Abstract
Members of the newly identified claudin gene family constitute tight junction (TJ) strands, which play a pivotal role in compartmentalization in multicellular organisms. We identified oligodendrocyte-specific protein (OSP) as claudin-11, a new claudin family member, due to its sequence similarity to claudins as well as its ability to form TJ strands in transfected fibroblasts. Claudin-11/OSP mRNA was expressed in the brain and testis. Immunofluorescence microscopy with anti–claudin-11/OSP polyclonal antibody (pAb) and anti-neurofilament mAb revealed that in the brain claudin-11/OSP-positive linear structures run in a gentle spiral around neurofilament-positive axons. At the electron microscopic level, these linear structures were identified as the so-called interlamellar strands in myelin sheaths of oligodendrocytes. In testis, well-developed TJ strands of Sertoli cells were specifically labeled with anti–claudin-11/OSP pAb both at immunofluorescence and electron microscopic levels. These findings indicated that the interlamellar strands of oligodendrocyte myelin sheaths can be regarded as a variant of TJ strands found in many other epithelial cells, and that these strands share a specific claudin species, claudin-11/OSP, with those in Sertoli cells to create and maintain the repeated compartments around axons by oligodendrocytes.
Keywords: oligodendrocyte, myelin sheath, tight junction, Sertoli cell, claudin-11, oligodendrocyte-specific protein
Tight junctions (TJs)1 are located at the most apical region of lateral membranes of epithelial cells, and are thought to function as a primary barrier to the diffusion of solutes through the paracellular pathway (for reviews see Schneeberger and Lynch, 1992; Gumbiner, 1987, 1993; Anderson and van Itallie, 1995). On thin-section electron microscopy, TJ appears as a series of discrete sites of apparent fusion, involving the outer leaflet of the plasma membrane of adjacent cells (Farquhar and Palade, 1963). On freeze-fracture electron microscopy, TJ appears as a set of continuous, anastomosing intramembranous strands or fibrils in the protoplasmic face (P-face; the outwardly facing cytoplasmic leaflet) with complementary grooves in the extracellular face (E-face; the inwardly facing extracytoplasmic leaflet; Staehelin, 1973, 1974). Recent technical progress has enabled the identification of several TJ-associated peripheral membrane proteins such as ZO-1 (Stevenson et al., 1986), ZO-2 (Gumbiner et al., 1991), ZO-3 (Balda et al., 1993; Haskins et al., 1998), cingulin (Citi et al., 1988), 7H6 antigen (Zhong et al., 1993), and symplekin (Keon et al., 1996), but the TJ-specific integral membrane protein, i.e., the component of TJ strands, had not been identified until recently.
Occludin, a 65-kD integral membrane protein bearing four transmembrane domains, is the first identified component of TJ strands (Furuse et al., 1993; Ando-Akatsuka et al., 1996). Occludin was shown to be a component of the TJ strand itself (Fujimoto, 1995; Furuse et al., 1996) and to be directly involved in the barrier functions of TJs (McCarthy et al., 1996; Balda et al., 1996; Chen et al., 1997; Wong and Gumbiner et al., 1997). Recently, occludin gene knockout was successfully performed, but unexpectedly occludin-deficient epithelial cells still demonstrated a well-developed network of TJ strands, suggesting the existence of as yet unidentified TJ-specific integral membrane proteins (Saitou et al., 1998).
As the second and third components of TJ strands, two 22-kD integral membrane proteins each with four transmembrane domains were identified from the isolated junctional fraction of the liver (Furuse et al., 1998a). Since these two proteins showed sequence similarity to each other (∼38% identical at the amino acid sequence level) but not to occludin, they were designated as claudin-1 and -2. Both proteins were targeted to and incorporated into preexisting TJ strands when expressed in cultured epithelial cells. Furthermore, when they were introduced singly into cultured L fibroblasts lacking TJs, they induced the formation of a well-developed network of TJ strands between stable transfectants (Furuse et al., 1998b). Furthermore, similarity searches in data bases identified many sequences similar to claudin-1 and -2, pointing to the existence of a new gene family, the claudin family (Morita et al., 1999). To date, we have identified eight members of this family (claudin-1 to -8), which were concentrated at TJs when introduced into cultured MDCK cells. However, Northern blotting showed that none of these molecules were expressed in large amounts in the central nervous system.
In the brain of vertebrates, two types of cells are known to bear TJ or TJ-like structures; vascular endothelial cells and oligodendrocytes. In endothelial cells in the brain, a typical TJ strand network is well developed, which is thought to be responsible for the blood-brain barrier (Rubin, 1991). In myelinated nerve fibers of central nervous system, each internodal segment between two consecutive nodes of Ranvier is composed of a cellular process of an oligodendrocyte with its myelin lamellae. For saltatory conduction along these myelinated axons, the intercellular spaces between axons and surrounding myelin sheaths must be electrically isolated. In the paranodal region, numerous rows of glial-axonal (septate-like) junctions bridge between the terminal loops of the ensheathing cell and the underlying axonal membrane, and these junctions were thought to be responsible for electrical sealing (Raine, 1984). Linear strands ∼10 nm thick were observed between lamellae of myelin sheaths by freeze-fracture electron microscopy (Dermietzel et al., 1974, 1980; Reale et al., 1975; Schnapp and Mugnaini, 1978; Tabira et al., 1978). These strands run parallel to the axon axis and radially through the myelin sheath, consisting of the so-called radial component of myelin (Peters, 1961, 1964; Dermietzel, 1974). Since these strands showed very similar morphological characteristics to TJ strands in epithelial and endothelial cells, the radial component was also supposed to be directly involved in isolating the extracellular compartment within myelin sheaths (Mugnaini and Schnapp, 1974; Gumbiner and Louvard, 1985). However, since any TJ-specific proteins, especially occludin or claudins, have not yet been detected in these strands, it remains unclear whether these strands can be regarded as TJ strands not only morphologically but also physiologically.
