Abstract
We have identified a maize homologue of yeast MAD2, an essential component in the spindle checkpoint pathway that ensures metaphase is complete before anaphase begins. Combined immunolocalization of MAD2 and a recently cloned maize CENPC homologue indicates that MAD2 localizes to an outer domain of the prometaphase kinetochore. MAD2 staining was primarily observed on mitotic kinetochores that lacked attached microtubules; i.e., at prometaphase or when the microtubules were depolymerized with oryzalin. In contrast, the loss of MAD2 staining in meiosis was not correlated with initial microtubule attachment but was correlated with a measure of tension: the distance between homologous or sister kinetochores (in meiosis I and II, respectively). Further, the tension-sensitive 3F3/2 phosphoepitope colocalized, and was lost concomitantly, with MAD2 staining at the meiotic kinetochore. The mechanism of spindle assembly (discussed here with respect to maize mitosis and meiosis) is likely to affect the relative contributions of attachment and tension. We support the idea that MAD2 is attachment-sensitive and that tension stabilizes microtubule attachments.
Keywords: MAD2, kinetochore, checkpoint, spindle assembly, meiosis
The spindle checkpoint is a surveillance pathway that ensures metaphase is complete before anaphase begins (Elledge, 1996; Rudner and Murray, 1996; Wells, 1996; Hardwick, 1998). The components of the spindle checkpoint were originally identified in budding yeast as nonessential genes that allowed cells to divide even in the absence of fully formed spindles. At least seven yeast genes have been identified in the pathway, including Bub1, 2, and 3, Mad1, 2, and 3, and Mps1 (Hardwick, 1998). One of the most thoroughly studied spindle checkpoint genes is Mad2, which encodes a highly conserved ∼24-kD protein. In yeast, the absence of MAD2 causes the fidelity of chromosome segregation to drop by 15-fold (Li and Murray, 1991), and in mammalian cells, microinjection of anti-MAD2 antibodies causes premature anaphase onset (Gorbsky et al., 1998).
A variety of evidence indicates that the signal for the spindle checkpoint emanates from the kinetochores (Nicklas, 1997), which are the organelles that bind to centromeres and interact with the spindle. Both the human and Xenopus homologues of MAD2 bind to kinetochores that are not attached by microtubules (Chen et al., 1996; Li and Benezra, 1996). As soon as the chromosomes properly attach to the spindle, MAD2 staining is lost and is not visible at kinetochores again until the next cell cycle. A single unaligned chromosome is sufficient to activate the spindle checkpoint (Nicklas, 1997), and only unaligned chromosomes stain positive for MAD2 (Chen et al., 1996; Waters et al., 1998). Apparently the availability of free microtubule binding sites or an absence of tension on the kinetochore causes MAD2 to be recruited to kinetochores where it activates the spindle checkpoint (Elledge, 1996). In a study of animal mitotic cells designed to differentiate between these two alternatives, the disappearance of MAD2 staining appeared to be more dependent on microtubule attachment than tension (Waters et al., 1998). No studies have yet been published on the localization of MAD2 in meiotic cells.
Recent studies have provided the necessary link between MAD2 and the cell cycle regulatory proteins that initiate anaphase (Elledge, 1998). The link is Cdc20 (with homologues known as Sleepy, p55CDC, and Fizzy), a protein that imparts substrate specificity to the anaphase-promoting complex (APC1; Visitin et al., 1997). The APC is involved in the ubiquitination and degradation of proteins such as Pds1 that inhibit the onset of anaphase (King et al., 1996). Evidence from a variety of sources suggest that Mad2 delays anaphase because it not only interacts with (Fang et al., 1998; Hwang et al., 1998; Kallio et al., 1998; Kim et al., 1998; Wassmann and Benezra, 1998), but inhibits the action of Cdc20 (Kim et al., 1998). Unattached kinetochores may act as catalytic sites for the activation of MAD2, allowing the active MAD2 or CDC20/MAD2 to diffuse and inhibit APC activity throughout the cell (Gorbsky et al., 1998; Kallio et al., 1998).
Cytological evidence in animal systems suggests that protein phosphorylation, perhaps regulated by tension, plays a key role in the spindle checkpoint pathway (Campbell and Gorbsky, 1995; Nicklas, 1997). The 3F3/2 antibody recognizes a phosphoepitope that is localized to prometaphase kinetochores until the chromosomes have aligned properly at the metaphase plate (Gorbsky and Ricketts, 1993; Nicklas et al., 1995). A strong correlation exists between 3F3/2 staining, tension at the kinetochore, and progression to anaphase. When tension is manually applied to a single unaligned chromosome, anaphase commences (Li and Nicklas, 1995) and 3F3/2 staining disappears (Li and Nicklas, 1997; Nicklas, 1997). Further, when the 3F3/2 antibody is injected into metaphase cells, anaphase onset is delayed (Campbell and Gorbsky, 1995). Although the 3F3/2 epitope appears to have an important checkpoint function, no information is yet available whether the epitope and its function are broadly conserved among eukaryotes.
Here we describe the identification of a maize homologue of MAD2 and detailed immunolocalization studies designed to investigate its role in the spindle checkpoint. The data are interpreted in the context of apparent differences in both kinetochore morphology and spindle formation between plants and animals. Whereas animal kinetochores have a three-layered morphology (Earnshaw, 1994), plant kinetochores have a nondescript ball-shaped structure (e.g., Braselton and Bowen, 1971; Jensen, 1982). Animal mitotic spindles are initiated from centrosomes at the spindle poles, whereas plant spindles and animal meiotic spindles are initiated from the nuclear envelope or the chromosomes (Baskin and Cande, 1990; Smirnova and Bajer, 1992; Rieder et al., 1993; Waters and Salmon, 1997). Our data indicate that MAD2 localization patterns in mitosis are basically conserved among eukaryotes, but that at least in maize, the localization patterns in meiosis differ from those in mitosis. Based on MAD2 as well as 3F3/2 staining, we argue that microtubule attachment has a major role in the mitotic spindle checkpoint but the meiotic spindle checkpoint may rely more heavily on sensing the amount of tension at the kinetochore.
Materials and Methods
Generation of Recombinant Proteins and Antibodies
The clones of two Mad2 ESTs (expressed sequence tags) were gifts from Pioneer Hi-Bred. At the time the EST database was queried, there were 80,000 sequences available. One clone, CGEUZ35 is described here (the other clone, CDPEE81 may identify a second locus but further studies are needed). Complete sequencing revealed CGEUZ35 to be a full-length Mad2 cDNA. To express MAD2 in E. coli, KpnI and XbaI restriction sites were incorporated into the 5′ and 3′ ends of the open reading frame by PCR, and the fragment inserted into the pThioHisC vector (Invitrogen). The pThioHisC vector is designed to fuse thioredoxin to the NH2 terminus of the expressed protein. Thioredoxin-MAD2 fusion protein was induced using 10 mM IPTG in E. coli TOP10 cultures grown at room temperature. The recombinant protein was partially purified on a nickel column (Invitrogen), and then purified to near-homogeneity by ion-exchange chromatography (Macro-Prep DEAE Support; BioRad). Polyclonal antibodies against the purified thioredoxin-MAD2 protein were produced in rabbits by the UGA polyclonal antibody facility. The resulting antibodies were either blot affinity purified (Tang, 1993) or column affinity purified against the recombinant maize MAD2 using an UltraLink Immobilization Kit (Pierce). Affinity-purified antibodies were extensively dialyzed in PBS at 4°C, and concentrated to ∼1 mg/ml with Centriplus concentrators (Amicon).
Protein Blotting
Different maize tissues from the W23 inbred, including young tassels (∼5 cm), young ears (∼8 cm), young leaves, and root tips (∼1–2 cm) were ground in liquid nitrogen and resuspended in 20 mM Tris-HCl (pH 6.8), 20 mM EDTA, 200 mM NaCl, and 1 mM PMSF. Standard SDS-PAGE was performed and proteins were blotted to nitrocellulose (Harlow and Lane, 1988). The protein blot was blocked with 5% Carnation nonfat milk and incubated with affinity-purified anti-MAD2 antibodies (final concentration ∼0.2 μg/ml) for at least 2 h at room temperature. After washing 3× in TBST, the membrane was incubated with peroxidase-conjugated goat anti–rabbit antibodies (Amersham) for one hour at room temperature, and chemiluminescent immunodetection carried out using an ECL Western-blotting kit (Amersham). When the affinity-purified antiserum was preincubated with the purified thioredoxin-MAD2 before use, no bands were detected on maize protein blots (data not shown).
Analysis of Living Meiocytes
The culture and data collection from live microsporocytes was performed as before (Yu et al., 1997). Meiocytes at the appropriate stages were dissected from anthers into a modified rye culture medium, stained with Syto 12 at ∼2.5 μM, and visualized using the DeltaVision system (described below). Four-dimensional data sets (three-dimensions over time) were collected and analyzed.
