Abstract
The Src-related tyrosine kinase p56lck (Lck) is primarily expressed in T lymphocytes where it localizes to the cytosolic side of the plasma membrane and associates with the T cell coreceptors CD4 and CD8. As a model for acylated proteins, we studied how this localization of Lck is achieved. We followed newly synthesized Lck by pulse–chase analysis and found that membrane association of Lck starts soon after synthesis, but is not complete until at least 30–45 min later. Membrane-binding kinetics are similar in CD4/CD8-positive and CD4/CD8-negative cells. In CD4-positive T cells, the interaction with CD4 rapidly follows membrane association of Lck. Studying the route via which Lck travels from its site of synthesis to the plasma membrane, we found that: CD4 associates with Lck within 10 min of synthesis, long before CD4 has reached the plasma membrane; Lck associates with intracellular CD4 early after synthesis and with cell surface CD4 at later times; and transport of CD4-bound Lck to the plasma membrane is inhibited by Brefeldin A. These data indicate that the initial association of newly synthesized Lck with CD4, and therefore with membranes, occurs on intracellular membranes of the exocytic pathway. From this location Lck is transported to the plasma membrane.
Keywords: p56lck, acylation, targeting, CD4, protein sorting
Acylation, the covalent attachment of fatty acids, is a modification that allows numerous cytosolic proteins to associate with the cytoplasmic leaflet of cellular membranes (Resh, 1994; Milligan et al., 1995; Wedegaertner et al., 1995; Bhatnagar and Gordon, 1997). Acylated proteins have specific subcellular localizations that are important for their functions, but very little is known about the mechanisms involved in targeting these proteins to specific sites in the cell. To obtain more insight into this, we are studying the acylated tyrosine kinase p56lck (Lck).1
Lck is a member of the Src-family of nonreceptor kinases and is expressed primarily in T lymphocytes and thymocytes. The protein is predominantly associated with the cytosolic side of the plasma membrane (Ley et al., 1994; Bijlmakers et al., 1997), a localization that is consistent with the importance of Lck in the early signaling events through the T cell receptor (TcR) (Weiss and Littman, 1994). The domain organization of Lck is identical to that of other Src-family kinases (Fgr, Hck, Blk, Fyn, Lyn, Yes, Src, Yrk) (Rudd et al., 1993). Each possesses a conserved Src-homology 2 (SH2), SH3, and kinase domain, while the NH2-terminal 50–70 amino acids (the unique domain) are unique to each individual member. Src-related kinases are modified by the attachment of myristic acid to an NH2-terminal glycine and, with the exception of Src and Blk, contain potential palmitoylation sites as well (Resh, 1994; Milligan et al., 1995). The short conserved NH2-terminal region that contains the acylation sites has been designated the SH4 domain (Resh, 1993). Despite the similarity between members of the Src-family, the subcellular distribution of Lck is not identical to that of its relatives. Many Src-related proteins localize to the plasma membrane but are also found at other locations in the cell: v-Src in focal adhesions (Rohrschneider, 1980), c-Src on endosomes (Kaplan et al., 1992) and synaptic vesicles (Linstedt et al., 1992), Fyn in the microtubule organizing center (Ley et al., 1994), and Hck on secretory granules (Mohn et al., 1995).
One feature that distinguishes Lck from other members of the Src-family is its association with the cytoplasmic domains of the cell surface proteins CD4 and CD8 (Rudd et al., 1988; Veillette et al., 1988), coreceptors of the TcR on helper and cytotoxic T cells, respectively. However, this noncovalent interaction, mediated by a pair of cysteines (C20 and C23) in the unique domain of Lck and a CXCP motif in the cytoplasmic tails of CD4 or CD8α (Shaw et al., 1990; Turner et al., 1990), is not required to target Lck to the plasma membrane (Bijlmakers et al., 1997). Rather, we observed that the unique domain of Lck contains plasma membrane targeting information that can operate in the absence of coreceptor expression (Bijlmakers et al., 1997). The identity of these targeting signal(s) and their mode of operation remain to be established.
In this study we investigated the route via which Lck travels to the plasma membrane. Two extreme possibilities can be envisaged: Lck could insert directly into the plasma membrane after synthesis. Alternatively, the newly synthesized protein could initially be targeted to intracellular membranes and subsequently travel to the plasma membrane. Lck is myristoylated during translation (Paige et al., 1993), but stable association with membranes requires posttranslational palmitoylation (Kwong and Lublin, 1995). Palmitoyl transferases are membrane-associated enzymes and there is evidence for their presence at the plasma membrane (Dunphy et al., 1996; Schroeder et al., 1996), intermediate compartment (Bonatti et al., 1989), and Golgi complex (Solimena et al., 1994). However, it is not yet known where the palmitoylation, and therefore the initial membrane association, of Lck occurs.
We followed newly synthesized Lck by pulse–chase analysis in the human leukemia T cell line SupT1 and established membrane- and CD4-binding kinetics. We observed that a large proportion of Lck is not targeted to the plasma membrane directly, but initially associates with intracellular membranes and is subsequently transported to the plasma membrane in a Brefeldin A (BFA)-sensitive manner. Our data describe a novel pathway for the trafficking of a newly synthesized acylated cytosolic protein.
Materials and Methods
Reagents
Tissue culture reagents and plastics were from Gibco Ltd. and other chemicals were from Sigma Chemical Co., unless indicated otherwise.
Cell Lines and Antibodies
The human T cell line SupT1 and Jurkat cells were cultured in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 0.1 mg/ml streptomycin (pen/strep). The mutant Jurkat cell line JCam.1 was provided by A. Weiss (University of California, San Francisco, CA). These cells express low levels of a shorter mutant form of Lck, but not wild-type (wt) Lck (Straus and Weiss, 1992). NIH-3T3 cells stably transfected with human Lck (Bijlmakers et al., 1997) were cultured in DMEM, 10% FCS, pen/strep, 1 mg/ml G418.
Two polyclonal rabbit anti-Lck sera, here designated LckN and LckC, were used. LckN (Brouns et al., 1993) was provided by J. Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands) and used in immunoprecipitation experiments. This serum was raised against an NH2-terminal Lck peptide comprising residues 39–58. In addition to Lck, LckN also immunoprecipitates and immunoblots an unidentified protein with a slightly higher molecular weight than Lck (Fig. 1). LckC (Ley et al., 1994), provided by S. Ley (National Institute for Medical Research, Mill Hill, United Kingdom), was used in Western blotting. This antibody was raised against a synthetic Lck peptide comprising residues 478–509. For immunoprecipitation of CD4, a mixture of two mouse mAbs, #4 and #19, provided by J. Hoxie (University of Philadelphia, Philadelphia, PA) was used. The anti-CD4 mouse mAb Q4120 was used for immunoblotting. Peroxidase-conjugated goat anti–rabbit antibodies were from Pierce and Warriner.