In this study, we analyzed the expression and subcellular distribution of oligodendrocyte-specific protein (OSP), partly because it shows sequence similarity to claudin-1 to -8, and partly because it is expressed in large amounts in the brain. The OSP cDNA was first identified as a cDNA expressed in oligodendrocytes by subtraction cloning, but its product has not been characterized in detail (Bronstein et al., 1996). Here, we showed that OSP should be regarded as a member of the claudin family, and that this molecule is a specific component of TJ strands in myelin sheaths of oligodendrocytes, and interestingly, also in Sertoli cells of the testis.
Materials and Methods
Antibodies and Cells
Rat anti–mouse occludin mAb (MOC37) was raised and characterized as described previously (Saitou et al., 1997). Mouse anti-neurofilament 200-kD mAb and rat anti-FLAG mAb were purchased from Boehringer Mannheim Biochemicals and Eastman Kodak Co., respectively. Mouse L cells and their transfectants were grown in DME supplemented with 10% fetal calf serum.
cDNA Cloning and Sequencing
Kidney total RNA was isolated according the method described by Chomczynski and Sacchi (1987). Poly(A)+ was prepared from the total RNA using oligo-dT cellulose (New England Biolabs, Inc.). First strand cDNA was prepared from this poly(A)+ RNA with Superscript™ II reverse transcriptase (GIBCO BRL) and used for PCR. Claudin-11/OSP cDNA was amplified from mouse kidney first strand DNA by PCR using the previously reported sequence of mouse OSP (Bronstein et al., 1996). The amplified cDNA was subcloned into pGEM-T Easy Vector (Promega). The lack of sequence errors was confirmed by DNA sequence analysis using a Dye Terminator Cycle Sequence Kit (Applied Biosystems, Inc.).
Mammalian Expression Vectors and Transfection
Mouse claudin-11/OSP was tagged with FLAG-peptide at its COOH terminus. To construct FLAG-tagged claudin-11/OSP-expression vectors, EcoRI site was introduced at the 3′-end of claudin-11/OSP cDNA by PCR, and amplified fragments were subcloned into pBluescript SK(−)- Flag-tag. The insert was excised by SalI-XbaI digestion followed by blunting with T4-polymerase, and then introduced into pCAGGSneodelEcoRI (Niwa et al., 1991), which was provided by Dr. J. Miyazaki (Osaka University). Mouse L cells were used for transfection as described previously (Furuse et al., 1998b), and the clones stably expressing tagged protein were screened by fluorescence microscopy with anti-FLAG mAb.
Northern Blotting
The expression of claudin-11/OSP in various mouse tissues was examined by Northern blotting using a Mouse Multiple Tissue Northern Blot (Clontech). The DNA fragment of mouse claudin-11/OSP (entire ORF) was radiolabeled with [32P]dCTP and used as a probe for Northern blotting. Hybridization was performed in ExpressHyb™ Hybridization Solution (Clontech) at 68°C for 12 h. The membranes were washed with 2× SSC containing 0.1% SDS at room temperature for 30 min and then with 0.1× SSC containing 0.1% SDS at 50°C for 30 min. The membranes were exposed to imaging plates for 12 h, and the signals were visualized using a BAS2000 Bio-Imaging Analyzer (Fuji Photo Film Co. Ltd.).
Polyclonal Antibody Production
A polypeptide, CNRFYYSSGSSSPTHAKSAHV, corresponding to the COOH-terminal cytoplasmic domain of mouse claudin-11/OSP was synthesized, and coupled via cysteine to keyhole limpet hemocyanin. This conjugated peptide was used as an antigen to generate polyclonal antibodies (pAb) in rabbits. The antiserum was affinity-purified with glutathione- S-transferase (GST) fusion protein with the COOH-terminal cytoplasmic domain of claudin-11/OSP.
SDS-PAGE and Immunoblotting
Lysates of E. coli expressing GST/claudin fusion proteins were subjected to one-dimensional SDS-PAGE (12.5%), according to the method of Laemmli (1970), and gels were stained with Coomassie brilliant blue R-250. For immunoblotting, proteins were electrophoretically transferred from gels onto nitrocellulose membranes, which were then incubated with the first antibody. Bound antibodies were detected with biotinylated second antibodies and streptavidin-conjugated alkaline phosphatase (Amersham Corp.). Nitroblue tetrazolium and bromochloroindolyl phosphate were used as substrates for detection of alkaline phosphatase.
Immunofluorescence Microscopy
L transfectants plated on glass coverslips were fixed with 1% formaldehyde in PBS for 10 min at room temperature. Mouse brain and testis were frozen using liquid nitrogen, and frozen sections ∼10–20 μm thick were cut on a cryostat, mounted on glass slides, air-dried, and fixed in 95% ethanol at 4°C for 30 min followed by 100% acetone at room temperature for 1 min. These samples were processed for immunofluorescence microscopy as described previously (Furuse et al., 1998b; Morita et al., 1999). Specimens were observed using a fluorescence microscope, Zeiss Axiophot photomicroscope (Carl Zeiss, Inc.), or a Bio-Rad MRC 1024 confocal fluorescence microscope (Bio-Rad Laboratories) equipped with a Zeiss Axiophot photomicroscope. For each stereoscopic image (see Fig. 5), 30 optical sections (0.3–0.4-μm interval) were accumulated in the computer.
Figure 5.
