Abstract
Neurons use kinesin and dynein microtubule-dependent motor proteins to transport essential cellular components along axonal and dendritic microtubules. In a search for new kinesin-like proteins, we identified two neuronally enriched mouse kinesins that provide insight into a unique intracellular kinesin targeting mechanism in neurons. KIF21A and KIF21B share colinear amino acid similarity to each other, but not to any previously identified kinesins outside of the motor domain. Each protein also contains a domain of seven WD-40 repeats, which may be involved in binding to cargoes. Despite the amino acid sequence similarity between KIF21A and KIF21B, these proteins localize differently to dendrites and axons. KIF21A protein is localized throughout neurons, while KIF21B protein is highly enriched in dendrites. The plus end-directed motor activity of KIF21B and its enrichment in dendrites indicate that models suggesting that minus end-directed motor activity is sufficient for dendrite specific motor localization are inadequate. We suggest that a novel kinesin sorting mechanism is used by neurons to localize KIF21B protein to dendrites since its mRNA is restricted to the cell body.
Keywords: kinesin, protein sorting, WD-40 repeats, neuron transport, dendrite
The extensive lengths of axons and dendrites present a unique intracellular transport problem for neurons. For example, proteins synthesized in the cell body of spinal motor neurons must be transported down the axon to the synapse where they function. In humans, these axons can be up to a meter in length. On the other hand, a Purkinje cell possesses an enormous, extensively branched dendritic array whose aggregate length rivals that of even the longest axons.
The identification of molecular motors such as dynein (Paschal et al., 1987; Schnapp and Reese, 1989; Hirokawa et al., 1990) and kinesin (Brady, 1985; Scholey et al., 1985; Vale et al., 1985) that are capable of movement toward opposite ends of microtubules provides a solution to the intracellular transport problems faced by neurons. Since the identification of kinesin and dynein, numerous related neuronal kinesin-like proteins (KLPs1; Aizawa et al., 1992; Nakagawa et al., 1997; Yang et al., 1997) have been identified indicating that kinesin and dynein are not the only molecular motors that function in neuronal transport. Some examples are Unc104/KIF1A, which transports a subset of synaptic vesicle precursors (Hall and Hedgecock, 1991; Okada et al., 1995); KIF1B, which transports mitochondria (Nangaku et al., 1994); KIF2, which transports the insulin like growth factor receptor βgc to extending neurites (Morfini et al., 1997); and KIFC2, which may transport multivesicular bodies in dendrites (Hanlon et al., 1997; Saito et al., 1997).
The uniform orientation of axonal microtubules, with plus ends toward the synapse and minus ends toward the cell body (Burton and Paige, 1981; Heidemann et al., 1981), allow plus end-directed kinesins to transport anterograde cargo to the synapse and minus end-directed kinesins and dyneins to transport retrograde cargo to the cell body (Black and Baas, 1989). Directed microtubule based transport within dendrites, however, is more complicated since microtubule polarity is mixed (Baas et al., 1988; Burton, 1988). Current models suggest that minus end-directed kinesins and dynein are responsible for localizing dendrite specific cargoes, since these motors largely will be excluded from the axon owing to the orientation of the axonal microtubules, unless these motors are first transported down the axon by a plus end-directed motor (Black and Baas, 1989).
Using a PCR based strategy, we identified two closely related plus end-directed KLPs, KIF21A and KIF21B, which provide insight into targeting of KLPs to neuronal processes. KIF21A protein distribution throughout neurons is similar to that observed for other plus end-directed KLPs. However, KIF21B protein is enriched in dendrites compared with the cell body and axon. This localization pattern has not been previously observed for any other kinesin motors and suggests that KIF21B utilizes a previously unrecognized dendritic protein sorting mechanism.