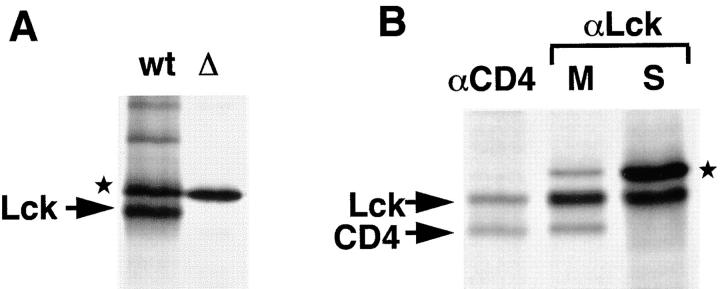
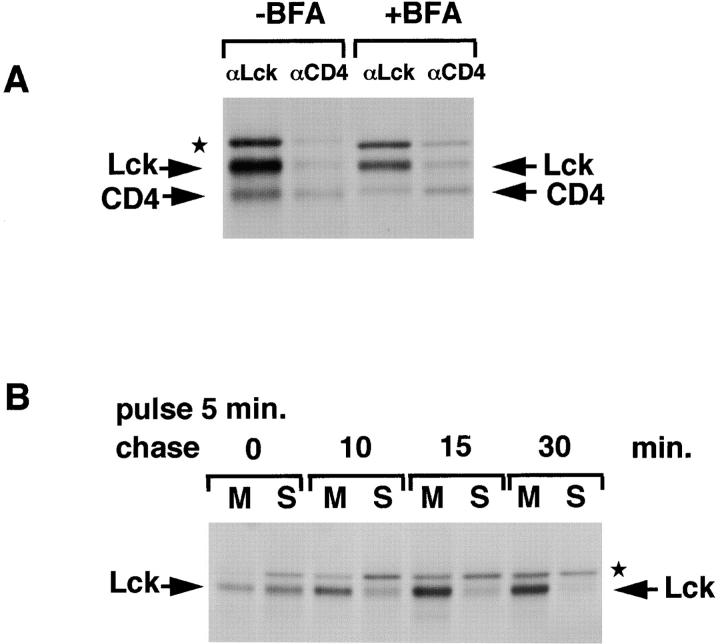
Figure 1.
Characterization of Lck antiserum. (A) Jurkat (wt) and mutant JCam.1 (Δ) cells (6 × 106 each) were labeled for 30 min with [35S]methionine/cysteine (0.5 mCi) and subjected to immunoprecipitation with LckN serum. JCam.1 does not express wt Lck (Straus and Weiss, 1992). In addition to Lck, the LckN antiserum recognizes a protein with an apparent molecular mass of 60 kD (★). (B) SupT1 cells were labeled with [35S]methionine/ cysteine for 5 min and chased for 15 min. Cells were broken in hypotonic buffer, nuclei removed by centrifugation, and membranes recovered by centrifugation at 100,000 g for 45 min. Membrane and soluble fractions were split into two: half was subjected to immunoprecipitation with anti-CD4 antibodies (αCD4), the other half with anti-Lck antibodies (αLck). The CD4 immunoprecipitation from membranes, and the Lck immunoprecipitation both from the membrane (M) and soluble (S) fractions are shown. CD4, Lck, and the background band (★) are indicated.
Pulse–Chase Labeling
Cells were washed once and incubated for 45 min in methionine- and cysteine-free DME medium (ICN Biomedicals Ltd.). For pulse-labeling of suspension cells (SupT1 and Jurkat cells), typically 1 mCi [35S]methionine/ cysteine (Express [1,175 Ci/mmol]; DuPont) was used per 108 cells in 1 ml methionine- and cysteine-free DME medium, 10% FCS. When chase times did not exceed 15 min, incorporation of label was terminated by the addition of nonradioactive methionine and cysteine to a final concentration of 1 mM each. For experiments with chase times >15 min, cells were transferred after the pulse to 10 ml warm (37°C) DMEM with methionine and cysteine at a final concentration of 1 mM. Where indicated, BFA was present at a final concentration of 10 μg/ml (added from a 2.5 mg/ml stock in EtOH) before and during the pulse, and at 2 μg/ml during the chase. Samples taken at indicated chase times were kept in 10 ml ice-cold DMEM until the final time point, pelleted by centrifugation (5 min at 1,500 rpm, 4°C), and further processed as described for individual experiments. For the adherent NIH-3T3 cells, per time point one 10-cm dish of cells (∼7 × 106) was labeled with 0.75 mCi [35S]methionine/cysteine in 1 ml methionine- and cysteine-free DME medium without FCS. The chase was started by replacing the radiolabel with prewarmed DMEM containing cysteine and methionine at 1 mM each. At the end of the chase this medium was replaced by ice-cold DMEM. The cells were put on ice, gently scraped, and recovered by centrifugation at 1,500 rpm for 5 min at 4°C.
Immunoprecipitation, Endoglycosidase H (Endo H) Digestion, and Gel Electrophoresis
Unless indicated otherwise, cells were lysed in NP-40 buffer (2% NP-40 [Pierce and Warriner], 20 mM Tris, pH 7.8, 150 mM NaCl, 2 mM MgCl2, 1 mM EDTA) containing the protease inhibitors PMSF (at 1 mM) and CLAP (5 μg/ml each of chymostatin, pepstatin A, antipain hydrochloride, and 10 μg/ml leupeptin hemisulphate). After removal of nuclei and cell debris by centrifugation at 13,000 g for 5 min at 4°C, the lysates were cleared of nonspecifically binding proteins by three rounds of incubations with normal rabbit serum (3 μl) and 20 μl packed protein A–Sepharose beads (Pharmacia Biotech AB) for 30 min at 4°C. For specific immunoprecipitations, samples were incubated on ice for 45 min with relevant antibodies: 1 μl of the polyclonal rabbit serum LckN for immunoprecipitation of Lck or a mixture of mAbs #4 (1.7 μg) and #19 (0.5 μg) for CD4. Immune complexes were recovered by incubation with protein A–Sepharose (25 μl packed beads) for 45 min at 4°C and washed five times in NP-40 lysis buffer. For Endo H digestion, washed immunoprecipitates were incubated for 1 h at 37°C with 1 mU Endo H (Boehringer Mann-heim) in 50 mM sodium citrate (pH 5.5), 0.02% SDS. Immune complexes were eluted by addition of nonreducing SDS sample buffer, incubated for 5 min at 95°C, and loaded on 8% SDS-polyacrylamide gels. After electrophoresis, gels were enhanced in salicylic acid (16% wt/vol in 30% methanol), dried, and exposed to Kodak X-Omat AR film (Eastman Kodak Co.) for 1–9 d.
Immunoblotting
After gel electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher and Schuell). The blots were incubated in blocking buffer (10% skimmed milk, 0.1% Tween 20 in PBS) for 1 h at room temperature. Incubations with primary and secondary antibodies were in blocking buffer for 1 h each at room temperature. To detect Lck, the rabbit antiserum LckC (1:1,000) and HRP-conjugated goat anti–rabbit antibodies (1:2,000) were used. Q4120 (1.6 μg/ml) and HRP-conjugated goat anti–mouse antibodies (1:2,000) were used to detect CD4. Blots were developed using enhanced chemiluminescence (Amersham International plc) and visualized with autoradiography film (Fuji Photo Film Co. Ltd.).