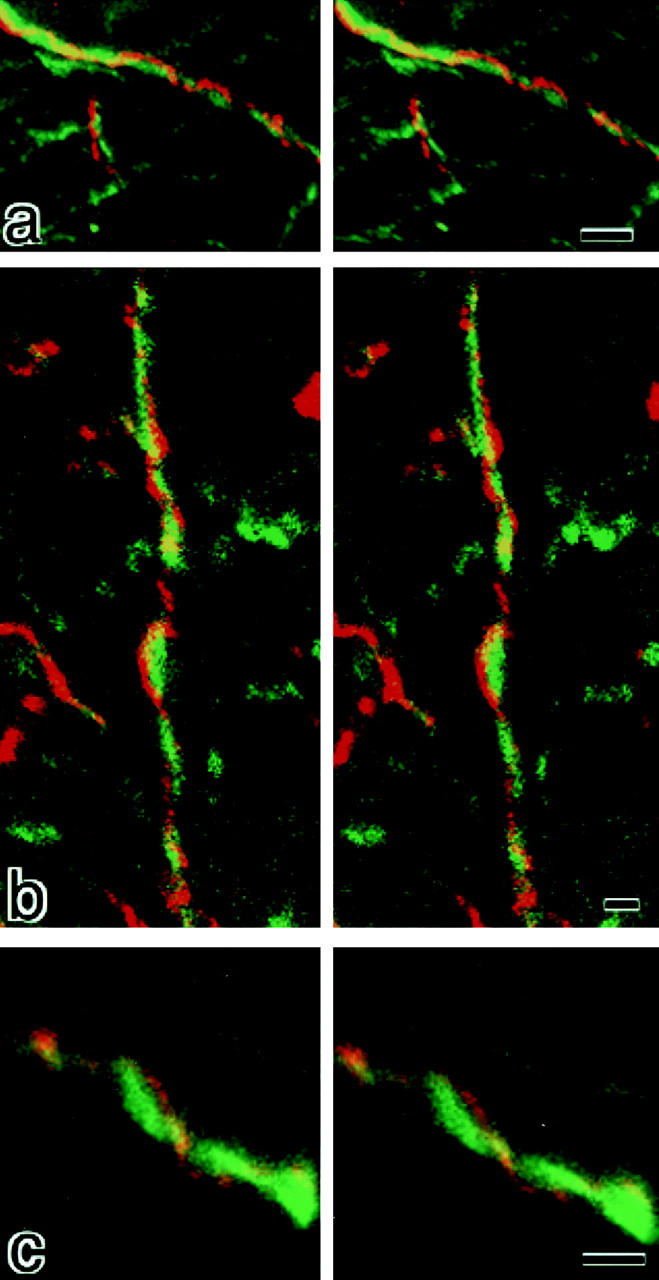
Stereoscopic comparison of subcellular distribution between claudin-11/OSP and neurofilaments. Frozen sections of the brain cortex were doubly stained with anti–claudin-11/OSP pAb (red) and anti-neurofilament mAb (green), examined by confocal microscopy, and stereoscopic images were generated. Note that each claudin-11/OSP-positive linear structure (red) ran in a gentle spiral around a neurofilament-positive axon (green). Bars: (a) 2 μm; (b) 1 μm; (c) 1 μm.
Freeze-Fracture Electron Microscopy
For conventional freeze-fracture analysis, tissues or cultured L fibroblasts were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) for 3 h at room temperature, washed with 0.1 M sodium cacodylate buffer three times, immersed in 30% glycerol in 0.1 M sodium cacodylate buffer for 2 h, and then frozen in liquid nitrogen. Frozen samples were fractured at −100°C and platinum-shadowed unidirectionally at an angle of 45° in Balzers Freeze Etching System (BAF060; Bal-Tec). The samples were then immersed in household bleach, and replicas floating off the samples were washed with distilled water. Replicas were picked up on formvar-filmed grids, and examined with a JEOL 1200EX electron microscope (JEOL) at an acceleration voltage of 100 kV.
Immunoelectron Microscopy
The immunoelectron microscopic technique for examining freeze-fracture replicas was described in detail previously (Fujimoto, 1995; Moroi et al., 1998), except that samples were frozen in a high-pressure freezer (Baltec HPM010; Bal-Tec). Immunoelectron microscopy using ultrathin cryo-sections was performed essentially according to the method developed by Tokuyasu (Tokuyasu, 1980; Fujimoto et al., 1992). Samples were examined with a JEOL 1200EX electron microscope (JEOL) at an acceleration voltage of 80 kV.
Results
Characterization of OSP as a Claudin Family Member, Claudin-11
Using the previously reported nucleotide sequence of mouse OSP (Bronstein et al., 1996), we amplified a full-length cDNA encoding mouse OSP by PCR, and confirmed that its open reading frame encoded a protein of 207 amino acids with a calculated molecular mass of 22.1 kD. OSP showed rather weak sequence similarity to claudins: it was almost equidistantly related to previously identified members of the claudin family (claudin-1 to -8; ∼30% identity at the amino acid sequence level to each member). As shown in Fig. 1, comparison between OSP and claudin-1 revealed that identical amino acids were almost evenly distributed throughout these molecules.
Figure 1.
Comparison of amino acid sequences of mouse OSP and claudin-1 by the GENETYX program. Identity and homology are indicated by | and :, respectively. Four putative transmembrane domains are indicated by boxes. They showed 31.7% identity at the amino acid sequence level. Note that identical residues are distributed almost evenly through the molecule, and that OSP and claudin-1 end in -H-V and -Y-V, respectively.