Materials and Methods
Identification of cDNA
Degenerate PCR was performed on a mouse retinal cDNA (oligo-dT primed) library using primers to conserved motor domain amino acids, MGKTY/FTM (EcoRI · GGG/A/T/CAAA/GACG/A/T/CTA/TT/ CACG/A/T/CATG) and DLAGSE (BamHI · TCA/GCTG/A/T/CCCG/ A/T/CGCC/TAAA/GTC). PCR products were cloned into pBluescript (Stratagene) using the EcoRI and BamHI sites from the oligos and sequenced. One novel cDNA (KIF21A) was identified and used to screen the retinal cDNA library from which it was isolated. Five overlapping clones containing the entire coding sequence of KIF21A were identified from the dT primed retinal library, as well as a random primed retinal library (B6 strain mouse retina), and sequenced. A second gene, KIF21B, was isolated due to its cross hybridization to a KIF21A cDNA probe. The entire KIF21B coding sequence is contained on two overlapping clones. The GCG sequence analysis software package (Devereux et al., 1984) was used to align the KIF21A and KIF21B amino acids sequences (gap program) as well as produce a dendrogram comparing the motor domain core amino acids of many KLPs (from IFAY to LAGSE).
Mapping of KIF21A and KIF21B to Chromosome Location
The murine chromosomal locations of KIF21A and KIF21B genes were determined using an interspecific backcross DNA panel obtained from The Jackson Laboratories (Rowe et al., 1994). The panel consists of 94 F2 progeny from a (C57BL/6J × SPRET/Ei) F1 female mated to a SPRET/Ei male and DNA from parental C57BL/6J and Mus spretus. ScaI polymorphisms between SPRET/Ei and C57BL/6J mouse strains were identified by Southern blotting for KIF21A (using bp 640–1800) and KIF21B (bp 2880–3900). The distribution of the C57BL/6J allele among the F1 progeny was used to estimate gene locus position and linkage distance by The Jackson Laboratories. Human chromosomal locations that are syntenic to the mouse regions were determined by using the map generated by DeBry and Seldon (1996).
Northern Blot Analysis
Total RNA was isolated from various mouse tissues using the guanidinium isothiocyanate extraction method as previously described (Chomczynski and Sacchi, 1987). RNA amount and purity was determined by absorbance at 260 nm and the 260/280 nm ratio using a spectrometer. 30 μg of total RNA was separated on a 1% formaldehyde agarose gel, transferred to GeneScreen Plus membrane (New England Nuclear), and fixed by baking. The blots were probed with random primed DNA (KIF21A; bp 3400–5000) and (KIF21B; bp 3000–5400) with α[32P]dATP incorporation in a buffer of 0.5 M NaPO4 and 7% SDS at 65°C for 16 h. Blots were washed in 0.1× SSC/1% SDS at 65°C, twice for 30 min. Washed blots were exposed to Biomax MS film (Kodak).
Preparation of Antibodies
A KIF21A-HIS fusion protein construct which contained amino acids 1124–1355 (KLCG to QINQ) was generated in pET-23b (Novagen, Inc.). A comparable KIF21B-HIS fusion protein containing amino acids 1135– 1419 (KFKG to QINQ) was also generated in pET-23b. These fusion proteins were grown in pLysS BL21(DE3) bacteria, induced with 0.5 mM IPTG, and purified using Ni-NTA–agarose (Qiagen, Inc.). Each fusion protein was further purified by SDS-PAGE. Gel slices (containing 300 μg of fusion protein) were injected into three rabbits to produce polyclonal sera against KIF21A or KIF21B. To generate antisera that recognize only KIF21A or KIF21B, each antiserum was incubated with the conflicting Affigel (Bio-Rad Laboratories) bound fusion protein to immunodeplete antibodies that recognize both KIF21 proteins. Immunodepletion was confirmed by Western immunoblot analysis of a dilution series of known amounts of KIF21A and KIF21B fusion proteins. Affinity-purified antibody was generated by incubating each antiserum with its Affigel-bound antigen used in its generation. The antibody was then released from the column by lowering the pH.
Western Blot Analysis
Tissue was homogenized in 25 mM sodium phosphate, 5 mM EDTA, 1% SDS, pH 7.5, buffer using a polytron. Protein concentrations were determined using the Bio-Rad Dc protein assay kit. Protein samples were separated on a SDS-PAGE gel using standard Laemmli method and then transferred to PVDF membrane (Bio-Rad), dried, and blocked in 5% dry milk in 1× TBS/0.05% Tween for 1 h. Primary antibodies were incubated 1 h in the same solution at room temperature, washed, incubated with an HRP-conjugated secondary antibody, and then washed again. ECL (Nycomed-Amersham Inc.) was used for detecting the antibodies by exposing the blots to X-OMAT-XAR5 (Kodak) film. Antibody concentrations used: polyclonals α-KIF21A and α-KIF21B whole serum, 1:1,000; α-KIF3A, 1:5,000; nKHC, 1:2,000 (a gift from Dr. Ron Vale, UCSF); monoclonals α-synaptotagmin, 1:500; Stressgen α-SV2, 1:50 (Hybridoma Bank, University of Iowa).
