binding protein which interacts with IgCAMs and is specifically localized at cell– cell adhesion sites has been reported. We have first shown here that l-afadin, an F-actin–binding protein, binds to the three members of the nectin family both in vitro and in vivo. The binding of l-afadin to nectin-1, -2α, and -2δ is stoichiometric and their affinities are apparently similar as estimated by the in vitro binding assay using the recombinant proteins and the immunoprecipitation experiment using COS7 cells, although it is not clear why nectin-1 shows ∼10-fold higher binding activity than nectin-2α and -2δ in the yeast two-hybrid assay. We have not directly shown here that nectin is associated with the actin cytoskeleton through l-afadin, but this possibility is likely because l-afadin binds F-actin in vitro and in vivo (Mandai et al., 1997).
We have shown here that nectin is colocalized with l-afadin at cadherin-based cell–cell AJs, but not at cell– matrix AJs, in various tissues and cell lines. Moreover, we have shown that nectin is recruited to cadherin-based cell– cell AJs through interaction with l-afadin. It is not known how these two proteins are recruited and colocalized with the cadherin-catenin system at cell–cell AJs. We have found recently that l-afadin does not directly bind to α-, β-catenin, or the cytoplasmic region of E-cadherin (Sakisaka et al., 1999). We have shown here that nectin does not interact directly with any of these proteins either. These results suggest that the nectin-l-afadin system is colocalized with the cadherin-catenin system through a still unidentified factor(s). Recently, we have isolated another l-afadin–binding protein, named ponsin, which is ubiquitously expressed and colocalized with vinculin at cell–cell and cell–matrix AJs (Mandai et al., 1999). Furthermore, ponsin binds vinculin. However, because ponsin forms a binary complex with either l-afadin or vinculin but hardly forms a ternary complex with l-afadin and vinculin, there should be an additional system which associates the nectin-l-afadin system to the cadherin-catenin system.
It may be noted that, in intestinal absorptive epithelial cells where ZO, ZA, and desmosome are well separated, nectin is specifically localized and more highly concentrated at ZA than E-cadherin which is distributed along the entire lateral membrane. This unique localization is also found for vinculin (Geiger et al., 1981; Yonemura et al., 1995), l-afadin (Mandai et al., 1997), and ponsin (Mandai et al., 1999). ZO and ZA in the junctional complex of polarized epithelial cells are closely aligned from the apical side to the basal side, suggesting that there are molecular interactions between these two junctional structures. Evidence is accumulating that the cadherin-catenin system plays essential roles for the assembly of the junctional complex (Gumbiner and Simons, 1986; Gumbiner et al., 1988; Watabe et al., 1994). It has been shown recently by use of an α-catenin–deficient colon carcinoma cell line that the interaction of α-catenin with vinculin is required for the organization of ZO (Watabe-Uchida et al., 1998). Furthermore, it has been shown that the junctional organization is impaired in vinculin-null F9 cells (Watabe-Uchida et al., 1998). The unique localization properties of nectin, l-afadin, ponsin, and vinculin suggest that the nectin-l-afadin system plays a role in the assembly of the junctional complex in cooperation with the cadherin-catenin system.
We have confirmed that nectin-2α has cell–cell adhesion activity as described (Aoki et al., 1997; Lopez et al., 1998) and have shown that nectin-1 also has this activity. In contrast to cadherin, most IgCAMs regulate cell–cell adhesion in a Ca2+-independent manner. Consistently, both nectin-1 and -2 show Ca2+-independent cell–cell adhesion. These results indicate that nectin is a Ca2+-independent CAM which is associated with l-afadin and specifically localized at ZA in epithelial cells and at cadherin-based cell–cell AJs in nonepithelial cells. Nectin-1 and -2 have been shown to be expressed in most tissues examined thus far (Morrison and Racaniello, 1992; Eberlé et al., 1995; Lopez et al., 1995). We have found here that the three members of the nectin family are expressed in MDCK cells. It remains to be clarified why the different nectin family members are expressed in the same cells, but the three members of the nectin family may be functionally redundant because of their common properties, including Ca2+-independent cell–cell adhesion activity, l-afadin–binding activity, and localization at cadherin-based cell–cell AJs.