Next, we introduced cDNA encoding OSP with a FLAG- sequence at its COOH terminus into cultured L fibroblasts which lacked TJs or the expression of claudins (Furuse et al., 1998b). Immunofluorescence microscopy of the stable transfectants with anti-FLAG mAb showed that expressed FLAG-OSP was concentrated at cell–cell borders as planes or on thin cellular protrusions (Fig. 2, a–d). This mAb gave no signal from parent L fibroblasts. Then, these stable L transfectants expressing FLAG-OSP were fixed with glutaraldehyde and examined by conventional freeze-fracture electron microscopy (Fig. 2 e). In these cells, TJ strand/groove-like structures were frequently observed to be arranged in a parallel manner, whereas in parent L cells these structures were not detected. These strands were associated with the P-face, and were mostly discontinuous with intervening spaces of various widths (Fig. 2 e, inset). On the E-face, complementary continuous grooves were identified, containing scattered particles (Fig. 2 e, inset). The OSP-induced strands did not bifurcate frequently and showed a tendency to run parallel to each other.
Figure 2.
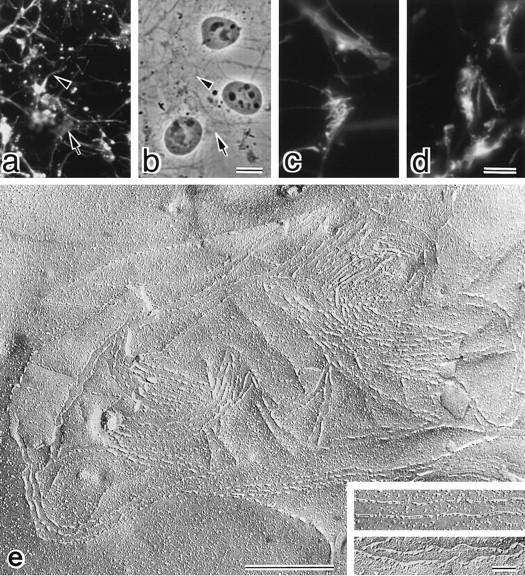
L transfectants expressing FLAG-tagged OSP. (a–d) Immunofluorescence (a) and corresponding phase contrast images (b) of stable L transfectants expressing FLAG-OSP. Cells were stained with anti-FLAG mAb. Expressed FLAG-OSP was concentrated at cell–cell borders as planes (arrow) or on thin cellular protrusions (arrowhead). At higher magnification (c and d), at cell–cell contact planes, FLAG-OSP was concentrated as networks or as thick lines. (e) Freeze-fracture images of cell–cell contact planes of stable L transfectants expressing FLAG-OSP. At low magnification, large numbers of TJ strand/ groove-like structures were observed. These strands scarcely branched, and showed a tendency to run parallel to each other. Inset, higher magnification of strands on P-face (top) and grooves on E-face (bottom). Bars: (a and b) 10 μm; (c and d) 4 μm; (e) 500 nm; (inset) 100 nm.
These characteristics of OSP, i.e., sequence similarity to claudins and ability to induce TJ strand/groove-like structures in L fibroblasts, led us to regard OSP as a member of the claudin family, and we tentatively designated it as claudin-11 after consulting the human gene nomenclature committee (http://www.gene.ucl.ac.uk/nomenclature/). (In the updated human gene database, at least 15 members of the claudin family were found, all of which were already assigned to claudin-1 to -15.)
Expression and Distribution of Claudin-11/OSP in Brain
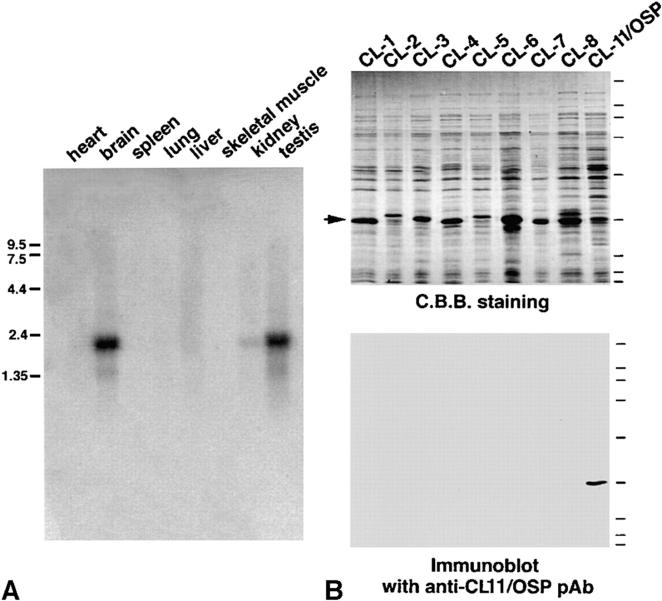
We next examined the expression of claudin-11/OSP in various tissues by Northern blotting. As shown in Fig. 3 A, claudin-11/OSP mRNA was detected as a 2.3-kb band in large amounts in the brain and testis and in only a trace amount in the kidney, suggesting that this type of claudin is involved in the TJ formation specifically in the brain and testis. Then, to examine the subcellular distribution of claudin-11/OSP, we generated polyclonal antibodies (pAbs) in rabbits using a synthesized peptide corresponding to COOH-terminal 20 amino acids of claudin-11/ OSP as an antigen. By immunoblotting, one affinity-purified pAb (pAb CL11-2) specifically recognized the GST fusion protein with COOH-terminal cytoplasmic domain of claudin-11/OSP, but not that of claudin-1 to -8, which were produced in E. coli (Fig. 3 B). Using this pAb, we first examined the subcellular distribution of claudin-11/OSP in the brain. When frozen sections of mouse brain were immunofluorescently stained with anti–claudin-11/OSP pAb, a large number of intensely stained linear structures were seen scattered in random directions in the cortex (Fig. 4 a). In deeper regions, these linear structures were occasionally arranged in a parallel manner to form thick bundles (Fig. 4 b). In the brain, TJs are known to be developed in vascular endothelial cells. Since these TJs were stained positively with anti-occludin antibody, we then stained frozen sections of brain doubly with anti–claudin-11/OSP pAb and anti-occludin mAb. However, as shown in Fig. 4, c and d, the claudin-11–positive linear structures did not overlap with occludin-positive endothelial TJs.