Oryzalin Treatment of Seedlings
Seeds from the KYS inbred line were germinated in a moist chamber at 26°C. 3-d-old seedlings with root tip lengths of ∼1 cm were treated with 1 μM oryzalin (Chem Service), a concentration that is sufficient to depolymerize all of the mitotic microtubule arrays in oat (Hoffman and Vaughn, 1994). Oryzalin-treated root tips were harvested at 0, 4, and 8 h, and processed for immunocytochemistry as described below.
Plant Material and Fixation
Meiocytes from the maize inbred W23 were extruded into PHEMS (Yu et al., 1997) containing 3% paraformaldehyde and 0.05% Triton X-100. In the experiments involving the 3F3/2 antibody, 100 nM microcystin (a phosphatase inhibitor; Sigma) was added to the fixation buffer. Cells were transferred to polylysine-coated coverslips for ∼15 min to allow fixation. Fixed cells were washed 3× in PBS (or MPS for 3F3/2 experiments; Gorbsky and Ricketts, 1993) before immunocytological experiments. Mitotic cells (untreated or oryzalin-treated) were prepared from the seedlings of W23 and KYS inbreds that had been cultured in a moist chamber at 26°C. Root tips ∼1 cm in length were cut from 3-d-old seedlings and transferred to the same buffer used to fix meiocytes for ∼30 min at room temperature. Fixed root tips were washed 2× in PBS and quickly frozen in PolyFreeze (PolySciences) using liquid nitrogen. Sections ∼10 μm thick were prepared on a cryostat at −20°C. The root tip sections were transferred to polylysine-coated slides for further study.
Indirect Immunocytochemistry
In double-labeling experiments for MAD2 and tubulin, or MAD2 and the 3F3/2 epitope, it was possible to use indirect immunolocalization. The procedure was performed essentially as before (Yu et al., 1997) except that the rabbit antiserum was detected with rhodamine-conjugated secondary antibodies (111-095-144; Jackson ImmunoResearch Laboratories). The mouse monoclonal antibody to α-tubulin, a generous gift of David Asai (Asai et al., 1982), and the monoclonal antibody 3F3/2, a generous gift of Gary Gorbsky (University of Virginia, Charlottesville), were detected by FITC-conjugated secondary antibodies (115-095-146; Jackson ImmunoResearch Laboratories). The 3F3/2 primary antibody was used at a 1:50– 1:100 dilution. In the experiment to determine the effect of phosphatase treatment on 3F3/2 staining, cells were first fixed in the presence of 100 nM microcystin and then treated with 100 units/ml phosphatase (Sigma; P3627) for 30 min at 37°C, either in the presence or absence of 5 μM microcystin. In cases where MAD2, CENPC, and tubulin were detected simultaneously (see Fig. 6, A and D), MAD2 and CENPC were direct-labeled (see below), and tubulin was detected using CY5-conjugated anti– mouse antibodies (115-175-146; Jackson ImmunoResearch Laboratories). Chromosomes were stained with DAPI at 0.1 μg/ml.
Figure 6.
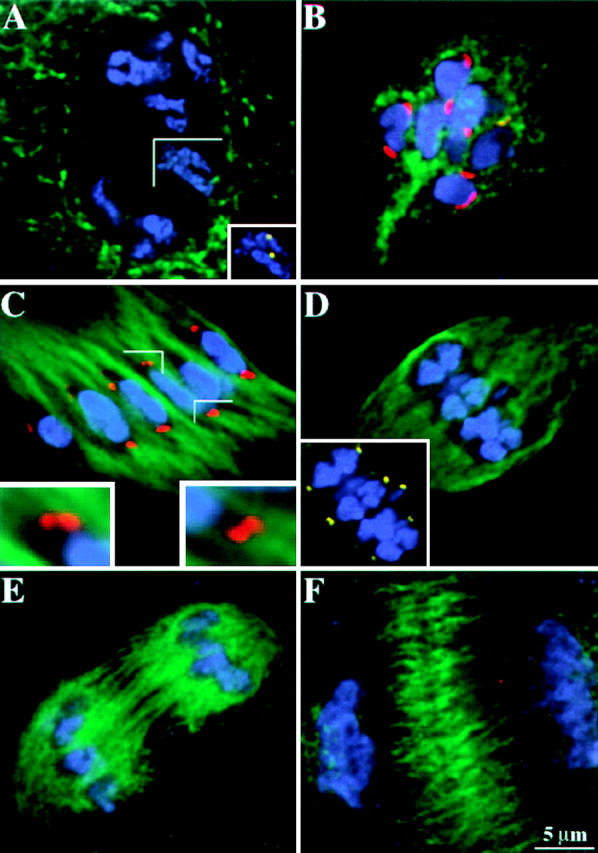
MAD2 localization in meiosis I. Chromosomes are shown in blue, microtubules are shown in green, MAD2 is shown in red, CENPC is shown in yellow. The MAD2 and CENPC antibodies were directly labeled in the cells presented in images A, D, and F. The images shown here are partial projections. (A) Prophase I. Note the absence of MAD2 staining. The inset shows CENPC staining in the same cell. (B) Early prometaphase I. MAD2 staining is apparent at kinetochores. (C) Late prometaphase I. MAD2 staining is conspicuous on extended kinetochores. The insets show enlarged views of strong MAD2 and associated microtubule staining. Under favorable conditions, sister kinetochores are visualized as doublets. (D) Metaphase I. Note the gaps between homologous chromosomes and the absence of MAD2 staining on kinetochores. The inset shows CENPC staining in the same cell. (E) Anaphase I. There is no MAD2 staining at anaphase. (F) Telophase I. There is no MAD2 staining at telophase.
Direct Immunocytochemistry
To visualize MAD2 and CENPC simultaneously (antibodies to both were generated in rabbits), fluorescent dyes were directly coupled to primary antibodies using the Alexa 546 and Alexa 488 Protein Labeling Kits (Molecular Probes). For the data in Fig. 3 A, affinity-purified anti-CENPC and affinity-purified anti-MAD2 antibodies were used for direct labeling. The efficiency of labeling for the CENPC antibody was poor at ∼1 mol dye/mol protein whereas the efficiency of labeling for the MAD2 antibody was excellent at ∼12 mol dye/mol protein. The poor labeling of the CENPC made it difficult to obtain high-contrast images. Therefore the CENPC antibody labeling was repeated on the proteins derived from a 40% ammonium sulfate precipitation of the crude antiserum (a method used for partial purification of antibodies; Harlow and Lane, 1988). When the labeled protein preparation was used to detect CENPC in double-labeling experiments with the directly labeled anti-MAD2, the CENPC staining was much brighter and qualitatively indiscernible from the results obtained when directly labeled affinity-purified CENPC antibodies were used. The labeled ammonium sulfate precipitate was used in most of the experiments where quantitative data was collected (see Fig. 8) and for Figs. 3 B, 6, and 7. Since the affinity-purified MAD2 antibodies were well-labeled with Alexa 546, ammonium sulfate precipitates were not used to detect MAD2. Chromosomes were stained with DAPI at 0.1 μg/ml. Kinetochores were not detected either by the crude rabbit CENPC or MAD2 preimmune sera, or by purified IgGs derived from these sera (data not shown).
Figure 3.
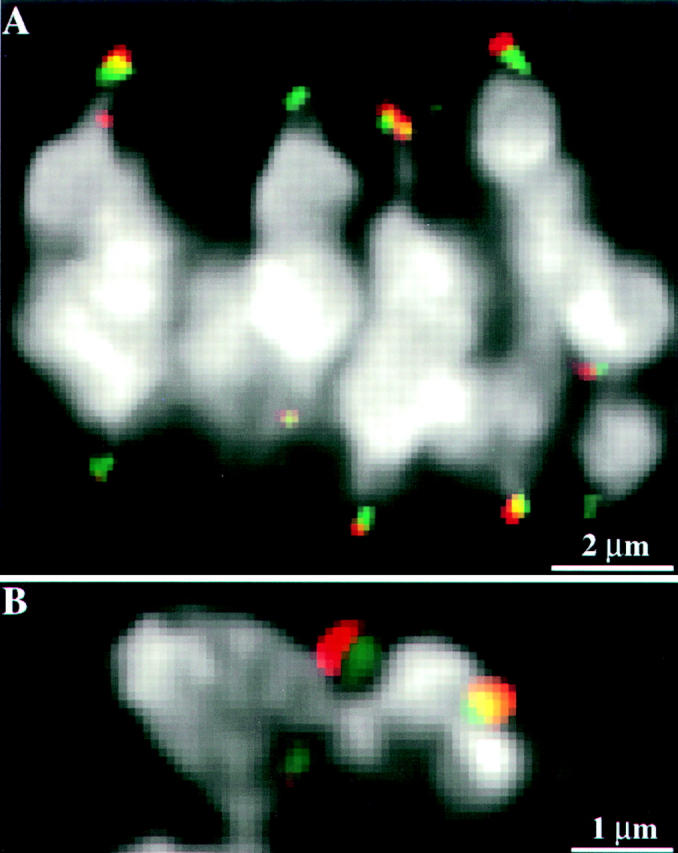
MAD2 localizes to an outer domain of the meiotic kinetochore. Chromosome staining is in white, CENPC staining is in green: MAD2 staining is in red. (A) A single optical section from a three-dimensional data set showing the relative localization of CENPC and MAD2 on prometaphase I kinetochores. CENPC is localized close to the chromosomes while MAD2 is localized to an outer domain. (B) A single optical section from a prometaphase II cell showing a similar localization pattern of CENPC and MAD2 on kinetochores in meiosis II. In B, there is a noticeable ∼0.1-μm shift of the MAD2 signal (leftward relative to the chromatin) caused by the polychroic mirror system used in data acquisition. Apparently because image displacement varies slightly with sample preparation (Hiraoka et al., 1991), it is not as noticeable in A.