Membrane Separation
After pulse–chase labeling, cells were incubated in 1 ml hypotonic buffer (20 mM Tris, pH 7.8, 2 mM MgCl2, 1 mM EDTA, 1 mM PMSF, CLAP as above) on ice for 12 min and homogenized by 15 strokes in a Dounce homogenizer (Wheaton Scientific). To remove nuclei, cell homogenates were centrifuged for 5 min at 1,500 rpm, 4°C. The postnuclear supernatant was centrifuged in an Optima TL Ultracentrifuge (Beckman Instruments) for 45 min at 100,000 g, 4°C, to recover total cellular membranes. The pellet (membrane fraction) was resuspended in hypotonic buffer, Dounce homogenized (20 strokes), and adjusted to 2% NP-40, 150 mM NaCl, 1 mM PMSF, and CLAP. Similarly, the soluble fractions were adjusted to 2% NP-40 and 150 mM NaCl. The final volume of both fractions was equivalent. Samples (2% of total volume) were analyzed by immunoblotting to check the efficiency of membrane separation and the remainder was subjected to immunoprecipitation. In control experiments, to examine the presence of Lck in the nuclear fraction, nuclei were resuspended in an equal volume of the same buffer as the membrane and soluble fractions and analyzed for Lck by immunoprecipitation and immunoblotting. The amount of Lck detected in this fraction was negligible (Fig. 2 A).
Figure 2.
Membrane-binding kinetics of Lck. (A) SupT1 cells were broken in hypotonic buffer and Dounce homogenized. After removal of nuclei (5 min at 1,500 rpm), membrane and soluble fractions were separated by centrifugation at 100,000 g. Equivalent amounts of total (T), nuclear (N), membrane (M), and soluble (S) fractions were analyzed by immunoblotting with LckC. (B) SupT1 cells (2 × 108) were labeled with [35S]methionine/cysteine (2 mCi) for 5 min and chased for the indicated times. Lck was immunoprecipitated from the membrane (M) and soluble (S) fractions with LckN and analyzed by SDS-PAGE and autoradiography. Lck and CD4 are indicated. ★ indicates the background band.
Sequential Immunoprecipitations of Cell Surface and Intracellular CD4
After pulse–chase labeling, SupT1 cells (2.5 × 107 for each time point) were washed once with ice-cold DMEM and incubated with the anti-CD4 antibodies #4 (1.7 μg/ml) and #19 (0.5 μg/ml) for 1 h at 4°C to absorb cell surface CD4. During incubation, the cells were kept in suspension by rotation. The cells were washed three times with 10 ml ice-cold DMEM to remove unbound antibody. Next, the cells were lysed in NP-40 buffer (see above) to which soluble nonlabeled CD4 (150 ng/ml, diluted from a 500 μg/ml stock; American Biotechnologies) was added to prevent binding of intracellular labeled CD4 to free antigen-binding sites. Nuclei and cell debris were removed by centrifugation (5 min at 13,000 g, 4°C) and cell surface CD4 was recovered by incubation with 25 μl packed protein A–Sepharose beads. To recover the remaining intracellular CD4, the lysate was precleared twice with normal rabbit serum and protein A–Sepharose and subsequently incubated with antibodies #4 and #19 and protein A–Sepharose again.
Quantitation
Autoradiograms were digitized using Sony XC-77CE CCD video camera and NIH Image, stored as TIFF files and imported into the Bio-Rad Molecular Analyst program for analysis. The relative amount of membrane-associated Lck (Figs. 2–4) was determined as follows: [M − BG1/(M − BG1) + (S − BG2)] × 100%, where M is the density of the membrane-associated Lck band, BG1 the background in the membrane lane, S the density of the soluble Lck band, and BG2 the background in the soluble fraction. The relative amount of CD4-associated Lck (Fig. 5 A) was determined as follows: [CD4 − BG1/(CD4 − BG1) + (Lck − BG2)] × 100%, with CD4 being the density of the Lck band in the anti-CD4 immunoprecipitation, and BG1 the background in the anti-CD4 immunoprecipitation; Lck the density of the Lck band in the anti-Lck immunoprecipitation, and BG2 the background in the anti-Lck immunoprecipitation. The relative amount of Lck associated with cell surface CD4 (Fig. 8) was determined as follows [Lck(CS) − BG1/{Lck(CS) − BG1} + {Lck(IC) − BG2}] × 100%, where Lck(CS) is the density of the Lck band in the immunoprecipitation of cell surface CD4, Lck(IC) the density of the Lck band in the immunoprecipitation of intracellular CD4, and BG1 and BG2 the background in cell surface and intracellular immunoprecipitation, respectively. The relative amount of CD4 at the plasma membrane was determined in the same way for the CD4 bands. We found that quantitation of autoradiograms leads to an overestimation of signals with low intensity, as a result of which curves do not reach the 0 and 100% as would be expected based on the autoradiograms. Similar curves were obtained when the gels were analyzed using a PhosphorImager.
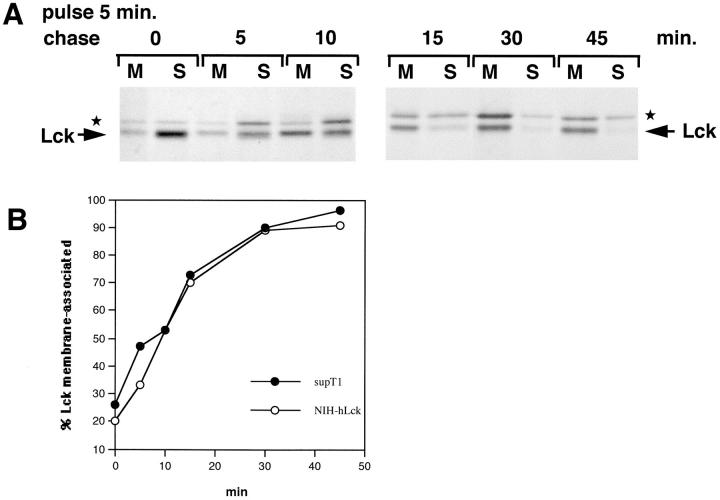
Figure 4.
Lck membrane-binding kinetics in NIH-3T3 cells. (A) NIH-3T3 cells stably transfected with Lck were pulse-labeled and chased as indicated and Lck was immunoprecipitated from the membrane (M) and soluble (S) fractions. ★ indicates the background band. (B) Comparison of Lck membrane-binding kinetics in SupT1 and NIH-3T3 cells. The experiments shown here and in Fig. 2 B were quantitated. The percentage of membrane-associated Lck is plotted against time.
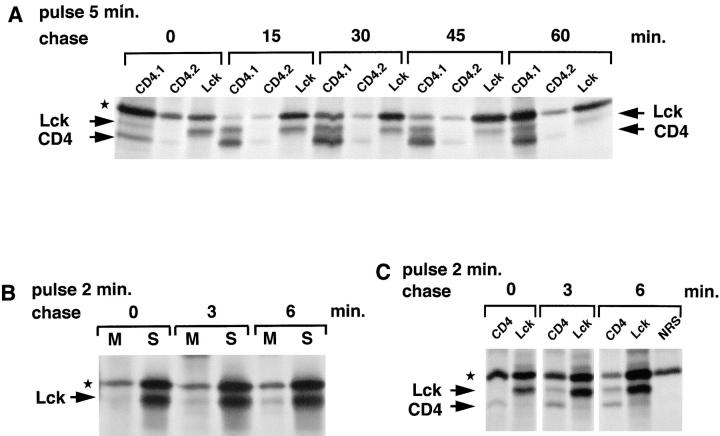
Figure 5.