Figure 3.
Northern blots of claudin-11/OSP expression and specificity of anti–claudin-11/OSP pAb. (A) Mouse Multiple Tissue Northern Blot (CLONTECH) was probed with a DNA fragment of mouse claudin-11/OSP. Claudin-11/OSP mRNA was detected as a 2.3-kb band in large amounts in the brain and testis and in only a trace amount in the kidney. (B) Immunoblots of total lysates of E. coli expressing GST fusion proteins with cytoplasmic domains of claudin-1 to -8 and claudin-11/OSP (arrow) confirmed the specificity of anti–claudin-11/OSP pAb. Bars indicate molecular masses of 200, 116, 97, 66, 45, 31, 21, 15, and 10 kD from the top.
Figure 4.
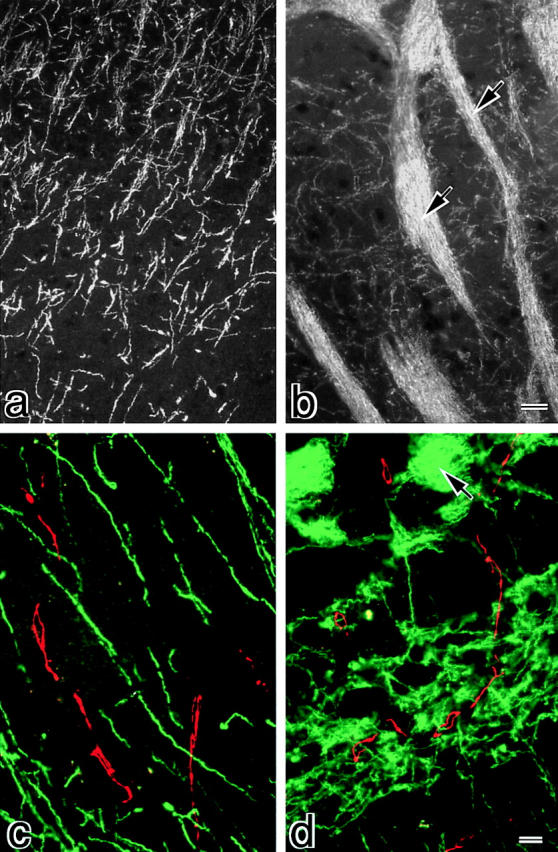
Distribution of claudin-11/OSP in mouse brain. Frozen sections of mouse brain, cortex region (a and c), and deeper region (b and d), were singly stained with anti–claudin-11/OSP pAb (a and b) or doubly stained with anti–claudin-11/OSP pAb (green in c and d) and anti-occludin mAb (red in c and d). In the cortex region (a and c), a large number of intensely stained linear structures was seen scattered in random directions, whereas in the deeper region (b and d) these claudin-11/OSP-positive structures were occasionally arranged in a parallel manner to form thick bundles (arrows). These claudin-11/OSP-positive linear structures did not overlap with occludin-positive endothelial TJs (c and d). Bars: (a and b) 10 μm; (c and d) 5 μm.
Then, frozen sections of the brain cortex were doubly stained with anti–claudin-11/OSP pAb and anti-neurofilament mAb, examined by confocal microscopy, and stereoscopic images were generated by computer (Fig. 5). At higher magnification, each claudin-11/OSP-positive linear structure was seen to run in a gentle spiral around a neurofilament-positive axon. These images suggested that these claudin-11/OSP signals were derived from myelin sheaths surrounding individual axons.
As reported previously (Dermietzel et al., 1974, 1980; Reale et al., 1975; Schnapp and Mugnaini, 1978; Tabira et al., 1978), conventional freeze-fracture electron microscopy of glutaraldehyde-fixed mouse brain revealed that so-called interlamellar strands of oligodendrocytes were arranged in a gentle spiral around axons (Fig. 6 a). Similarly to TJ strands in L transfectants expressing claudin-11/OSP (see Fig. 2), these interlamellar strands were associated with the P-face, and were mostly discontinuous with intervening spaces of various widths (Fig. 6 a, inset). When freeze-fracture replicas from unfixed brains or optic nerves were immunolabeled with anti–claudin-11/OSP pAb, these interlamellar strands were specifically labeled (Fig. 6, b and c). Furthermore, as shown in Fig. 7, when ultrathin cryo-sections of the brain were incubated with the same pAb, transverse sections of myelin sheaths were labeled radially, which may correspond to the so-called radial component of myelin (Peters, 1961, 1964; Dermietzel, 1974).
Figure 6.