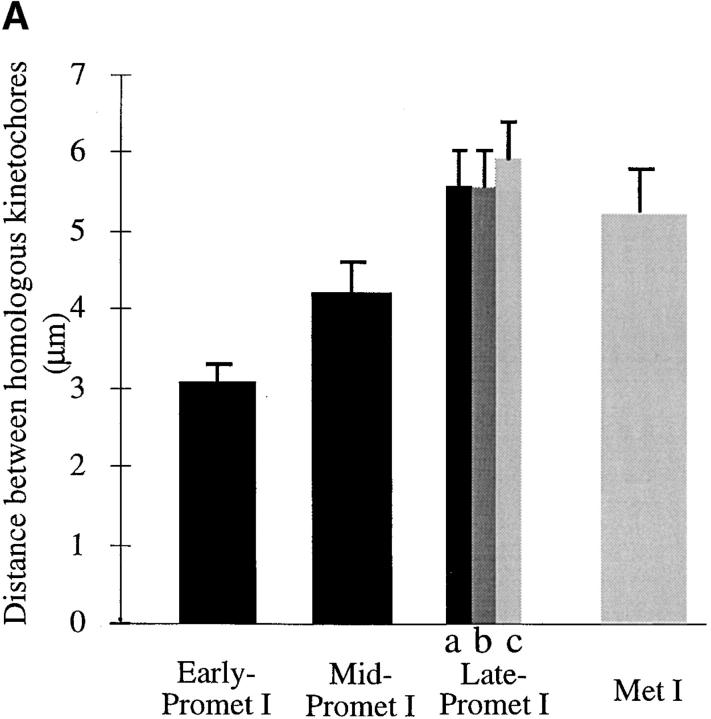
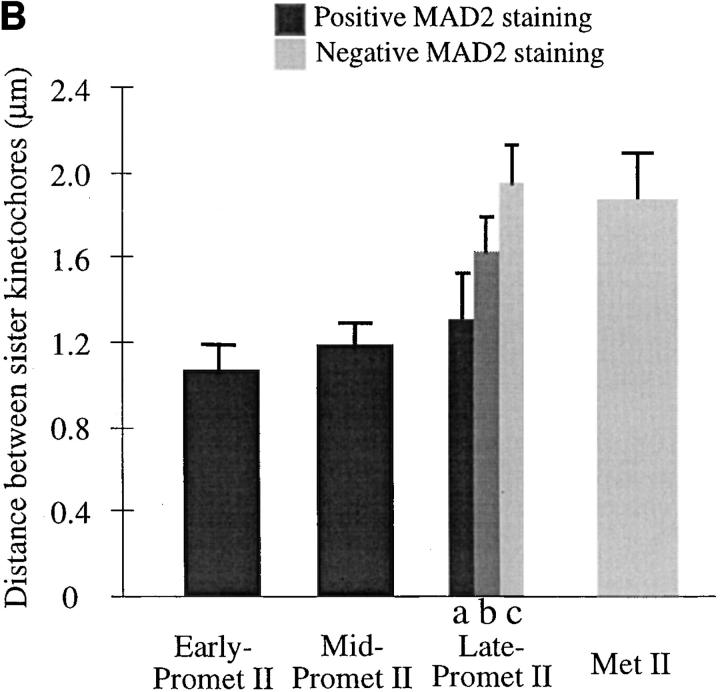
Figure 8.
MAD2 staining at prometaphase-metaphase I and II is correlated with the distance between homologous/sister kinetochores. The stage definition in this figure is as follows: (Early prometaphase) The nuclear envelope has broken down but there is no bipolar spindle established. (Mid-prometaphase) A bipolar spindle has formed but the chromosomes are randomly dispersed in the cell. (Late-prometaphase) The chromosomes are located in the vicinity of the metaphase-plate but are not fully aligned (Metaphase) Chromosomes are perfectly aligned at the metaphase-plate. (A) Summary of MAD2 staining at prometaphase-metaphase I. (B) Summary of MAD2 staining at prometaphase-metaphase II. (a) Both homologous/sister kinetochores are positively stained by MAD2; (b) only one homologous/sister kinetochore is stained; (c) neither homologous/sister kinetochores are stained. A kinetochore was counted as positively stained when the fluorescence signal could be detected over background; by this method, even the most weakly stained kinetochores were counted as positive. This scoring method was used only as a means to categorize the data and is not meant to imply that MAD2 staining is an all or nothing event. Error bars indicate the standard deviation (n > 5 in each case).
Data Collection and Presentation
Data from both living and fixed cells were collected using an Applied Precision, Inc. SA3.1 multidimensional light microscope system (see Yu et al., 1997). Optical sections were taken using a 60× Nikon objective at 0.2 μm to 0.4 μm intervals either with or without a 1.5× Optivar (pixel size = 0.065 μm or 0.097 μm, respectively). The data from living cells were binned, resulting in an effective pixel size of 0.196 μm (Yu et al., 1997). The three dimensional data sets were mathematically deconvolved, to remove the out-of-focus information, with software supplied with the DeltaVision system. The images were scaled to optimize contrast but not enhanced further. The intensity of MAD2 staining at kinetochores (see Results on MAD2 staining in mitosis) was measured by identifying optical sections with the most intense MAD2 staining, and averaging the grey level values from 9 pixels in a 3 × 3 square. The kinetochore-kinetochore distances (see Fig. 8) were measured using software supplied with the DeltaVision system. Image color was modified using the GraphicConverter program (Lemke Software) and printed using a Techtronix Phaser IIsdx dye sublimation printer.
Results
The MAD2 Spindle Checkpoint Protein Is Broadly Conserved among Eukaryotes
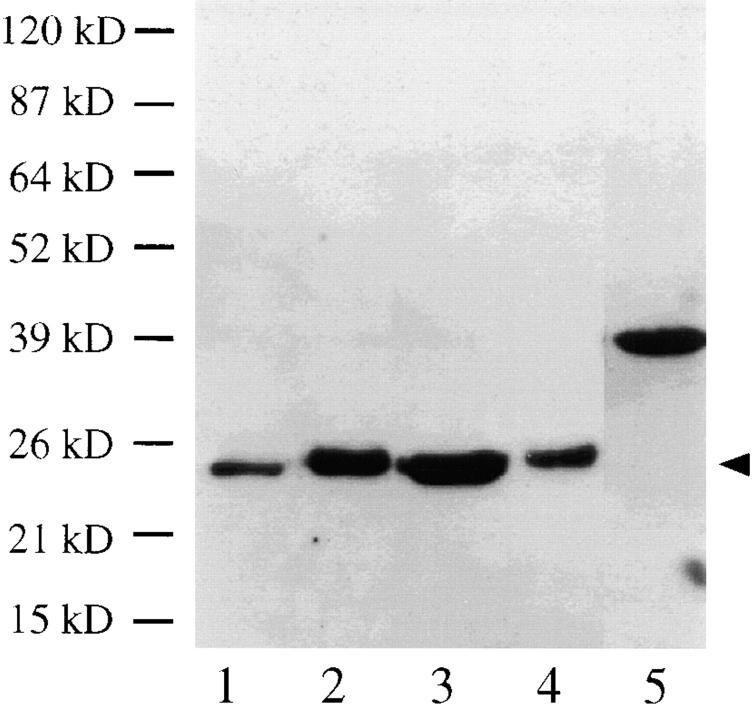
A full-length cDNA homologous to Saccharomyces cerevisiae Mad2 was identified in a maize EST data base compiled by Pioneer Hi-bred. Complete sequencing revealed that the Mad2 gene encodes a polypeptide of 208 amino acids with a predicted molecular mass of 24 kD. BLAST alignments (Atschul et al., 1990) revealed 42% identity (64% similarity) to yeast Mad2, 45% identity (65% similarity) to human MAD2 and 46% identity (70% similarity) to Xenopus XMAD2. The sequence alignment is shown in Fig. 1. Using the full-length maize Mad2 cDNA, a thioredoxin-MAD2 fusion protein was generated, purified, and injected into rabbits. The resulting antibodies were affinity-purified against the recombinant MAD2 protein and used on protein blots. As shown in Fig. 2, the purified antibody preparation recognized a single 24-kD protein in tassel, ear, and root tissue. The results are consistent with the predicted molecular mass of maize MAD2 as well as previous animal studies where 24-kD MAD2 proteins have been consistently observed (Chen et al., 1996; Li and Benezra, 1996; Gorbsky et al., 1998; Waters et al., 1998).