Kinetics of the association of Lck with CD4. (A) SupT1 cells (1.25 × 108) were labeled for 5 min with [35S]methionine/cysteine (1.5 mCi) and chased as indicated. The cells were lysed in NP-40 buffer and subjected to sequential immunoprecipitations, first with two rounds of anti-CD4 antibodies (CD4.1 and CD4.2) and next with anti-Lck antibodies (Lck). (B) SupT1 cells (108) were labeled with [35S]methionine/cysteine (1 mCi) for 2 min and chased for 0, 3, or 6 min. Lck was immunoprecipitated from membrane (M) and soluble (S) fractions with LckN and analyzed by SDS-PAGE and autoradiography. (C) SupT1 cells (7.5 × 107) were labeled with [35S]methionine/cysteine (1 mCi) for 2 min and chased for 0, 3, or 6 min. Cell lysates were subjected to sequential immunoprecipitations, first with anti-CD4 antibodies (CD4) and next with anti-Lck antibodies (Lck). The last of three rounds of preclears with normal rabbit serum (NRS) and protein A–Sepharose is shown for the 6-min chase (NRS). Lck, CD4, and the background band (★) are indicated.
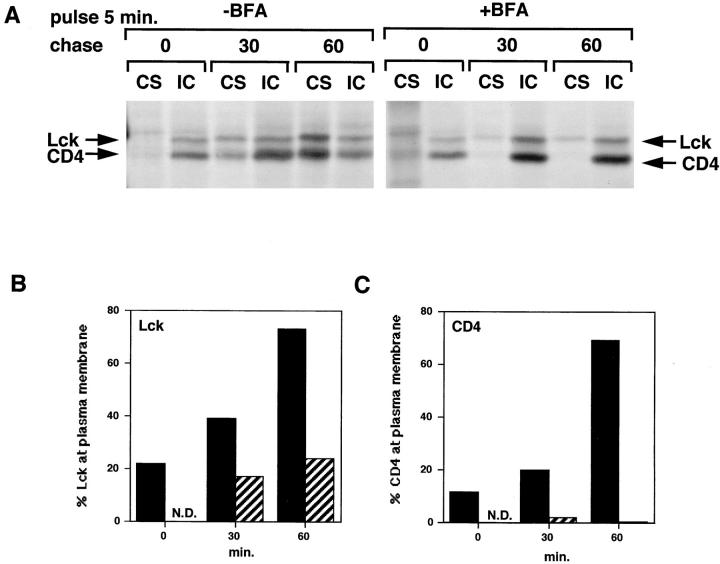
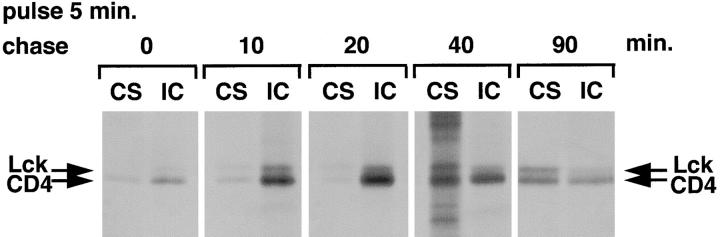
Figure 8.
Transport of CD4-associated Lck is inhibited in the presence of BFA. (A) SupT1 cells were labeled in the absence or presence of BFA. BFA was added 2 min before pulse-labeling at a concentration of 10 μg/ml and was present during the chase at 2 μg/ml. Cell surface (CS) and intracellular (IC) CD4 were immunoprecipitated separately as described for Fig. 7. (B) Quantitation of the autoradiogram in A for Lck. Per time point, the amount of Lck associated with cell surface (CS) CD4 was expressed as a percentage of the total (CS + IC) Lck coimmunoprecipitated with CD4. Black bars, without BFA; striped bars, with BFA. (C) Quantitation of the autoradiogram in A for CD4. Per time point the relative amount of CD4 detected in the cell surface (CS) immunoprecipitation was expressed as a percentage of the total (CS + IC) amount of CD4. Black bars, without BFA; striped bars, with BFA.
Results
Membrane-Association Kinetics of Lck
We used the human leukemia T cell line SupT1 to study the mechanism by which newly synthesized Lck reaches the plasma membrane. In these cells, the majority of Lck is detected at the cytosolic side of the plasma membrane by immunofluorescence (Bijlmakers et al., 1997). In the study described here, we used pulse–chase labeling and immunoprecipitation to follow newly synthesized Lck. The rabbit antiserum used for immunoprecipitations (Brouns et al., 1993) recognizes Lck and, in addition, a protein with a slightly higher molecular weight. Lck was identified by comparing Jurkat cells that express wt Lck, with JCam.1 cells, a Jurkat derivative that expresses very low levels of a shorter, mutant Lck (Fig. 1 A). The background band was detected in both cell lysates whereas wt Lck was only detected in Jurkat lysates (the shorter mutant Lck in JCam.1 was only visible after long exposures of the autoradiogram, not shown). The background band (marked with ★) was also detected in a variety of Lck-negative cell lines including HeLa and NIH-3T3 (not shown), indicating that this protein is not associated with, nor related to, Lck. Lck was further identified by its association with CD4 in SupT1 cells. Lck and CD4 have similar molecular weights; however, in SDS-PAGE, under nonreducing conditions, CD4 migrates slightly faster than Lck and the two proteins can be clearly resolved. Immunoprecipitation with anti-CD4 antibodies resulted in coimmunoprecipitation of Lck, but not of the background band (Fig. 1 B). Vice versa, immunoprecipitation with anti-Lck antibodies coimmunoprecipitated CD4. As expected, CD4 only coimmunoprecipitated from a membrane, but not from a soluble fraction (Fig. 1 B).
To establish the kinetics with which newly synthesized Lck becomes membrane-associated, SupT1 cells were pulse-labeled for 5 min with [35S]methionine/cysteine and chased for various times. The cells were then broken in hypotonic buffer, the nuclei removed by centrifugation, and total cellular membranes recovered by centrifugation at 100,000 g. By immunoblotting, total cellular Lck was detected exclusively in the membrane fraction (Fig. 2 A), indicating that virtually all Lck is membrane-associated at steady state. To study newly synthesized labeled molecules, Lck was immunoprecipitated from the membrane and soluble fractions and analyzed by SDS-PAGE. After 5 min of pulse-labeling only a small amount of Lck (∼15%) was found at membranes (Fig. 2 B). This amount increased with time, and membrane association was complete after 30–45 min of chase (Fig. 2 B). Newly synthesized CD4 coimmunoprecipitated with Lck already at early chase times (5 min), and, as expected, was only detected in membrane fractions (Fig. 2 B). This coimmunoprecipitation was easier to detect when cells were directly lysed in NP-40 buffer (see Fig. 6). Quantitation showed that the kinetics of Lck membrane association were consistent between experiments and occurred with a calculated half time of 9 min.
Figure 6.
Kinetics of the association of CD4 with Lck. (A) SupT1 cells (5 × 107) were labeled for 5 min with [35S]methionine/cysteine (0.5 mCi) and chased for the indicated times. The cells were lysed in NP-40 buffer and subjected to immunoprecipitation with anti-LckN serum. Association of CD4 with Lck was detected by coimmunoprecipitation with Lck. Lck, CD4, and the background band (★) are indicated. (B) SupT1 cells were labeled as described in A, lysed, and CD4 was immunoprecipitated from the cell lysates. Immunoprecipitates were incubated with Endo H (1 mU) for 1 h at 37°C before separation by SDS-PAGE. Endo H–sensitive (S) forms of CD4 from which two carbohydrate chains were removed and resistant (R) forms from which only one carbohydrate chain was removed are indicated. Nondigested CD4, containing two carbohydrate chains, migrates slower than Endo H–resistant CD4 (not shown). Coimmunoprecipitated Lck is indicated and is unaffected by Endo H treatment.