Microtubule Binding Assay
A mouse brain was homogenized in 3 ml microtubule binding buffer (0.1 M Pipes, 0.9 M glycerol, 5 mM EGTA, 0.5 mM EDTA, 2.5 mM MgSO4, pH 6.4) using a glass dounce homogenizer (Kontes). The homogenate was centrifuged at 25,000 rpm and 25°C in a Sorvall 1270 rotor for 20 min. The supernatant was recentrifuged for 30 min to remove vesicles and endogenous microtubules. 90 μl of supernatant was supplemented with 10 μl of purified taxol stabilized mouse microtubules (3 mg/ml). To this, 10 μl of either 0.1 M Mg · ATP, or 1 μl 0.5 M Mg · AMP-PNP was added for 15 min at room temperature. Then the samples were layered onto 100 μl of a 40% sucrose cushion in the same buffer and centrifuged for 30 min at 25,000 rpm and 25°C in a Sorvall 42.2 TI rotor. Taxol was added to 10 mM in all the buffers. Pellets were resuspended in 1× SDS-PAGE sample buffer (10% glycerol, 62.5 mM Tris-HCl, pH 6.8, 2% SDS, 700 mM β-mercaptoethanol) and analyzed by Western blotting.
Immunoprecipitations
A mouse brain was homogenized in 4 ml cell fractionation buffer (20 mM Hepes, 100 mM sodium aspartate, 40 mM KCl, 5 mM EGTA, 5 mM MgCl2, 2 mM Mg-ATP, 1 mM DTT, pH 7.2) supplemented with protease inhibitors (to 1 mM PMSF, 0.7 ng/ml pepstatin A, 10 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor; final concentrations). The homogenate was centrifuged twice at 8,800 rpm 10 min each in a Sorvall SS34 rotor. 1.2 ml of lysate was preabsorbed with 100 μl of protein A–Sepharose beads (Pharmacia Biotech, Inc.). 5 μl of immunodepleted KIF21A or KIF21B whole serum was added to the lysate and incubated 1.5 h at 4°C on a rotator. 20 μl of protein A beads were added to lysate for 2 h at 4°C on a rotator. Proteins were eluted by boiling 5 min with 75 μl of 1× SDS-PAGE sample buffer without β-mercaptoethanol and spun in microfuge. To the supernatant, 25 μl of 4× buffer + β-mercaptoethanol was added and boiled for 5 min more. 15 μl of each sample was analyzed by Western immunoblotting.
Cell Fractionation
Cell fractionation was performed using a modified version of the protocol described by Okada et al. (1995). In brief, one mouse brain was homogenized on ice with a glass dounce homogenizer in 3 ml of CF buffer (see Immunoprecipitations section). The homogenate was spun at 3,000 gavg for 5 min, 9,000 gavg for 10 min, then centrifuged in a Sorvall 1270 rotor at 100,000 gavg for 1 h. 50 μg of total protein from each fraction, as determined using Bio-Rad Dc protein assay kit, was separated on 7.5% polyacrylamide gels and transferred to PVDF membrane (Bio-Rad Laboratories) for Western immunoblotting. P3 pellets were extracted by homogenization with a dounce homogenizer and recentrifuged at 100,000 gavg for 1 h. The pellet was resuspended in the starting volume and equal volumes of the pellet and supernatant were analyzed by Western immunoblotting.