We have analyzed here the binding regions of l-afadin and nectin and found that the PDZ domain of l-afadin and the cytoplasmic regions of nectin directly interact with each other. PDZ domains are modular domains that bind to specific COOH-terminal peptide sequences (Saras and Heldin, 1996; Ponting et al., 1997; Hata et al., 1998). Many PDZ domain–containing proteins and their binding partners have been isolated recently, and peptide sequences for various PDZ domains have been reported. Using the oriented peptide library technique, PDZ domains are assigned into classes according to their peptide-binding specificities (Songyang et al., 1997). The PDZ domain of AF-6 (s-afadin) is classified as the class II, selecting peptides with hydrophobic or aromatic aa residues at position −2 relative to the COOH terminus. The PDZ domain binds preferentially to a peptide which terminates in the sequence, E-F-Y-V (Songyang et al., 1997). Nectin terminates in the sequence, E/A-X-Y-V (X indicates W, V, and M for nectin-1, -2α, and -2δ, respectively). Our finding is consistent with these earlier observations (Songyang et al., 1997), but we have shown here by the yeast two-hybrid assay that the PDZ domain of l-afadin does not bind to neurexin-2α which terminates in the sequence, E-Y-Y-V. A recent study of the third PDZ domain of PSD-95/SAP90 indicates that X residues at position −1 in the consensus sequence (X-S/T-X-V) and the upstream residues of the tetrapeptide determine the specificity and affinity for the binding of the PDZ domain to its binding partner (Niethammer et al., 1998). By analogy, unique aromatic or hydrophobic X residues at position −2 in the sequence (E/A-X-Y-V), such as W, V, and M, may be necessary to bind to the PDZ domain of l-afadin. It is also possible that the upstream residues of the tetrapeptide are crucial for the specificity and affinity for the PDZ domain. It has been shown recently that the PDZ domain of AF-6 (s-afadin) binds to neurexin as well as the Eph receptor tyrosine kinase family members (Hock et al., 1998), but this result is not consistent with ours and the reason for this discrepancy is not known at present.
Acknowledgments
We thank Drs. S. Tsukita, A. Nagafuchi, and M. Itoh (Kyoto University, Kyoto, Japan) for helpful discussions and for providing us with L cells, EL cells, and the GST-fusion vectors, containing α-, β-catenins, and the cytoplasmic region of E-cadherin. We also thank Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany) for providing us with MDCK cells and Drs. J. Miyazaki and H. Niwa (Osaka University, Osaka, Japan) for providing us with the pCAGGS vector.
Abbreviations used in this paper
- AJs
adherens junctions
- CAM
cell adhesion molecule
- F-actin
actin filament
- GST
glutathione S-transferase
- PRR
poliovirus receptor–related protein
- ZA
zonula adherens
- ZO
zonula occludens
Footnotes
Junken Aoki's present address is Graduate School of Pharmaceutical Sciences, Tokyo University, Tokyo 113-0033, Japan. Akio Nomoto's present address is Department of Microbiology, Institute of Medical Science, Tokyo University, Tokyo 108-0071, Japan.