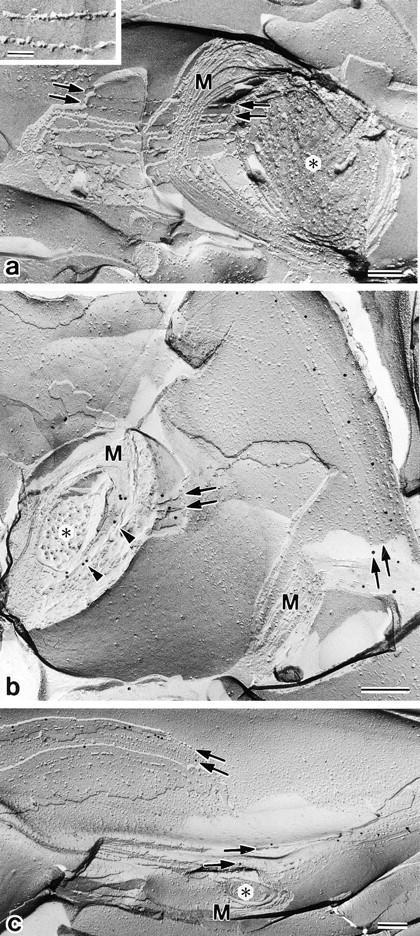
Localization of claudin-11/OSP at interlamellar strands in oligodendrocytes. (a) Mouse brain was fixed with glutaraldehyde and then processed for conventional freeze-fracture analysis. So-called interlamellar strands (arrows) were observed in each lamella of myelin sheaths. Asterisk, transversely fractured image of axoplasm; M, transversely fractured image of myelin sheaths. Inset, enlarged image of interlamellar strands. (b and c) Mouse brain (b) or optic nerve (c) was quickly frozen without chemical fixation, and then processed for freeze-fracture analysis. Freeze-fracture replicas were labeled with anti–claudin-11/OSP pAb. Interlamellar strands (arrows) were specifically labeled with the pAb (10-nm gold particles). Note that the transversely fractured myelin sheaths were also labeled with the pAb (arrowheads), and that this labeling pattern was very similar to Fig. 7. Asterisk, transversely fractured image of axoplasm; M, transversely fractured image of myelin sheaths. Bars: (a) 100 nm; (b and c) 200 nm.
Figure 7.
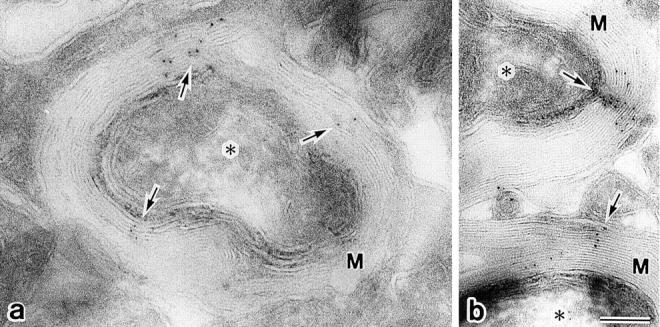
Localization of claudin-11/OSP at radial components in myelin sheaths. (a and b) Ultrathin cryo-sections of the mouse brain were labeled with anti–claudin-11/ OSP pAb. Note that transverse sections of myelin sheaths were specifically labeled radially (10-nm gold particles; arrows). Asterisk, transverse section of axoplasm; M, transverse section of myelin sheaths. Bar, 100 nm.
Distribution of Claudin-11/OSP in Testis
Finally, frozen sections of mouse testis were doubly stained with anti–claudin-11/OSP pAb and anti-occludin mAb. As shown in Fig. 8, a and b, both claudin-11/OSP and occludin were concentrated and precisely colocalized in a linear fashion at the most basal region of lateral membranes of adjacent Sertoli cells where TJs were reported to be well developed. Endothelial cells of microvessels were occludin-positive, but claudin-11/OSP-negative (Fig. 8, a and b). Immunofreeze-fracture electron microscopy revealed that claudin-11/OSP was exclusively localized on well-developed TJ strands of Sertoli cells (Fig. 8 c).
Figure 8.
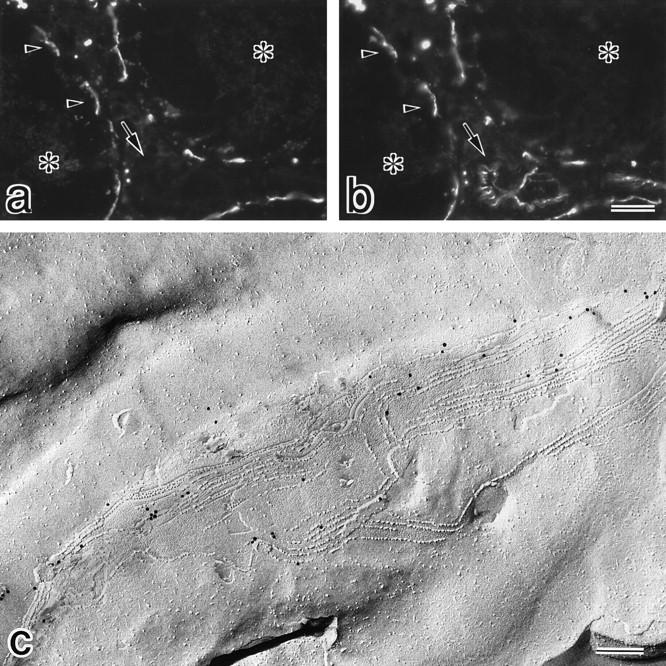
Subcellular localization of claudin-11/OSP at TJ strands in Sertoli cells. (a and b) Frozen sections of mouse testis were doubly stained with anti–claudin-11/ OSP pAb (a) and anti-occludin mAb (b). Both claudin-11/OSP and occludin were concentrated and precisely colocalized in a linear fashion at the most basal region of lateral membranes of adjacent Sertoli cells (arrowheads). Note that vascular endothelial cells were stained positively for occludin but were negative for claudin-11/ OSP (arrow). Asterisks, centers of seminiferous tubules. (c) Mouse testes were quickly frozen without chemical fixation, and then processed for freeze-fracture. Freeze-fracture replicas were labeled with anti–claudin-11/OSP pAb. Characteristic Sertoli TJs were exclusively labeled with the pAb (10-nm gold particles). Bars: (a and b) 25 μm; (c) 200 nm.