Figure 1.
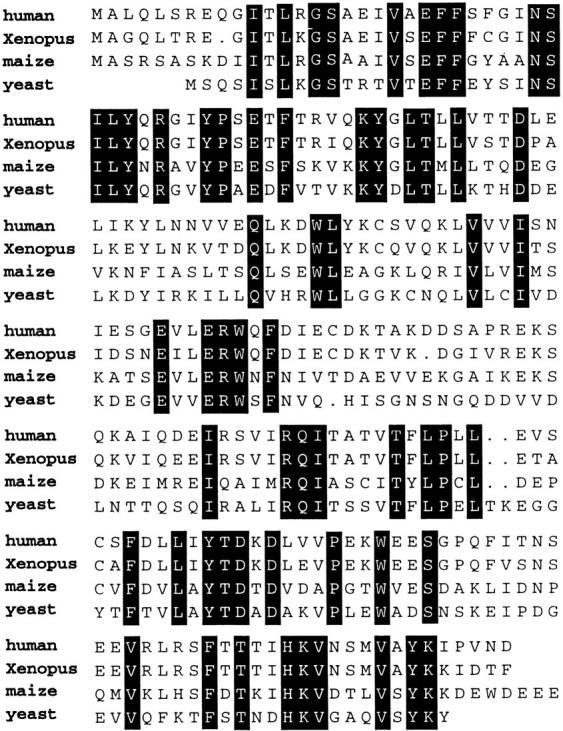
Protein sequence comparisons among human, Xenopus, maize, and budding yeast MAD2 homologues. Shaded amino acids are conserved. These sequence data are also available from GenBank under accession number AF143681.
Figure 2.
MAD2 protein blot. Lane 1, root; lane 2, young leaf; lane 3, young ear; lane 4, tassel; lane 5, recombinant thioredoxin-MAD2 fusion protein. A single 24-kD protein (arrowhead) was identified in all maize tissues. Roughly equal amounts of protein were loaded in each lane. The relative intensity differences (and apparent slight mobility differences) were observed in two separate experiments. The thioredoxin-MAD2 fusion protein is 39 kD due to the added weight of the thioredoxin moiety.
MAD2 Localizes to an Outer Domain of the Meiotic Kinetochore
To determine the precise subcellular localization of MAD2, we made use of a recently generated antibody to maize CENPC (Dawe et al., 1999). CENPC is a kinetochore structural component in yeast, mammals and maize, where it appears to localize close to the centromeric DNA (Saitoh et al., 1992; Meluh and Koshland, 1997; Dawe et al., 1999). Because the MAD2 and CENPC antisera were both prepared in rabbits, the affinity-purified antisera were differentiated from each other by direct labeling using differing fluorochromes. Fig. 3 A shows a triple-labeled prometaphase I cell, where DNA, MAD2, and CENPC were each detected using different wavelengths on a three-dimensional light microscope workstation. The data demonstrate that MAD2 and CENPC occupy essentially nonoverlapping domains in the maize kinetochore: CENPC occupies an inner domain close to the chromosome while MAD2 occupies an outer domain. As shown in Fig. 3 B, similar results were obtained in prometaphase II cells.
The affinity-purified MAD2 antiserum also recognized mitotic kinetochores from maize root tip cells (see below). Because the directly labeled anti-CENPC antibodies only weakly recognized these kinetochores, however, we were unable to determine whether mitotic kinetochores have a substructure similar to meiotic kinetochores.
MAD2 Staining in Mitosis Is Negatively Correlated with Microtubule Attachment
In mitotic root tip cells, we observed a cell cycle–specific pattern of MAD2 staining that is essentially the same as that reported for mammalian cultured cells and newt lung cells (Chen et al., 1996; Li and Benezra, 1996). The important features of the MAD2 localization patterns are shown in Fig. 4. The interphase and prophase stages can be identified by the state of chromatin condensation and the characteristic perinuclear array of microtubules that mark the presence of the nuclear envelope (Lambert, 1993). In prometaphase, the nuclear envelope and perinuclear array are broken down and the chromosomes begin to interact directly with microtubules. Using indirect immunofluorescence we were unable to detect MAD2 staining at the interphase and prophase stages (Fig. 4 A and data not shown), but detected intense kinetochore staining in prometaphase (Fig. 4 B). All 20 kinetochores were visible in some prometaphase cells, with most chromosomes containing paired spots representing sister kinetochores. The identity of the MAD2-positive regions as kinetochores was further verified in several cases by combined direct immunofluorescence of MAD2 and CENPC. MAD2 staining became undetectable in metaphase (Fig. 4 C), and remained undetectable in anaphase (Fig. 4 D) and telophase (data not shown).
Figure 4.
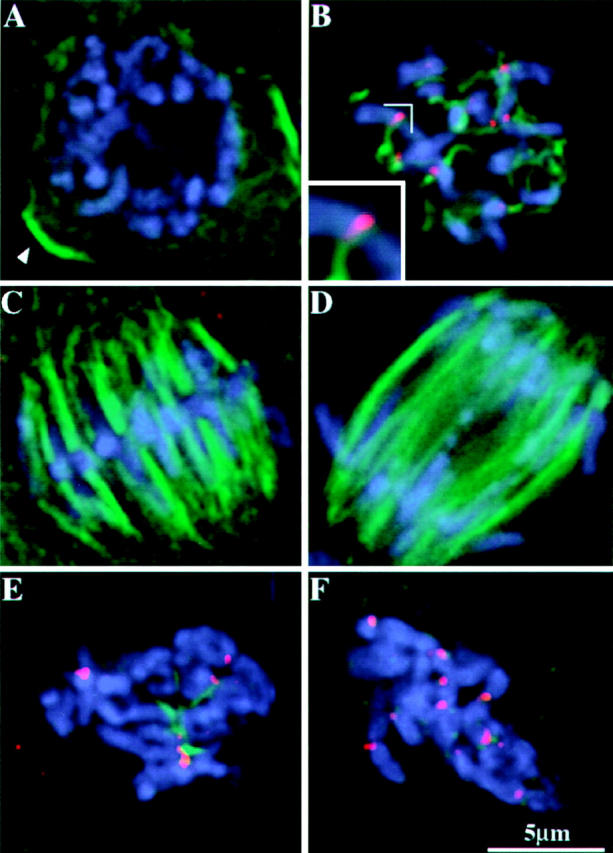
MAD2 localization in mitotic root tip cells. Images shown here are partial projections. Chromosomes are shown in blue, microtubules are shown in green, MAD2 is shown in red. (A) Prophase. Note the absence of MAD2 staining. The remnant of a preprophase band is still visible in cross-section (see arrow; the preprophase band is a microtubule-containing structure that predicts the future division plane in plant mitosis). (B) Prometaphase. Short segments of spindle fiber are present, and MAD2 is evident at centromeric regions. Inset shows that the association of a spindle fiber reduces the intensity of MAD2 staining (this partial projection shows all the MAD2 staining on the chromosome). (C) Metaphase. MAD2 is not detectable on metaphase chromosomes. (D) Anaphase. MAD2 staining is not detectable on anaphase chromosomes. (E) Root meristematic cells treated for 4 h with oryzalin. A prometaphase-like stage is apparent with the most intense MAD2 staining on chromosomes that lack associated K-fiber remnants. (F) Root meristematic cells treated for 8 h with oryzalin. All microtubules are depolymerized and all kinetochores stain brightly for MAD2.