Membrane-Binding Kinetics of Lck in the Absence of CD4/CD8 Interaction
In SupT1 cells, the majority of Lck (at least 70%) is associated with CD4, as determined by coimmunoprecipitation experiments (not shown). However, this interaction is not essential for membrane binding of Lck, since also in CD4/ CD8-negative T cells, Lck is completely membrane-associated at steady state (Bijlmakers et al., 1997). Nevertheless, CD4 or CD8 could influence the rate of membrane binding of newly synthesized Lck. To examine this, we studied Lck membrane binding in BC7 cells, a CD4-negative derivative of SupT1. The kinetics of membrane association were identical to those seen in SupT1 cells (not shown), indicating that membrane recruitment of Lck is not dependent on the presence of CD4. However, BC7 and SupT1 cells do express CD8, and therefore a role for this protein could not be excluded, although an interaction with CD8 was not observed by immunoprecipitation.
Therefore, we also determined Lck membrane-binding kinetics in the CD8-negative Jurkat cells. Three different Jurkat clones were screened by FACS® analysis for CD4 expression. Two were found to express very little if any CD4, whereas one expressed considerable amounts of CD4 (approximately half the amount of CD4 in SupT1 cells, which is ∼30,000 copies per cell; Pelchen-Matthews et al., 1991). This was confirmed by immunoblotting (Fig. 3 C). Again, we did not find a difference in membrane association of newly synthesized Lck between CD4-positive and -negative cells (Fig. 3, A, B, and D). However, membrane-binding kinetics of Lck were slower in Jurkat cells than in SupT1 cells (t 1/2 21 min in Jurkat vs. 9 min in SupT1), indicating that cell type–specific differences can influence these kinetics.
Figure 3.
Lck membrane-binding kinetics in the presence and absence of CD4. (A) CD4-negative Jurkat cells were labeled for 5 min and chased as indicated. Lck was immunoprecipitated from membrane (M) and soluble (S) fractions as described for Fig. 2 B. ★ indicates the background band. (B) The same experiments as in A, now for CD4-positive Jurkat cells. (C) Cell lysates of CD4-negative (−) and CD4-positive (+) Jurkat cells were analyzed for CD4 expression by immunoblotting. (D) Quantitation of Lck membrane-binding experiments for two CD4-negative and one CD4-positive Jurkat cell line. Autoradiograms were digitized (as described in Materials and Methods) and analyzed using the Molecular Analyst program (Bio-Rad). Membrane-associated Lck is expressed as a percentage of the total amount of Lck and plotted against time.
The membrane-binding kinetics measured here for Lck differ from those reported recently for another member of the Src-family, Fyn (van't Hof and Resh, 1997), which is also myristoylated and palmitoylated. Membrane binding of Fyn was studied in transfected NIH-3T3 fibroblasts and COS cells and found to be complete within 5 min of synthesis. To investigate whether this apparent difference between Lck and Fyn was due to the different cellular backgrounds, we also analyzed Lck in stably transfected NIH-3T3 cells. Previously, we established that at steady state all the Lck is membrane bound in these cells (Bijlmakers et al., 1997). We found little Lck on membranes after 5 min of labeling, whereas membrane association of newly synthesized Lck proceeded at rates similar to those seen in SupT1 cells, with 50% membrane-associated after 10 min (Fig. 4, A and B). Thus, T cell–specific proteins do not enhance membrane binding of newly synthesized Lck and Lck differs markedly from Fyn in its rate of membrane association.
Kinetics of Lck Association with CD4
In mammalian cells, nonpalmitoylated soluble Lck does not interact with CD4 (Turner et al., 1990), suggesting that membrane binding of Lck is necessary for stable association with CD4. To establish the kinetics of CD4 interaction, SupT1 cells were pulse-labeled and chased, and cell lysates were then subjected to sequential immunoprecipitations, first with anti-CD4 antibodies and then with anti-Lck antibodies. With the anti-CD4 antibodies we recovered CD4 and Lck and, in addition, a background band with a molecular weight similar to the one seen in Lck immunoprecipitates (Fig. 5, A and C). The latter band (★) was also present in the normal rabbit serum control (Fig. 5 C), showing that it is precipitated nonspecifically. In contrast to the anti-Lck immunoprecipitates, the background band is not consistently found in anti-CD4 immunoprecipitates (see Figs. 1 B and 7 for example).
Directly after 5 min of pulse-labeling, some Lck coimmunoprecipitated with CD4, while at 15 min chase ∼50% interacted with CD4 (Fig. 5 A, CD4.1 vs. Lck lanes). Previously, we determined that in SupT1 cells, at least 70% of the total Lck is associated with CD4 at steady state. This situation is apparently reached 60 min after synthesis (Fig. 5 A). The kinetics of the interaction with CD4 are similar to those seen for membrane association of Lck, suggesting that the two processes are closely linked.
We next labeled the cells for only 2 min to detect the earliest membrane and CD4 association of Lck. On long exposures of autoradiograms, a small amount of Lck can be seen associated with membranes directly after the 2-min pulse (Fig. 5 B). Association of Lck with CD4, by contrast, can be detected only after a 3-min chase (Fig. 5 C), with a further increase in both the amounts of membrane- and CD4-associated Lck after a 6-min chase. Thus, membrane association of Lck starts soon after synthesis and is rapidly followed by CD4 association. Note that in these experiments, all the CD4 is immunoprecipitated and therefore no distinction can be made between Lck binding to newly or previously synthesized CD4.
CD4 Associates with Lck Early after Synthesis
To determine at which time after synthesis CD4 associates with Lck, we followed the coimmunoprecipitation of labeled CD4 with anti-Lck antibodies. CD4 was detected in Lck immunoprecipitates already at 5-min chase after 5 min of pulse-labeling, and possibly even at 0-min chase (Fig. 6 A). This is similar to the CD4 coimmunoprecipitation with Lck in membrane fractions shown in Fig. 2 B. Human CD4 carries two N-linked oligosaccharides (Konig et al., 1988), one of which becomes resistant to Endo H, an enzyme that selectively removes high mannose N-linked oligosaccharides (Crise and Rose, 1992b). Endo H resistance is acquired when the protein is delivered to the cis-Golgi where trimming of the high mannose carbohydrate occurs. Directly after pulse-labeling and at 5 min of chase, the majority of CD4 is Endo H–sensitive, indicating that the protein has not yet reached the cis-Golgi (Fig. 6 B). Endo H–resistant CD4 first appeared at 10 and 15 min of chase and coincided with a decrease in the amount of Endo H–sensitive CD4 (Fig. 6 B). We also detected small amounts of Endo H–sensitive CD4 in Lck immunoprecipitates at 5 min of chase (not shown). Together, the data show that CD4 interacts with Lck early after synthesis, possibly on the ER or intermediate compartment.