Discussion
Interlamellar strands (radial components) of myelin sheaths are of great interest in terms of the physiological functions of oligodendrocytes (see Schnapp and Mugnaini, 1978). Although their appearance on freeze-fracture images is similar to those of epithelial and/or endothelial TJs, TJ-specific proteins, especially occludin, have not been shown to be localized at these interlamellar strands. On the other hand, we recently identified novel gene family members, claudin-1 to -8, which are major constituents of TJ strands, and found that single or several claudins are copolymerized to form TJ strands in various tissues (Furuse et al., 1998a,b; Morita et al., 1999). However, Northern blotting revealed that they were not expressed in large amounts in the brain as compared with non-neuronal tissues (Furuse et al., 1998a; Morita et al., 1999). In this study, we found that OSP (oligodendrocyte-specific protein) shows a sequence similarity to claudins, although the degree of identity was at most ∼30% (see Fig. 1). OSP was first identified as a protein specifically expressing in oligodendrocytes, but it has not been characterized in detail (Bronstein et al., 1996). We have demonstrated that OSP has the ability to form TJ strand-like structures in L fibroblasts, and that it is exclusively localized at interlamellar strands. Based on these observations, we concluded that interlamellar strands can be regarded as a variant of TJ strands composed of a new claudin, claudin-11/OSP.
As previously reported, when claudin-1 or -2 was singly expressed in L fibroblasts, TJ strands were induced, and through their ramification a huge network of strands was formed (Furuse et al., 1998b). In contrast, the claudin-11/ OSP-induced strands scarcely branched in L fibroblasts, and showed a tendency to run parallel to each other. Interestingly, interlamellar strands (and also TJ strands in Sertoli cells [Gilula et al., 1976]) run parallel without branching (see Figs. 6 and 8), while typical TJ strands in epithelial cells frequently ramify to form networks (Staehelin, 1973, 1974). These findings favored the notion that claudin-11/ OSP is a major constituent of interlamellar strands (and also TJ strands in Sertoli cells) and that the frequency of ramification of strands depends on the intrinsic nature of each claudin species.
It is intriguing that TJ strands in oligodendrocytes (interlamellar strands) share a specific claudin species, claudin-11/OSP, with those in Sertoli cells. In the testis, well-developed TJ strands in Sertoli cells (so-called Sertoli junctions) closely protect spermatogenic cells from the external environment, which is known as the blood-testis barrier (Dym and Fawcett, 1970; Russell and Peterson, 1985). Similarly to the brain, Northern blotting analysis indicated that the testis does not express claudin-1 to -8 in large amounts (Furuse et al., 1998a; Morita et al., 1999). Therefore, it is likely that in the testis claudin-11/OSP is a major component of TJ strands, which function as a tight barrier, and that in the brain interlamellar strands of oligodendrocytes also function as a barrier to closely isolate the extracellular compartment within myelin sheaths. In the central nervous system, neurons are also closely protected from the external environment, but this blood-brain barrier is known to be established by TJ strands in vascular endothelial cells in vertebrates (Rubin, 1991). However, in invertebrates such as insects, TJs of the perineurial sheath in the central nervous system (Lane et al., 1994) and/or pleated septate junctions (possible invertebrate equivalent of TJs) of glia cells were reported to be directly involved in the blood-nerve barrier (Lane et al., 1977; Juang and Carlson, 1992; Baumgartner et al., 1996). It is still premature to discuss this issue further, but it would be interesting to examine the phylogenetical relationship between claudin-11/ OSP and the other claudins in future studies.
Sertoli junctions were initially called “the junctional specializations of Sertoli cells” (Flickinger and Fawcett, 1967), and thought to be different from typical TJs in other epithelial cells (Gilula et al., 1976). These Sertoli junctions were characterized by (a) discrete bundles of filaments running parallel to the junction membranes, (b) cisternae of the endoplasmic reticulum located deeper to the layer of filaments, and (c) the parallelly arranged strands that did not anastomose extensively. However, recent findings on the localization of ZO-1 (Byers et al., 1991; Balda and Anderson, 1993), symplekin (Keon et al., 1996), and occludin (Moroi et al., 1998) in Sertoli junctions indicated that this specialization is a variant of TJs found in many other epithelia. This study, i.e., the occurrence of claudin-11/ OSP in Sertoli junctions, conclusively supported this notion.
Another issue that we should discuss here is the sequence differences in the cytoplasmic tail between claudin-11/OSP and the other claudins. All of claudin-1 to -8 end in -Y-V at their COOH termini (Furuse et al., 1998a; Morita et al., 1999), whereas only claudin-11/OSP ends in -H-V. In this sense, claudin-11/OSP is rather distantly related to the other claudins. The COOH-terminal -E-S/T-D-V motif in the Shaker K+ channel (-ETDV)/NMDA R2 subunit (-ESDV) and -E-Y-Y-V motif in neurexins were shown to be responsible for their binding to PDZ domains of Dlg/PSD-95 family proteins and LIN-2/CASK, respectively (Kim et al., 1995; Komau et al., 1995; Hata et al., 1996; Niethammer et al., 1996). Considering that three PDZ-containing MAGUK family proteins, ZO-1, ZO-2, and ZO-3, are exclusively concentrated at the cytoplasmic surface of TJ strands in epithelial and endothelial cells (Stevenson et al., 1986; Gumbiner et al., 1991; Balda et al., 1993; Itoh et al., 1993; Willott et al., 1993; Jesaitis and Goodenough, 1994; Haskins et al., 1998), it is expected that the COOH-terminal -Y-V in claudin-1 to -8 binds to some of the PDZ domains of the MAGUK family proteins to recruit them to TJ strands. So far, occludin has been regarded as a membrane binding partner for ZO-1 and ZO-2 (Furuse et al., 1994; Itoh et al., 1999), but it was shown that even in occludin-deficient TJ strands ZO-1 (and also ZO-2; unpublished data) was still recruited (Saitou et al., 1998). These findings are consistent with the above expectation, and led to the further speculation that the COOH-terminal -H-V of claudin-11/OSP shows distinct affinity to ZO-1, ZO-2, and/or ZO-3 from the other claudins. Actually, ZO-1 and ZO-2 were not concentrated at the interlamellar strands of oligodendrocytes (data not shown; see Saitou et al., 1997).