Further inspection of prometaphase cells revealed that the MAD2 staining on sister kinetochores was frequently unequal. Bright MAD2 staining was correlated with weak staining of associated microtubules (known as kinetochore fibers, or K-fibers) and weak MAD2 staining was correlated with bright staining of the associated K-fibers (Fig 4 B, inset). A quantitative analysis of 10 chromosomes with a single attached kinetochore fiber indicated that, on average, microtubule attachment caused an ∼5.7-fold reduction in the intensity of MAD2 staining (SD = 4.0, with a low of 2.8 and a high of 14.4). Based on these data, we expected that artificial depolymerization of microtubules would result in bright MAD2 staining at all kinetochores, as had been established in previous studies (Chen et al., 1996; Li and Benezra, 1996). A variety of microtubule destabilizing agents is available for plants, many of which are used as herbicides. One particularly effective microtubule destabilizing agent is oryzalin (see Hoffman and Vaughn, 1994; Anthony et al., 1998). As shown in Fig. 4 E, a 4-h treatment of oryzalin disrupted mitotic spindles and arrested the cells at a prometaphase-like stage. Nearly all the microtubules were depolymerized with only short K-fibers remaining at the kinetochores of some chromosomes (Fig. 4 E). MAD2 staining was relatively bright at kinetochores with thin or no K-fibers attached and weak or absent on kinetochores with short K-fibers. An 8-h oryzalin treatment depolymerized all the microtubules, including the short K-fibers visible in four-hour treated cells. Consistent with expectations, all kinetochores stained brightly with the anti-MAD2 antibodies in the 8-h arrested cells (Fig. 4 F). These data lend support to the argument that microtubule attachment is sufficient to cause the dissociation of MAD2 and the inactivation of the spindle checkpoint (Waters et al., 1998).
MAD2 Staining in Meiosis Is Negatively Correlated with the Distance between Kinetochores
To further test the idea that microtubule attachment is associated with a loss of MAD2 staining, we extended our studies to the more specialized meiotic cell divisions. We began our analysis of meiosis I with living meiocytes to determine the timing and characteristic chromosome movements of this cell division. Using the cell culture and chromosome staining techniques that were developed in an earlier study of meiosis II (Yu et al., 1997), we successfully recorded the prometaphase-metaphase transition in six living meiosis I cells. Time-lapse data from one of these cells is illustrated in Fig. 5. The data indicate that during meiotic prometaphase I the chromosomes accumulate at the spindle midzone relatively quickly, but can take up to 60 min to form a definite metaphase plate. The metaphase I plate was characterized by a continuous gap that appeared to separate the homologues (the chiasmata were apparently stretched to the point that they were difficult to see). Once such a plate was formed, anaphase onset occurred within 30 min.
Figure 5.

Time-lapse sequence showing a maize meiosis I cell undergoing prometaphase, metaphase, and metaphase/anaphase transition. Columns represent the same optical section at subsequent indicated time points. Note the gap between homologues is visible after chromosome alignment (arrowhead).
Immunocytochemical analysis of fixed cells indicated that MAD2 was not detectable during the prophase stages of meiosis I. This is illustrated by the cell in Fig. 6 A, which at a late stage of prophase I (diplotene-diakinesis) lacks any evidence of MAD2 staining on the chromosomes. It is unlikely that the absence of prophase staining was a consequence of poor antibody penetration, because when the cells were counterstained with anti-CENPC antibodies the kinetochores were clearly visible (Fig. 6 A, inset). MAD2 was readily detectable on congressing chromosomes during prometaphase I (Fig. 6 B). Unlike in mitosis, however, the association of microtubules with the kinetochores did not appear to dim the intensity of MAD2 staining (Fig. 6 C). The result was that the paired homologous kinetochores appeared to stain brightly and with nearly equal intensity well into late prometaphase. A hallmark of late prometaphase-metaphase I transition is that the sister kinetochores begin to separate from each other (Lima-de-Faria, 1956; Dawe et al., 1999); the fact that we were able to detect sister kinetochore separation using MAD2 antibodies (Fig. 6 C, insets) is one indication that the chromosomes are MAD2-positive until immediately before metaphase. The dissociation of MAD2 appeared to occur gradually on a chromosome-by-chromosome basis, such that during the transition from prometaphase to metaphase some chromosomes had MAD2 staining while others lacked MAD2 staining (discussed below with respect to 3F3/2 staining). In cells that fit our strict definition of metaphase I (a gap between homologues) MAD2 was undetectable.
The patterns of MAD2 staining in meiosis II were similar in most respects to the staining in meiosis I. As with the meiosis I analysis, we determined the stages of the fixed meiosis II cells by comparing the images to data derived from live studies (Yu et al., 1997). MAD2 staining was not observed until prometaphase II, at which point the kinetochores stained brightly for MAD2 even though they were clearly attached to microtubules. Fig. 7, A and B illustrate a cell in mid-prometaphase II. By viewing this cell in stereo, we were able to identify 10 pairs of kinetochores by MAD2 staining alone (Fig. 7 C). The images in Fig. 7, D–F further illustrate that MAD2 was lost on a chromosome-by-chromosome basis as the cells approached metaphase II. Once the chromosomes had fully aligned at the metaphase plate, MAD2 staining was no longer detectable and remained undetectable on chromosomes throughout anaphase and telophase (not shown).
Figure 7.
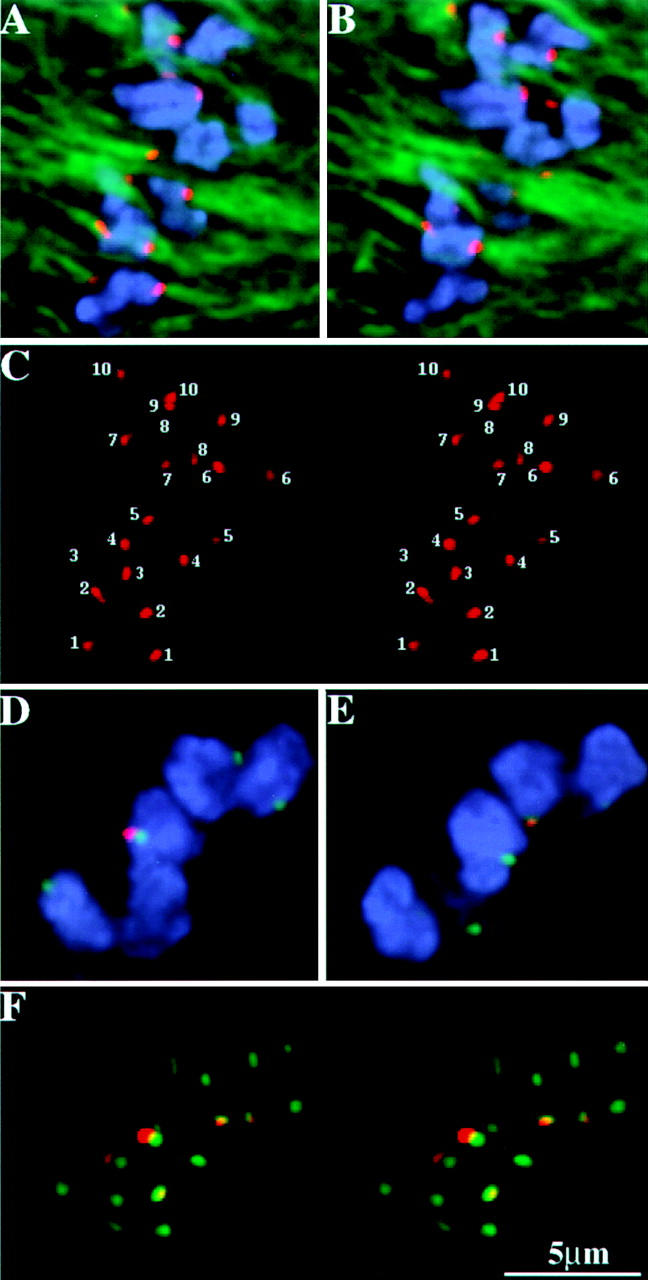
MAD2 localization in meiosis II. The chromosomes are shown in blue, microtubules (A and B) and CENPC (D–F) are shown in green, and MAD2 is shown in red. (A and B) Two optical sections from a prometaphase II cell showing MAD2 staining of roughly equal intensity on sister kinetochores. (C) A stereo-pair of all the kinetochores in the cell presented in A and B showing positive MAD2 staining (each pair of kinetochores is labeled with a different number). (D and E) Two optical sections from a late-prometaphase II cell. (F) A stereo-pair of all kinetochores in this cell presented in D and E. Note that MAD2 is only present on a few of the kinetochores.