References
- Albelda SM, Buck CA. Integrin and other cell adhesion molecules. FASEB (Fed Am Soc Exp Biol) J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- Barth AIM, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck CA. Immunoglobulin superfamily: structure, function and relationship to other receptor molecules. Semin Cell Biol. 1992;3:179–188. doi: 10.1016/s1043-4682(10)80014-5. [DOI] [PubMed] [Google Scholar]
- Cimino G, Moir DT, Canaani O, Williams K, Crist WM, Katzav S, Cannizzaro L, Lange B, Nowell PC, Croce CM, Canaani E. Cloning of ALL-1, the locus involved in leukemias with the t(4;11)(q21;q23), t(9; 11)(p22;q23), and t(11;19)(q23;p13) chromosome translocations. Cancer Res. 1991;51:6712–6714. [PubMed] [Google Scholar]
- Eberlé F, Dubreuil P, Mattei M-G, Devilard E, Lopez M. The human PRR2 gene, related to the poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Ginsberg D. The cytoplasmic domain of adherens-type junctions. Cell Motil Cytoskel. 1991;20:1–6. doi: 10.1002/cm.970200102. [DOI] [PubMed] [Google Scholar]
- Geiger B, Dutton AH, Tokuyasu KT, Singer SJ. Immunoelectron microscope studies of membrane-microfilament interactions: distribution of α-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981;91:614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 522–523.
- Hata Y, Südhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J Biol Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Südhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Nakanishi H, Takai Y. Synaptic PDZ domain-containing proteins. Neurosci Res. 1998;32:1–7. doi: 10.1016/s0168-0102(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Hock B, Böhme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, Holland S, Pawson T, Rübsamen-Waigmann H, Strebhardt K. PDZ-domain-mediated interaction of the eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA. 1998;95:9779–9784. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez M, Eberlé F, Mattei M-G, Gabert J, Birg F, Birdin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin– and vinculin-binding protein localized at cell–cell and cell–matrix adherens junctions. J Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Asou H, Kobayashi M, Uyemura K. Functional expression of a full-length cDNA coding for rat neural cell adhesion molecule L1 mediates homophilic intercellular adhesion and migration of cerebellar neurons. J Biol Chem. 1992;267:10752–10758. [PubMed] [Google Scholar]
- Mizoguchi A, Yano Y, Hamaguchi H, Yanagida H, Ide C, Zahraoui A, Shirataki H, Sasaki T, Takai Y. Localization of Rabphilin-3A on the synaptic vesicle. Biochem Biophys Res Commun. 1994;202:1235–1243. doi: 10.1006/bbrc.1994.2063. [DOI] [PubMed] [Google Scholar]
- Morrison ME, Racaniello VR. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kD cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J Cell Biol. 1997;139:951–961. doi: 10.1083/jcb.139.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Valtschanoff JG, Kapoor TM, Alloson DW, Weinberg RJ, Craig AM, Sheng M. CRIPT, a novel postsynaptic protein that binds to the third PDZ domain of PSD-95/SAP90. Neuron. 1998;20:693–707. doi: 10.1016/s0896-6273(00)81009-0. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- O'Halloran T, Molony L, Burridge K. Purification and assay of vinculin, metavinculin, and talin. Methods Enzymol. 1986;134:69–77. doi: 10.1016/0076-6879(86)34076-x. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur Mol Biol Organ) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Otey CA. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc Soc Exp Biol Med. 1994;205:282–293. doi: 10.3181/00379727-205-43709. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Phillips C, Davies KE, Blake DJ. PDZ domains: targeting signalling molecules to sub-membranous sites. BioEssays. 1997;19:469–479. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- Prasad R, Gu Y, Alder H, Nakamura T, Canaani O, Saito H, Huebner K, Gale RP, Nowell PC, Kuriyama K, et al. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Nakanishi H, Takahashi K, Mandai K, Miyahara M, Satoh A, Takaishi K, Takai Y. Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junction. Oncogene. 1999;18:1609–1619. doi: 10.1038/sj.onc.1202451. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Saras J, Heldin C-H. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem Sci. 1996;21:455–458. doi: 10.1016/s0968-0004(96)30044-3. [DOI] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/ spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxy-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Suter DM, Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr Opin Neurobiol. 1998;8:106–116. doi: 10.1016/s0959-4388(98)80014-7. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. J Virol. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. α-Catenin–vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rüdiger A-H, Jockusch BM, Rüdiger M. Vinculin is part of the cadherin–catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AF, Barclay AN. The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]