Shortly after Synthesis, Lck Associates Predominantly with Intracellular CD4
The previous experiment indicates that CD4 associates with Lck early in the exocytic pathway; however, this experiment does not discriminate between association of CD4 with newly or previously synthesized Lck. To determine the route through which Lck traffics to the plasma membrane, it is necessary to follow the pool of newly synthesized Lck exclusively. Therefore, we investigated the arrival of Lck at the plasma membrane at various times after pulse-labeling by selectively immunoprecipitating cell surface CD4. For this purpose, intact SupT1 cells were incubated with anti-CD4 antibodies on ice, thereby ensuring that only cell surface CD4 was complexed with antibody. Next the cells were lysed in the presence of soluble unlabeled CD4 to prevent binding of intracellular labeled CD4 to antibodies during lysis. Cell surface CD4 was then isolated by incubation with protein A–Sepharose. Intracellular CD4 was subsequently recovered by a second round of immunoprecipitation with anti-CD4 antibodies. In agreement with reported transport rates of CD4 (Crise and Rose, 1992b), we observed that at 0, 10, and 20 min of chase, the majority of newly synthesized CD4 was found inside the cell (Fig. 7). Approximately 50% was at the cell surface after 40 min of chase and at 90 min the majority was at the cell surface. At 0 min of chase, CD4 is completely Endo H–sensitive (Fig. 6 B) and has therefore not yet reached the cis-Golgi. Therefore, the small amount of labeled CD4 in the cell surface immunoprecipitation at this time most likely reflects an incomplete block of free antibody-binding sites during cell lysis and, consequently, the recovery of some intracellular CD4. A similar explanation accounts for the small amount of labeled CD4 in the cell surface immunoprecipitation at 10 and 20 min of chase.
Figure 7.
Lck associates predominantly with intracellular CD4 early after synthesis. SupT1 cells (1.5 × 108) were labeled with [35S]methionine/cysteine (1.5 mCi) for 5 min and chased for the indicated times. The intact cells were incubated with antibodies against CD4 for 1 h at 4°C. After extensive washing, the cells were lysed in the presence of soluble CD4 to block free antibody-binding sites, and cell surface CD4 (CS) was recovered by incubation with protein A–Sepharose. Subsequently, anti-CD4 antibodies were added to the lysates to recover intracellular CD4 (IC). CD4 and coimmunoprecipitated Lck are indicated.
Like CD4, newly synthesized Lck can be seen to move from the intracellular to the plasma membrane fraction with time. Lck predominantly associated with intracellular CD4 at early time points (0, 10, and 20 min of chase), while the majority was associated with cell surface CD4 at 40 and 90 min of chase (Fig. 7). Thus, it appears that the initial association between newly synthesized Lck and CD4 occurs on intracellular membranes and that Lck is subsequently transported to the plasma membrane. Little if any newly synthesized Lck associates directly with CD4 at the plasma membrane although >95% of the total amount of CD4 is located at this site. Given our observation that binding of Lck to CD4 occurs very rapidly after membrane binding (Fig. 5, B and C), the initial membrane association of Lck most likely occurs at the same intracellular site as CD4 binding.
BFA Blocks Transport of CD4-associated Lck
The transfer of newly synthesized Lck from intracellular membranes to the plasma membrane with time suggests that CD4-associated Lck is transported via the exocytic pathway. To further investigate this, we studied transport of Lck in the presence and absence of the drug BFA. BFA causes disruption of the Golgi apparatus and blocks transport from the ER and Golgi complex, but not from the TGN to the plasma membrane (Klausner et al., 1992). The drug was added 2 min before pulse-labeling and remained present during the chase. We again followed transport of Lck by its association with cell surface versus intracellular CD4. In the absence of BFA, the amount of cell surface CD4 increased with time (Fig. 8, A and C), while in its presence all CD4 remained intracellular, consistent with an inhibition of transport via the secretory pathway. Similarly, the relative amount of cell surface–bound Lck at 30 and 60 min of chase was significantly reduced in the presence of BFA (Fig. 8, A and B), suggesting that the newly synthesized Lck was associated with early compartments of the exocytic pathway and its transport to the plasma membrane inhibited by BFA. The block in transport caused by BFA was not as complete for Lck as for CD4: some Lck associated with nonlabeled cell surface CD4 at 30 and 60 min chase (Fig. 8, A and B). This minor fraction of Lck might be targeted from its site of synthesis to the plasma membrane directly.
CD4 synthesized in the presence of BFA coimmunoprecipitated with anti-Lck antibodies albeit to a lower extent than in the absence of BFA (Fig. 9 A). This is consistent with the notion that CD4 associates with Lck on an early exocytic compartment.
Figure 9.
Binding of Lck to newly synthesized CD4 and membranes in the presence of BFA. (A) SupT1 cells were pulse-labeled for 5 min and chased for 40 min in the absence or presence of BFA (10 μg/ml during pulse, 2 μg/ml during chase). Pretreatment with BFA was 2 min before the pulse. NP-40 cell lysates were subjected to immunoprecipitation, first with anti-Lck (αLck lanes), next with anti-CD4 antibodies (αCD4). Lck, CD4, and a background band (★) are indicated. (B) SupT1 cells were pretreated and labeled for 5 min in the presence of BFA (10 μg/ml) and chased for indicated times in the presence of 2 μg/ml BFA. Lck was immunoprecipitated at indicated chase times from membrane (M) and soluble (S) fractions.
The palmitoylation of the cytosolic proteins SNAP-25 and GAP43, but not of the alpha subunits of heterotrimeric G proteins, was shown to be inhibited in the presence of BFA (Gonzalo and Linder, 1998). Our data suggest that palmitoylation of Lck is not affected by BFA since its association with CD4 occurs normally (Fig. 8). We also studied membrane binding of Lck in the presence of BFA and found no difference in rates compared with untreated cells (Fig. 9 B), suggesting that Lck palmitoylation indeed occurred normally. Furthermore, incorporation of [3H]palmitic acid into Lck was not decreased in the presence of BFA (not shown). Thus, the reduction in the amount of Lck associated with cell surface CD4 in the presence of BFA (Fig. 8, A and B) is most likely caused only by the inhibition of transport through the exocytic pathway.
Discussion
To understand the mechanism(s) through which acylated cytosolic proteins are targeted to their functional locations in cells, we have studied the early events in the membrane binding and cellular localization of the Src-related tyrosine kinase Lck. We have shown previously that Lck contains intrinsic targeting signals in its NH2-terminal unique domain that direct the protein to the cytosolic leaflet of the plasma membrane (Bijlmakers et al., 1997). In the present study we have used pulse–chase analysis to investigate how newly synthesized Lck is delivered to this location. Our data indicate that in the lymphoid T cell line SupT1, a large proportion of Lck is initially targeted to an intracellular compartment, most likely an early station of the exocytic pathway, where it becomes stably membrane-bound and can interact with CD4, before it is transported to the cell surface.