The data from both meiosis I and II (Figs. 6 and 7) appear to be at odds with the idea that microtubule attachment results in the dissociation of MAD2. An alternative proposal for the mechanism of MAD2 action involves the idea that kinetochores can in some way sense the tension that is applied to kinetochores by the attached microtubules (McIntosh, 1991; Nicklas, 1997). Since chromatin is elastic (Nicklas, 1988), one measure of the tension applied to kinetochores during metaphase is the distance between sister kinetochores (Waters et al., 1996). By analyzing fixed meiocytes in a variety of stages, we could demonstrate that the kinetochore-to-kinetochore distance varied by a factor of two in both meiosis I and II. Further, as shown in Fig. 8, the variation in kinetochore-to-kinetochore distance was correlated with MAD2 staining. The kinetochores stained positive for MAD2 staining when the distance was beneath a specific threshold (∼5.6 μm in meiosis I and ∼1.7 μm in meiosis II), and stained negative when the distance was above the threshold. Thus, while the meiotic data are not consistent with the hypothesis that microtubule attachment is sufficient for the dissociation of MAD2, they are consistent with the hypothesis that a tension threshold must be reached before MAD2 is released or destroyed.
Dissociation of MAD2 Occurs Concomitantly with the Loss of the 3F3/2 Antigen at the Meiotic Kinetochore
The 3F3/2 antibody recognizes a kinetochore phosphoepitope that disappears when tension is applied to the kinetochore (Nicklas et al., 1995; Nicklas, 1997; Waters et al., 1998). Previously, 3F3/2 staining had only been demonstrated in animal systems. We were able to obtain reproducible staining of the 3F3/2 epitope in maize, but only in meiotic cells (I and II), and only with an ∼10-fold higher concentration of antibody than is normally used in animal cells. When cells were treated with phosphatase, weak or no staining was observed, whereas staining was preserved when the phosphatase treatment was accompanied by the phosphatase inhibitor microcystin (data not shown).
Strong staining with the 3F3/2 antibody was observed at prometaphase kinetochores in both meiosis I and II. Double labeling for CENPC and the 3F3/2 antigen (Fig. 9, A–C) revealed the same kinetochore substructure observed when CENPC and MAD2 were observed together (Fig. 3). When cells were double-labeled for MAD2 and the 3F3/2 antigen, the two signals almost perfectly colocalized (Fig. 9, D–F). We also observed 3F3/2 staining in the vicinity of chiasmata (Fig. 9, arrows). Not all of the maize bivalents stained at presumed chiasmata, and frequently only one arm of a bivalent demonstrated staining, even though the other appeared to have crossed over. The staining patterns may indicate that tension at meiosis I is registered not only at kinetochores but at the chiasmata as well. We are pursuing this idea with further studies involving meiotic mutants.
Figure 9.
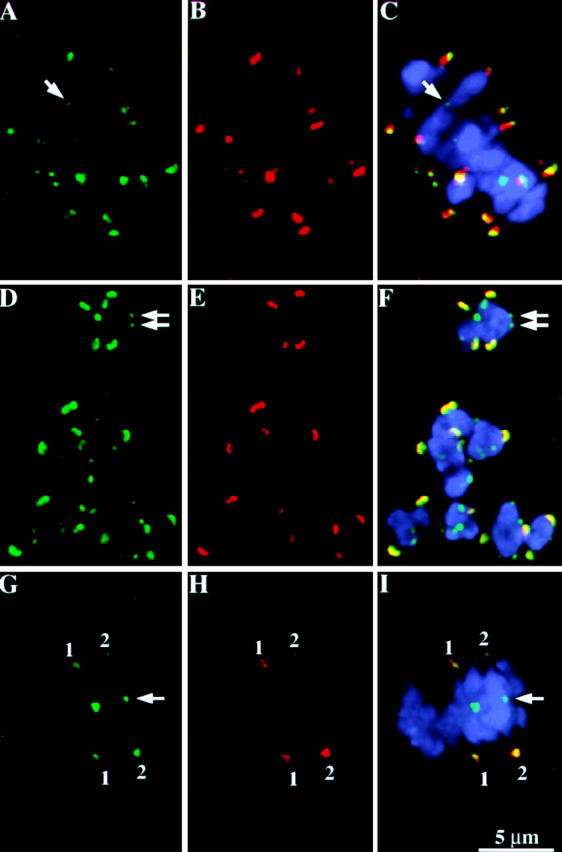
MAD2 and the 3F3/2 antigen colocalize to an outer domain of the meiotic kinetochore. Each row represents data from a single cell, with single-wavelength images shown in the first two panels followed by a three-color overlay (including chromosomes) in the third panel. The 3F3/2 antigen is localized at kinetochores as well as non-kinetochore locations, which may represent chiasmata (noted by arrows). (A–C) Double labeling of the 3F3/2 antigen and CENPC at mid prometaphase I. 3F3/2 staining is shown in green; CENPC in red; and chromosomes in blue. Note that the 3F3/2 antigen and CENPC (like MAD2 and CENPC) do not colocalize; the 3F3/2 antigen lies in domain outside of the CENPC domain. (D–F) Double labeling of the 3F3/2 antigen and MAD2 at early prometaphase I. 3F3/2 staining is shown in green; MAD2 in red; and chromosomes in blue. This is a partial projection of a prometaphase I cell including eight optical sections (2.4 μm). (G–I) Double labeling of the 3F3/2 antigen and MAD2 at late prometaphase I. 3F3/2 staining is shown in green; MAD2 in red; and chromosomes in blue. This is a partial projection of a prometaphase I cell including 10 optical sections (3 μm). 3F3/2 staining is shown in green; MAD2 in red; and chromosomes in blue. Note that only two pairs of homologous kinetochores are positively stained by both antibodies (pairs 1 and 2). The lower kinetochore of pair 2 is strongly stained by both antibodies, while the upper kinetochore of this pair is weakly stained simultaneously by both antibodies.
Like MAD2, the 3F3/2 staining disappeared from kinetochores as the cell exited prometaphase and was undetectable when all the chromosomes had properly aligned at the plate. We observed that kinetochores lost MAD2 and 3F3/2 staining concomitantly, such that the majority of kinetochores at prometaphase-metaphase were either both positively stained or both negatively stained. This is shown in Fig. 9 (G–I), which shows a cell with two pairs of homologous kinetochores that stained positively for both antibodies, and eight pairs of homologous kinetochores that failed to stain for either antibody. Among 240 kinetochores from 12 similar cells, only 1.3% stained singly for MAD2 and 7.1% stained singly for 3F3/2 (28.3% did not stain for either and 63.3% stained for both MAD2 and 3F3/2). In cases where only one or the other protein were detectable, the staining was usually very weak (data not shown). These observations suggest that dissociation of MAD2 from the meiotic kinetochore occurs contemporaneously with the dephosphorylation of the 3F3/2 antigen. Assuming that the 3F3/2 epitope reports tension in maize as it does in animals, these data support the idea that MAD2 dissociation occurs in response to tension applied at the meiotic kinetochore.
Discussion
MAD2 plays a key role in the evolutionarily conserved process of spindle checkpoint activation (Hardwick, 1998). As a member of a group of kinetochore proteins that senses the presence of unaligned chromosomes, MAD2 relays a stop anaphase signal through the action of CDC20 and the APC (Elledge, 1998; Fang et al., 1998). Once metaphase is achieved, MAD2 is degraded or released from kinetochores and anaphase is allowed to proceed. Here we report the identification of the maize homologue of MAD2, show that it is an outer kinetochore protein, and demonstrate differing localization patterns in the mitotic and meiotic cell cycles.
MAD2 Localizes to an Outer Domain of the Maize Kinetochore
Plant kinetochores have an unorganized ball-shaped appearance when viewed under the electron microscope (e.g., Braselton and Bowen, 1971; Bajer and Molé-Bajer, 1972; Jensen, 1982). In contrast, animal kinetochores have an highly ordered trilamellar construction, composed of an inner, middle, and outer plate (Choo, 1997). A major focus of mammalian kinetochore research has been to ascribe functions to these conspicuous domains. A case in point is CENPC, which is thought to be involved in the early stages of kinetochore assembly and is a component of the inner kinetochore plate (Saitoh et al., 1992). In a separate report we demonstrate that the maize homologue of CENPC is localized to an inner domain of the maize kinetochore close to the centromeric DNA (Dawe et al., 1999). We show here that maize MAD2 (Fig. 3) and 3F3/2 antigen (Fig. 9 C) localize to a domain of the kinetochore that lies outside of the region containing CENPC. These data provide encouraging evidence of a functional homology among eukaryotic kinetochores and of a domain structure within the plant kinetochore that can be observed using appropriate antisera.
MAD2 Staining in Mitosis Implies an Attachment-sensing Mechanism
The first studies of MAD2 in higher eukaryotes clearly demonstrated that its presence at kinetochores was limited to prometaphase when the chromosomes were aligning on the metaphase plate (Chen et al., 1996; Li and Benezra, 1996). Waters et al. (1998) subsequently demonstrated that the dissociation of MAD2 was not immediate but occurred over a period of minutes, with the intensity of MAD2 staining decreasing over time. Since the initial interaction of kinetochores with the animal spindle is a stochastic process, this often resulted in a distinct difference in the intensity of MAD2 staining between the two kinetochores of a chromosome. The authors went on to use the microtubule stabilizing drug taxol to release the tension applied during chromosome alignment, and did not observe an effect on MAD2 staining. Based on these data, they argued that microtubule attachment, not tension, is responsible for disappearance of MAD2 staining in metaphase (Waters et al., 1998).