In this study, we found that although the amino acid sequence of claudin-11/OSP is fairly diversified from the eight previously identified claudins, it has the ability to induce TJ strands in L fibroblasts and constitutes the interlamellar strands in oligodendrocytes as well as TJ strands of Sertoli cells in the testis. Molecular manipulation of claudin-11/OSP including targeted gene disruption will more directly unravel not only the physiological functions of this unique claudin family member in the brain and testis, but also the etiologies of some myelin-related as well as spermatogenesis-related disorders.
Acknowledgments
We thank all the members of our laboratory (Department of Cell Biology, Faculty of Medicine, Kyoto University) for helpful discussions. Our thanks are also due to Ms. K. Furuse for her excellent technical assistance.
This study was supported in part by a Grant-in-Aid for Cancer Research and a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science and Culture of Japan to Sh. Tsukita.
Abbreviations used in this paper
- E-face
extracellular face
- GST
glutathione-S-transferase
- OSP
oligodendrocyte-specific protein
- P-face
protoplasmic face
- pAb
polyclonal antibody
- TJ
tight junction
Footnotes
The first two authors contributed equally to this work.
References
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita Sh. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–C924. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Balda MS, González-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophilaneurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Popper P, Micevych PE, Farber DB. Isolation and characterization of a novel oligodendrocyte-specific protein. Neurology. 1996;47:772–778. doi: 10.1212/wnl.47.3.772. [DOI] [PubMed] [Google Scholar]
- Byers S, Graham R, Dai HN, Hoxter B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am J Anat. 1991;191:35–47. doi: 10.1002/aja.1001910104. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chen Y-H, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopusembryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Dermietzel R. Junctions in the central nervous system of the cat. I. Membrane fusion in central myelin. Cell Tissue Res. 1974;148:565–576. doi: 10.1007/BF00221940. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Leibstein AG, Schunke D. Interlamellar tight junctions of central myelin: II. A freeze-fracture and cytochemical study on their arrangement and composition. Cell Tissue Res. 1980;213:95–108. doi: 10.1007/BF00236923. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger C, Fawcett DW. The junctional specializations of Sertoli cells in the seminiferous epithelium. Anat Rec. 1967;158:207–222. doi: 10.1002/ar.1091580210. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita Sa, Tsukita Sh. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita Sh. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998a;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita Sh. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998b;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Fawcett DW, Aoki A. The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev Biol. 1976;50:142–168. doi: 10.1016/0012-1606(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Louvard D. Localized barriers in the plasma membrane: a common way to form domains. Trends Biochem Sci. 1985;10:435–438. [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160 kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita Sa, Tsukita Sh. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita Sh. Characterization of ZO-2 as a MAGUK family member associated with tight and adherens junctions with a binding affinity to occludin and α catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophiladiscs-large tumor suppresser protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang J-L, Carlson SD. A blood-brain barrier without tight junctions in the fly central nervous system in the early postembryonic stage. Cell Tissue Res. 1992;270:95–103. [Google Scholar]
- Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Komau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Skaer HI, Swales LS. Intercellular junctions in the central nervous system of insects. J Cell Sci. 1977;26:175–199. doi: 10.1242/jcs.26.1.175. [DOI] [PubMed] [Google Scholar]
- Lane, N.J., R. Dallai, G. Martinucci, and P. Burigbel. 1994. Electron microscopic structure and evolution of epithelial junctions. In Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease. S. Citi, editor. R.G. Landes, Austin, TX. 23–44.
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita Sh, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita Sh. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita Sh. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am J Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Schnapp B. Possible role of zonula occludens of the myelin sheath in demyelination condition. Nature. 1974;251:725–727. doi: 10.1038/251725a0. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Peters A. A radial component of central myelin sheaths. J Biophys Biochem Cytol. 1961;11:733–735. doi: 10.1083/jcb.11.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. Further observations on the structure of myelin sheaths in the central nervous system. J Cell Biol. 1964;20:281–296. doi: 10.1083/jcb.20.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane, C.S. 1984. Morphology of myelin and myelination. In Myelin. P. Morell, editor. 2nd edition. Plenum Press, New York. 1–50.
- Reale E, Luciano L, Spitznas M. Zonulae occludentes of the myelin lamellae in the nerve fibre layer of the retina and in the optic nerve of the rabbit: a demonstration by the freeze-fracture method. J Neurocytol. 1975;4:131–140. doi: 10.1007/BF01098778. [DOI] [PubMed] [Google Scholar]
- Rubin LL. The blood-brain barrier in and out of cell culture. Curr Opin Neurobiol. 1991;1:360–363. doi: 10.1016/0959-4388(91)90053-a. [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita Sh. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222–231. [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita Sh. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp, B., and E. Mugnaini. 1978. Membrane architecture of myelinated fibers as seen by freeze-fracture. In Physiology and Pathology of Axons. S.G. Waxman, editor. Raven Press, New York. 83–123.
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular mass polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabira T, Cullen MJ, Reier PJ, de Webster HF. An experimental analysis of interlamellar tight junctions in amphibian and mammalian C.N.S. myelin. J Neurocytol. 1978;7:489–503. doi: 10.1007/BF01173993. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT. Immunochemistry on ultrathin frozen sections. Histochem J. 1980;12:381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophiladiscs-large tumor suppresser protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin, and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]