Membrane binding of Lck is a relatively slow process; it starts soon after synthesis but is not complete until 30–45 min later. An Lck mutant that cannot be palmitoylated is unable to associate with membranes (Kwong and Lublin, 1995; Yurchak and Sefton, 1995; Bijlmakers et al., 1997; Zlatkine et al., 1997), indicating that palmitoylation is required for stable membrane interaction of Lck. Membrane association has similar kinetics in the presence or absence of CD4 and CD8 (Fig. 3). Thus, the rate of membrane association likely reflects the rate of Lck palmitoylation and not its interaction with these transmembrane proteins. Membrane binding has been studied for only a few acylated proteins and at present it remains unclear what elements are important for the kinetics of this process. Between different T cell lines, we did observe slight differences in membrane-binding rates. However, all our kinetics for Lck differ significantly from those measured by others for another Src-kinase, Fyn, which is completely membrane-bound within 5 min of synthesis (van't Hof and Resh, 1997). The rapid membrane association of Fyn was shown to require myristoylation at the NH2-terminal glycine and palmitoylation at cysteine 3 (van't Hof and Resh, 1997). Significantly, both Lck and Fyn are myristoylated and can be palmitoylated at cysteine 3 (Resh, 1994). In addition, Lck can also be palmitoylated at cysteine 5 and Fyn at cysteine 6. Cysteine 3 was found to be the major palmitoylation site for both proteins (Alland et al., 1994; Shenoy-Scaria et al., 1994; Yurchak and Sefton, 1995), although a conflicting report exists for Lck (Rodgers et al., 1994). Ostensibly, the modifications appear similar for the two proteins, suggesting that the acylations alone do not determine the rate of membrane association and that other features of the unique domains might be important. One difference between the unique domains of Lck and Fyn is the presence of two cysteines in Lck (Cys 20 and 23) that are required for the interaction with the cytoplasmic domains of CD4 and CD8. However, we excluded a role for this interaction in membrane-binding kinetics of Lck, since the kinetics are similar in the presence and absence of CD4/CD8. Another difference between the unique domains of Lck and Fyn is the absence of positively charged amino acids in the NH2-terminal 30 residues of Lck, while five positively charged amino acids are present in the corresponding region of Fyn. Given that Src requires positively charged amino acids for membrane association (Silverman and Resh, 1992), it is possible that these charges in Fyn also facilitate membrane binding. It is noteworthy that a mutant Fyn which is myristoylated but not palmitoylated can associate with membranes to some extent (Alland et al., 1994; Shenoy-Scaria et al., 1994; Wolven et al., 1997), whereas the corresponding Lck mutant does not (Kwong and Lublin, 1995; Yurchak and Sefton, 1995; Bijlmakers et al., 1997; Zlatkine et al., 1997). This implies that, in contrast to Lck, newly synthesized, myristoylated Fyn might have some affinity for membranes and as a result may translocate faster to membranes than Lck. van't Hof and Resh (1997) showed that NH2-terminal amino acid substitutions, apart from the myristoylation and palmitoylation sites at Gly 2 and Cys 3, respectively, did not change membrane-binding rates for Fyn. However, all their reported mutants contained at least one positively charged amino acid in the NH2-terminal 10 residues.
The interaction of newly synthesized Lck with CD4 occurred with similar kinetics to its interaction with membranes (Figs. 2 and 5). CD4 binding must succeed membrane association since nonpalmitoylated Lck is unable to associate with CD4 (Turner et al., 1990). Consistent with this, we observed the earliest CD4 association of Lck shortly after the earliest detectable membrane association (Fig. 5). This suggests that CD4 binding follows rapidly upon membrane binding, and therefore upon palmitoylation of Lck. Thus, it is likely that all three events (palmitoylation, membrane binding, and CD4 binding) occur at the same location in the cell. While we have no direct data concerning the site where palmitoylation or initial membrane association occurs, several results suggest that the initial interaction with CD4 takes place on an early compartment of the exocytic pathway. First, the association of newly synthesized CD4 with Lck starts within 10 min of CD4 synthesis (Fig. 6 A), at a time when CD4 has not yet reached the plasma membrane and is located in the exocytic pathway (Fig. 6 B). Secondly, newly synthesized Lck associates initially with intracellular CD4 and only at later times (after 40 min of chase) with cell surface CD4 (Fig. 7). Thirdly, in the presence of BFA, transport of CD4-associated Lck to the plasma membrane was inhibited (Fig. 8). Previously, Crise and Rose (1992a) showed that Lck can interact with CD4 retained in the ER. Interaction at the ER was also found in cells overexpressing both Lck and a chimera containing the VSV G protein ectodomain and the cytoplasmic tail of CD4 (Shaw et al., 1989). However, in these latter experiments the CD4 construct resides in the ER for a long time, at least 60 min after synthesis (Shaw et al., 1989). The pulse–chase experiments described here show that the interaction of CD4 with Lck takes place early in the exocytic pathway irrespective of expression levels or mislocalizations of CD4 and/or Lck. Our previous immunofluorescence experiments with transfected HeLa cells indicate that CD4 and Lck can interact at the Golgi complex and travel to the plasma membrane together (Bijlmakers et al., 1997). Thus, the normal route for the bulk of newly synthesized Lck is initial association with intracellular CD4 followed by delivery to the plasma membrane. The BFA experiments (Figs. 8 and 9 A) support this conclusion and further indicate that newly synthesized Lck associates with CD4 at a compartment located before the TGN (Klausner et al., 1992).
The ability of newly synthesized CD4 to associate with Lck in the presence of BFA (Fig. 9 A) and the observation that Endo H–sensitive CD4 coimmunoprecipitates with Lck (not shown) suggest that Lck can associate with membranes of the ER or intermediate compartment. Since palmitoylation is required for stable membrane association of Lck, our results imply that palmitoylation of newly synthesized Lck occurs on intracellular membranes of the early exocytic pathway. To date, palmitoyl transferase activity has been detected at the plasma membrane (Dunphy et al., 1996; Schroeder et al., 1996), intermediate compartment (Bonatti et al., 1989), Golgi complex (Solimena et al., 1994; Dunphy et al., 1996), and mitochondria (Dunphy et al., 1996). Palmitoyl transferase activities have been partially purified (Berthiaume and Resh, 1995; Dunphy et al., 1996; Liu et al., 1996), but the number of different palmitoyl transferases in the cell, their specificities, and subcellular distributions are unknown. Several transmembrane proteins are palmitoylated early after synthesis in the ER or intermediate compartment (Bonatti et al., 1989; Veit and Schmidt, 1993) and the cytosolic GAD65 (Solimena et al., 1994) requires targeting to the Golgi for palmitoylation. Possibly, newly synthesized Lck uses the same palmitoyl transferase(s) as these proteins. Palmitoylation of two cytosolic proteins, SNAP25 and GAP43, is inhibited by BFA, suggesting that an intact secretory pathway is required for the palmitoylation of these proteins (Gonzalo and Linder, 1998). On the other hand, palmitoylation of several viral transmembrane proteins is not affected by BFA (Veit and Schmidt, 1993; Uleato et al., 1995). Also for Lck, palmitoylation in the presence of BFA is apparently normal since membrane-binding kinetics (Fig. 9 B) and palmitic acid incorporation (not shown) were unaffected. Currently, there are no direct data concerning the cellular sites of palmitoylation of Src-family proteins. It has been suggested that this modification can occur at the plasma membrane since a short fluorescent peptide containing the first three amino acids of Lck (myrGlyCysGly) is palmitoylated and incorporates into the plasma membrane when added to intact cells (Schroeder et al., 1996). Palmitoylation of Lck is reversible (Paige et al., 1993), suggesting that the protein undergoes cycles of de-palmitoylation and re-palmitoylation. The re-palmitoylation might well occur at the plasma membrane where most steady-state Lck is located. However, the experiments described here indicate that newly synthesized Lck is palmitoylated on early membranes of the exocytic pathway.