The results of MAD2 immunolocalization in maize mitotic cells are consistent with the idea that microtubule attachment is an important factor in the dissociation of MAD2 at kinetochores. The disappearance of MAD2 staining at prometaphase was correlated with the interaction of kinetochores with microtubules. Those kinetochores that lacked an associated bundle of microtubules (K-fiber) had intense MAD2 staining, whereas in the presence of a K-fiber the MAD2 staining was reduced or absent (Fig. 4 B). A 4 h incubation of the mitotic cells with oryzalin destabilized the microtubules and produced short K-fiber remnants at the kinetochores (Fig. 4 E). Significantly, even these short K-fibers were sufficient to reduce or abolish MAD2 staining. The fact that MAD2 staining was negatively correlated with the presence of K-fibers even in the absence of an intact spindle apparatus suggests that microtubule attachment has a major role in the dissociation of MAD2 during mitosis.
MAD2 Staining in Meiosis Implies a Tension-sensing Mechanism
Tension, applied to the kinetochore by the attached kinetochore fiber, is an important component of the spindle checkpoint in animal meiotic cells (reviewed by Nicklas, 1997). In an elegant study, Li and Nicklas (1995) demonstrated that if a fine needle was used to apply tension to a mal-oriented chromosome the cell could be induced to proceed from an arrested metaphase state into anaphase. The mechanism for tension-sensing is not known. Recent studies indicate that at least one kinetochore protein, recognized by the 3F3/2 antibody, becomes dephosphorylated in response to tension (Gorbsky and Ricketts, 1993; Nicklas et al., 1995; Nicklas, 1997). The as yet unidentified antigen recognized by the 3F3/2 antibody appears to be phosphorylated in animals by the mitogen-activated protein (MAP) kinase pathway (Shapiro et al., 1998). Our experiments provide evidence that tension is also correlated with a loss of MAD2 staining in maize meiosis.
Unlike in mitosis, immunolocalization of meiotic cells revealed uniformly MAD2-stained kinetochores interacting with thick K-fibers throughout early to mid prometaphase I and II (Figs. 6 C and 7 C). Despite the fact that kinetochores interacted with microtubules from the earliest stages of prometaphase, there was no noticeable reduction of MAD2 staining until late prometaphase when opposing kinetochores began to visibly separate from each other. The loss of MAD2 was positively correlated with the kinetochore-kinetochore distance: beneath a threshold value, MAD2 was readily detectable, whereas above the threshold MAD2 was undetectable (Fig. 8). These observations, that MAD2 was detected throughout prometaphase regardless of microtubule attachment, and that MAD2 only became undetectable after poleward forces were sufficient to separate opposing kinetochores, suggest that tension is involved in the dissociation of MAD2 from meiotic kinetochores. In addition, we show that MAD2 and the 3F3/2 epitope are colocalized on the meiotic kinetochore both spatially and temporally (Fig. 9, D–I). This correlation provides further evidence that MAD2 and the 3F3/2 antigen are involved in a common process and that tension is a prerequisite for the timely initiation of anaphase.
Differences in Mitotic/Meiotic Spindle Assembly May Underlie Different MAD2 Staining Patterns
The distinct differences in MAD2 staining between mitotic and meiotic cells led us to consider the differences in spindle formation between the two cell types (see Rieder et al., 1993, for a similar discussion of animal cells). A comparison of the mitotic and meiotic spindle formation in maize is presented here for reference only (Fig. 10), since similar data have been illustrated and discussed in previous reports (e.g., Baskin and Cande, 1990; Smirnova and Bajer, 1992; Chan and Cande, 1998). All higher plant spindles lack centrosomes (Baskin and Cande, 1990), but in mitosis a fusiform spindle apparatus is nevertheless apparent before nuclear envelope breakdown (Fig. 10, A–D; the mechanism of pole formation is not understood; Lambert and Lloyd, 1994). In animals, where a similar pattern of spindle morphogenesis is mediated by centrosomes, microtubules originate from poles and search for kinetochores in a random fashion (Kirschner and Mitchison, 1986). Upon encountering a kinetochore, the inherently unstable microtubules are captured and stabilized. This stable interaction of microtubules with kinetochores, in conjunction with microtubule-based motor proteins, is thought to generate chromosome congression (Hardwick et al., 1996). In the mitotic search and capture type of spindle assembly, many correct chromosome-spindle interactions are likely to occur early in prometaphase.
Figure 10.
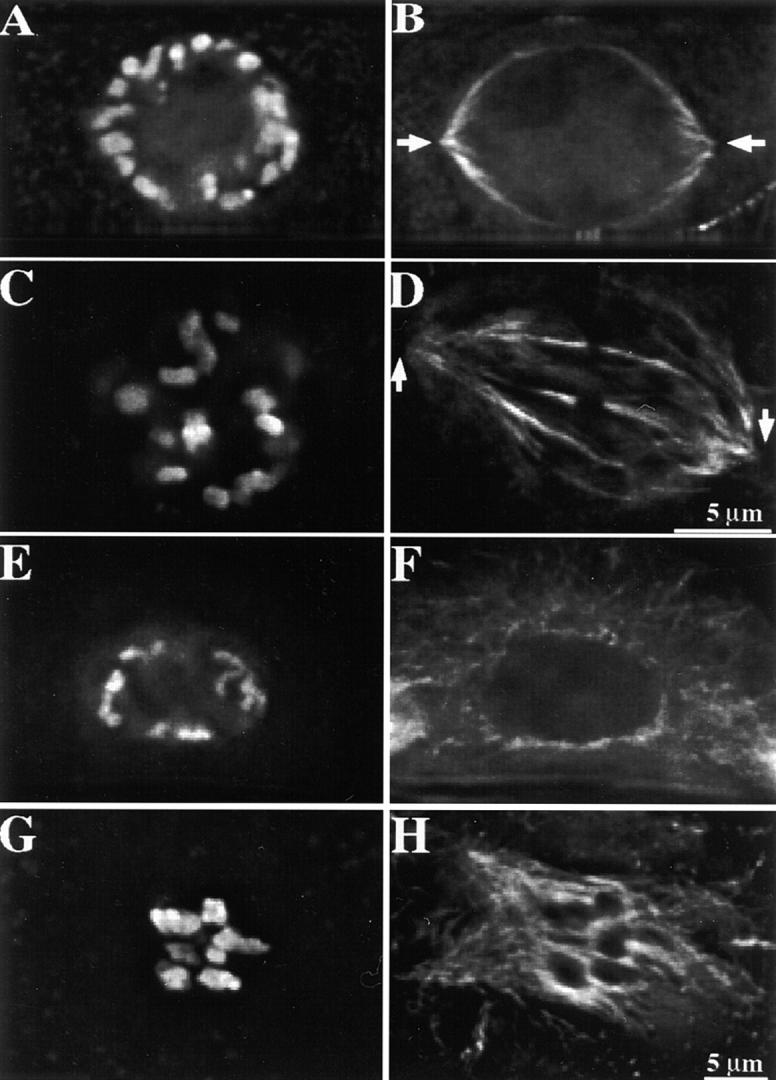
Spindle formation in maize mitosis and meiosis. Partial projections from two to three optical sections are shown here. Chromosomes are shown in A, C, E, and G; microtubules in B, D, F, and H. (A and B) A mitotic cell at late prophase. Note the formation of the perinuclear spindle apparatus. Arrows indicate the defined spindle poles. (C and D) A mitotic cell at prometaphase. Note the tapered spindle poles at this stage (arrows). (E and F) A meiotic cell at prophase II. No spindle apparatus is visible at this stage. Note microtubules radiating from the nuclear envelope. (G and H) A meiotic cell at prometaphase II. An amorphous spindle is forming around the chromosome mass.
In contrast, maize and many other animal meiotic spindles appear to form after nuclear envelope breakdown (Fig. 10, E–H) by an inside-out mechanism (Rieder et al., 1993; Merdes and Cleveland, 1997). The spindles assemble around the mass of chromosomes and initially appear as poorly organized, often multipolar structures (Fig. 10, G–H). Bipolar meiotic spindles emerge at mid-prometaphase both in meiosis I and II (Staiger and Cande, 1990). The progressive self-organization of the focused bipolar spindle probably occurs through the combined effects of microtubule bundling and specific motor activities (Vernos and Karsenti, 1995; Waters and Salmon, 1997). Since in meiosis spindle assembly begins at chromatin/kinetochores, initial microtubule attachment is a poor indicator of correct spindle formation; and tension is likely to have an important role. Therefore, while we believe that attachment and tension are both factors in the mitotic and meiotic spindle checkpoints, differences in the timing and/or relative contributions of the two factors may exist to accommodate basic differences in spindle assembly.
How a single protein or signal transduction pathway can detect both attachment and tension remains an interesting question. The answer may lie in the observation that in vivo, tension is required to stabilize the attachment of kinetochores to the spindle. Ault and Nicklas (1989) carried out an ultrastructural study of mal-oriented chromosomes that were undergoing reorientation. They consistently observed that reorienting kinetochores (not under tension) lost microtubule attachments at both the pole and the kinetochore (but see Nicklas et al., 1995). Since unattached microtubules are highly unstable, the loss of attachment is expected to result in the rapid loss of the microtubules (see also McIntosh and Hering, 1991). Under this view, MAD2 and its associated checkpoint proteins are dissociated by stable microtubule attachment (Waters et al., 1998), and the role of tension in the spindle checkpoint is to increase the stability or number of microtubule attachments. Supporting this proposition is the fact that a destabilization of kinetochore microtubules by vinblastine delays anaphase onset (Wendell et al., 1993) and the fact that the microtubule stabilizing agent taxol results in a near-complete loss of MAD2 staining from kinetochores (Waters et al., 1998). An additional prediction, which should be testable by current electron microscopic imaging techniques, is that there is a reciprocal relationship between MAD2 and attached microtubules in both mitosis and meiosis.
Acknowledgments
We thank Dr. Gary Gorbsky for providing the 3F3/2 antibody and Lisa Reed for purifying the maize CENPC antibody.
This work was supported by an NSF grant (9513556) to R.K. Dawe.
Abbreviations used in this paper
- APC
anaphase-promoting complex
- EST
expressed sequence tags
- MAP
mitogen-activated protein
References
- Anthony RG, Waldin TR, Ray JA, Bright SWJ, Hussey PJ. Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature. 1998;393:260–263. doi: 10.1038/30484. [DOI] [PubMed] [Google Scholar]
- Asai DJ, Brokaw CJ, Thompson WC, Wilson L. Two different monoclonal antibodies to tubulin inhibit the bending of reactivated sea urchin spermatozoa. Cell Motil. 1982;2:599–614. doi: 10.1002/cm.970020608. [DOI] [PubMed] [Google Scholar]
- Atschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 1989;98:33–39. doi: 10.1007/BF00293332. [DOI] [PubMed] [Google Scholar]
- Bajer, A.S., and J. Molé-Bajer. 1972. Spindle Dynamics and Chromosome Movements. Academic Press, New York.
- Baskin TI, Cande WZ. The structure and function of the mitotic spindle in flowering plants. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:277–315. [Google Scholar]
- Braselton JP, Bowen CC. The ultrastructure of the kinetochores of Lilium longiflorumduring the first meiotic division. Caryologia. 1971;24:49–58. [Google Scholar]
- Campbell MS, Gorbsky GJ. Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J Cell Biol. 1995;129:1195–1204. doi: 10.1083/jcb.129.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Cande WZ. Maize meiotic spindles assemble around chromatin and do not require paired chromosomes. J Cell Sci. 1998;111:3507–3515. doi: 10.1242/jcs.111.23.3507. [DOI] [PubMed] [Google Scholar]
- Chen R-H, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Choo, K.H.A. 1997. The Centromere. Oxford University, New York.
- Dawe, R.K., L.M. Reed, H.-G. Yu, M.G. Muszynski, and E.N. Hiatt. 1999. A maize homolog of mammalian CENP-C is a constitutive component of the inner kinetochore. Plant Cell. In press. [DOI] [PMC free article] [PubMed]
- Earnshaw, W.C. 1994. Structure and molecular biology of the kinetochore. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, New York.
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Mitotic arrest: Mad2 prevents sleepy from waking up the APC. Science. 1998;279:999–1000. doi: 10.1126/science.279.5353.999. [DOI] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner M. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Chen R-H, Murray AW. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Ricketts WA. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993;122:1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG. The spindle checkpoint. Trends Genet. 1998;14:1–4. doi: 10.1016/S0168-9525(97)01340-1. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies, A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- He X, Patterson TE, Sazer S. The Schizosaccharomyces pombespindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc Nat Acad Sci USA. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Swedlow JR, Paddy MR, Agard DA, Sedat JW. Three-dimensional multiple-wavelength fluorescence microscopy for the structural analysis of biological phenomena. Semin Cell Biol. 1991;2:153–165. [PubMed] [Google Scholar]
- Hoffman JC, Vaughn KC. Mitotic disrupter herbicides act by a single mechanism but vary in efficacy. Protoplasma. 1994;179:16–25. [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Jensen CG. Dynamics of spindle microtubule organization: kinetochore fiber microtubules of plant endosperm. J Cell Biol. 1982;92:540–558. doi: 10.1083/jcb.92.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol. 1998;141:1393–1406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lin D, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, Mitchison TJ. Beyond self assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Lambert A-M. Microtubule organizing centers in higher plants. Curr Opin Cell Biol. 1993;5:116–122. doi: 10.1016/s0955-0674(05)80016-x. [DOI] [PubMed] [Google Scholar]
- Lambert, A.-M., and C.W. Lloyd. 1994. The higher plant microtubule cycle. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, Inc., New York. 325–341.
- Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Li XT, Nicklas RB. Tension sensitive kinetochore phosphorylation and the chromosome distribution checkpoint in praying mantid spermatocytes. J Cell Sci. 1997;110:537–545. doi: 10.1242/jcs.110.5.537. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Lima-de-Faria A. The role of the kinetochore in chromosome organization. Hereditas. 1956;42:85–160. [Google Scholar]
- McIntosh JR. Structural and mechanical control of mitotic progression. Cold Spring Harbor Symp Quant Biol. 1991;56:613–619. doi: 10.1101/sqb.1991.056.01.070. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Hering GE. Spindle fiber action and chromosome movement. Annu Rev Cell Biol. 1991;7:403–426. doi: 10.1146/annurev.cb.07.110191.002155. [DOI] [PubMed] [Google Scholar]
- Meluh PB, Koshland D. Budding yeast centromere composition and assembly as revealed by in vivo cross-linking. Genes Dev. 1997;11:3401–3412. doi: 10.1101/gad.11.24.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms; conserved components. J Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. The forces that move chromosomes in mitosis. Annu Rev Biophys Biophys Chem. 1988;17:431–449. doi: 10.1146/annurev.bb.17.060188.002243. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Ward S, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cyt. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., J. Ault, U. Eichenlaub-Ritter, and G. Sluder. 1993. Morphogenesis of the mitotic and meiotic spindle: conclusions from one system are not necessarily applicable to the other. In Chromosome Segregation and Aneuploidy. B.K. Vig, editor. Springer-Verlag, Berlin. 183–197.
- Rudner AD, Murray AW. The spindle assembly checkpoint. Curr Opin Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Tomkiel J, Cooke CA, Ratrie H, III, Maurer M, Rothfield NF, Earnshaw WC. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70:115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, Ahn NG. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142:1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova EA, Bajer AS. Spindle poles in higher plant mitosis. Cell Motil Cytoskeleton. 1992;23:1–7. doi: 10.1002/cm.970230102. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Cande WZ. Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev Biol. 1990;138:213–242. doi: 10.1016/0012-1606(90)90193-m. [DOI] [PubMed] [Google Scholar]
- Tang W-JY. Blot-affinity purification of antibodies. Methods Cell Biol. 1993;37:95–103. doi: 10.1016/s0091-679x(08)60245-9. [DOI] [PubMed] [Google Scholar]
- Vernos I, Karsenti E. Chromosomes take the lead in spindle assembly. Trends Cell Biol. 1995;5:297–301. doi: 10.1016/s0962-8924(00)89045-5. [DOI] [PubMed] [Google Scholar]
- Visitin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Benezra R. Mad2 transiently associates with an APC/ p55Cdc complex during mitosis. Proc Natl Acad Sci USA. 1998;95:11193–11198. doi: 10.1073/pnas.95.19.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Salmon ED. Pathways of spindle assembly. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Waters JC, Skibbens RV, Salmon ED. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J Cell Sci. 1996;109:2823–2831. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- Waters JC, Chen R-H, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WAE. The spindle assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- Wendell KL, Wilson L, Jordan MA. Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-microtubule attachment and in centrosomes. J Cell Sci. 1993;104:261–274. doi: 10.1242/jcs.104.2.261. [DOI] [PubMed] [Google Scholar]
- Yu H-G, Hiatt EN, Chan A, Sweeney M, Dawe RK. Neocentromere-mediated chromosome movement in maize. J Cell Biol. 1997;139:831–840. doi: 10.1083/jcb.139.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]