The delivery of Lck from an intracellular membrane compartment to the plasma membrane in a BFA-sensitive manner presents a novel transport route for an acylated, cytosolic protein. The routes of only two other acylated proteins have been studied: Fyn which is myristoylated and palmitoylated, and the Gag protein of Moloney murine leukemia virus, which is myristoylated only. In contrast to Lck, both of these proteins were found to be targeted to the plasma membrane directly (Suomalainen et al., 1996; van't Hof and Resh, 1997). Fyn resembles Lck in the nature of its acylations, its expression in T lymphocytes, and its role in signaling through the TcR. However, while Lck interacts with CD4 and/or CD8 (Rudd et al., 1988; Veillette et al., 1988), no high stoichiometry interactions with transmembrane proteins have been identified for Fyn. Although expression of CD4 and CD8 does not affect steady-state localization nor membrane-binding kinetics of Lck, these proteins could still influence the transport route of Lck. If, for instance, newly synthesized CD4/CD8 is the only pool capable of interacting with Lck, targeting of Lck to early exocytic compartments would be required to facilitate efficient assembly of the coreceptor–Lck complexes. Possibly, Lck contains intrinsic signals for targeting to an intracellular membrane compartment. Alternatively, the presence of available CD4/CD8 might recruit newly synthesized Lck to early exocytic compartments and thereby favor palmitoylation and membrane association at these sites. Newly synthesized, nonpalmitoylated Lck is located primarily in the cytosol and shows no stable interaction with CD4. However, a weak interaction might occur since soluble Lck associates with CD4 in the yeast two-hybrid system (Campbell et al., 1995) and when coexpressed in Escherichia coli (Huse et al., 1998). Determining the precise site(s) of the initial Lck interaction with membranes and the influence of CD4/CD8 in defining this site will require further analysis. Nevertheless, the experiments reported here indicate that palmitoylation of Lck occurs on intracellular membranes and that Lck is transported to the plasma membrane via the exocytic pathway. Further characterization of the cellular and molecular mechanisms that underlie this transport will be essential for understanding the properties and functions of acylated proteins.
Abbreviations used in this paper
- BFA
Brefeldin A
- Endo H
endoglycosidase H
- Lck
p56lck
- TcR
T cell receptor
- wt
wild-type
Footnotes
We especially thank Jannie Borst for providing the anti-Lck serum which was crucial for immunoprecipitations, Karin Römisch and Kate Bowers for critically reading the manuscript, and our colleagues in the MRC-LMCB for helpful discussions. We would further like to thank Philippe Benaroch (Curie Institut, Paris, France) for Jurkat cells.
The work was supported by the UK Medical Research Council and by a long-term EMBO fellowship to M.-J.J.E. Bijlmakers.
References
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;24:16701–16705. [PubMed] [Google Scholar]
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–21. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJE, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S, Migliaccio G, Simons K. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989;264:12590–12595. [PubMed] [Google Scholar]
- Brouns GS, de Vries E, van Noesel CJ, Mason DY, van Lier RA, Borst J. The structure of the mu/pseudo light chain complex on human pre-B cells is consistent with a function in signal transduction. Eur J Immunol. 1993;23:1088–1097. doi: 10.1002/eji.1830230517. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Buder A, Deuschle U. Interactions between the amino-terminal domain of p56lck and cytoplasmic domains of CD4 and CD8 alpha in yeast. Eur J Immunol. 1995;25:2408–2412. doi: 10.1002/eji.1830250842. [DOI] [PubMed] [Google Scholar]
- Crise B, Rose JK. Human immunodeficiency virus type 1 glycoprotein precursor retains a CD4-p56lckcomplex in the endoplasmic reticulum. J Virol. 1992a;66:2296–2301. doi: 10.1128/jvi.66.4.2296-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crise B, Rose JK. Identification of palmitoylation sites on CD4, the human immunodeficiency virus receptor. J Biol Chem. 1992b;267:13593–13597. [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Eck MJ, Harrison SC. A Zn2+ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J Biol Chem. 1998;273:18729–18733. doi: 10.1074/jbc.273.30.18729. [DOI] [PubMed] [Google Scholar]
- Kaplan K, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Ashwell G, Hanover JA. Glycosylation of CD4. Tunicamycin inhibits surface expression. J Biol Chem. 1988;263:9502–9507. [PubMed] [Google Scholar]
- Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal for membrane binding of p56lck . Biochem Biophys Res Commun. 1995;207:868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Vetter ML, Bishop JM, Kelly RB. Specific association of the proto-oncogene product pp60c-src with an intracellular organelle, the PC12 synaptic vesicle. J Cell Biol. 1992;117:1077–1084. doi: 10.1083/jcb.117.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Dudler T, Gelb MH. Purification of a protein palmitoyltransferase that acts on H-Ras protein and on a C-terminal N-Ras peptide. J Biol Chem. 1996;271:23269–23276. doi: 10.1074/jbc.271.38.23269. [DOI] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Mohn H, Le Cabec V, Fischer S, Maridonneau-Parini I. The src-family protein-tyrosine kinase p59hck is located on the secretory granules in human neutrophils and translocates towards the phagosome during cell activation. Biochem J. 1995;309:657–665. doi: 10.1042/bj3090657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige LA, Nadler MJS, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993;268:8669–8674. [PubMed] [Google Scholar]
- Pelchen-Matthews A, Armes JE, Griffiths G, Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991;173:575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochim Biophys Acta. 1993;1155:307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider LR. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci USA. 1980;77:3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci USA. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Janssen O, Prasad KVS, Raab M, da Silva A, Telfer JC, Yamamoto M. Src-related protein tyrosine kinases and their surface receptors. Biochim Biophys Acta. 1993;1155:239–266. doi: 10.1016/0304-419x(93)90007-y. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Leventis R, Shahinian S, Walton PA, Silvius JR. Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently S-acylated at the plasma membrane of mammalian cells. J Cell Biol. 1996;134:647–660. doi: 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AS, Amrein KE, Hammond C, Stern DF, Sefton BM, Rose JK. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Chalupny J, Whitney JA, Hammond C, Amrein KE, Kavathas P, Sefton BM, Rose JK. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lcktyrosine protein kinase. Mol Cell Biol. 1990;10:1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L, Resh MD. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristoylated pp60v-src. J Cell Biol. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Jr, Radzynski M, Mundigl O, De Camilli P. A signal located within amino acids 1–27 of GAD65 is required for its targeting to the Golgi complex region. J Cell Biol. 1994;126:331–341. doi: 10.1083/jcb.126.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Hultenby K, Garoff H. Targeting of Moloney murine leukemia virus gag precursor to the site of virus budding. J Cell Biol. 1996;135:1841–1852. doi: 10.1083/jcb.135.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Uleato D, Grosenbach D, Hruby DE. Brefeldin A inhibits vaccinia virus envelopment but does not prevent normal processing and localization of the putative envelopment receptor P37. J Gen Virol. 1995;76:103–111. doi: 10.1099/0022-1317-76-1-103. [DOI] [PubMed] [Google Scholar]
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veit M, Schmidt MF. Timing of palmitoylation of influenza virus hemagglutin. FEBS Lett. 1993;336:243–247. doi: 10.1016/0014-5793(93)80812-9. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol. 1995;15:6914–6922. doi: 10.1128/mcb.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]