Construction of KIF21B Motor Protein
A KIF21B motor construct (amino acids 1–750) was generated by PCR with the following primers that contained either a NdeI or XhoI restriction enzyme site (5′-CTG GTG CCG GAG CAT ATG GCT GGC CAG GGC, and 3′-CGC TTG TAG CTT CTC GAG CTC CCT TTC ATA). The PCR product was cloned into the NdeI and XhoI sites of pET-23b (Novagen Inc.). The construct was introduced into BL21 (DE3) bacteria and cells were grown at 37°C until an OD600 ∼1.5 and then induced with 0.5 mM IPTG overnight at room temperature. Cells were harvested by centrifugation and resuspended in lysis buffer (300 mM NaCl, 50 mM sodium phosphate, 0.5 mM MgCl2, 0.01% NP-40, 10 μg/ml soybean trypsin inhibitor, 0.7 μl/ml β-ME, 1 mM PMSF, 0.1 μM ATP, pH 7.4) at 1 g/5 ml. Cells were lysed three times with a French press and then spun for 45 min at 30,000 rpm in a 647.5 Sorvall rotor at 4°C. KIF21B-HIS protein was isolated by incubating the high speed supernatant with 0.5 ml of Ni-NTA– agarose beads (Qiagen Inc.) for 2 h. The beads were washed three times with lysis buffer supplemented with 25 mM imidazole and 1 μM ATP, and protein was eluted with lysis buffer + 200 mM imidazole and 1 μM ATP. Protein was concentrated by centrifuging the protein in a Millipore-4 concentrator.
In Vitro Motility Assay
Polarity marked microtubules were generated by the method described in Howard and Hyman (1993), using unlabeled bovine tubulin, rhodamine-labeled tubulin (Cytoskeleton), and N-ethyl maleimide tubulin (Hyman et al., 1991). 5 μl of 0.5 mg/ml KIF21B motor protein was absorbed onto a coverslip for 3 min and then washed with PEM40 buffer (40 mM Pipes, pH 6.9, 1 mM EGTA, 1 MgCl2) for 1 min. The motility assay was performed by adding the following components: 1:1,000 polarity marked microtubules; 1 mM MgATP; 10 μM taxol; and an oxygen scavenging system (0.1 mg/ml catalase, 0.03 mg/ml glucose oxidase, 10 mM glucose, 0.1% β-ME; Kishino and Yanagida, 1988) to PEM40 buffer. Images were gathered using a Zeiss Axioplan fluorescence microscope, a Princeton Instruments cooled CCD, and the MetaMorph software package (Universal Imaging Corp.).
Immunofluorescence
Animals were killed by suffocation with carbon dioxide and their circulatory system flushed by intracardial perfusion of 1× PBS, followed by fixation with 4% paraformaldehyde in 0.1 M PO4. Tissue was dissected and cryoprotected by overnight incubation in 20% sucrose/1× PBS at 4°C. Tissue was then embedded in OCT compound and 10–15 μm sections were cut on a cryostat. Sections were dried 1 h at room temperature and then blocked with 1× PBS/0.1% Triton X-100/1% BSA for 1 h. Tissue was incubated in 1° antibody for 1 h at room temperature in incubation buffer (1× PBS/0.1% Triton X-100/1% BSA), washed, incubated with the appropriate 2° antibody for 1 h, washed, and then mounted with Vectashield (Vector Labs, Inc.). Tissue was observed using a Bio-Rad MRC-1000 scanning confocal imaging system. Antibody concentrations used: affinity-purified KIF21A and KIF21B, 1 μg/ml; SMI-31, 1:1,000 (Sternberger and Sternberger); MAP2, 1:400 (Boehringer Mannheim); goat α–rabbit Cy5 1:200 (Jackson ImmunoResearch Laboratories); and goat α–mouse Texas red, 1:200 (Jackson ImmunoResearch Laboratories).
In Situ Hybridization
Adult BALB/C mice were killed by cervical dislocation and isolated brains were frozen using Tissue-Tek OCT (Miles Inc.) and Histofreeze (Fisher Scientific Co.). Parasagittal cryostat sections (20 μm) were cut, thaw-mounted onto charged microscope slides (Superfrost Plus; Fisher Scientific Co.), fixed, and processed as previously described (Chun et al., 1991). Digoxigenin-labeled riboprobes were transcribed in the sense and antisense orientations from linearized plasmids containing: KIF21A, nucleotide (nt) 2720 (BamHI) to nt 4260 (EcoO109); KIF21B, nt 3020 (HindIII) to nt 4430 (EcoRV); and est W46113 for MAP2 cDNAs using standard protocols (Boehringer Mannheim Corp.). Hybridization and color reaction were carried out as previously described (Weiner and Chun, 1997).
Results
Identification and Chromosomal Mapping of KIF21A and KIF21B Genes
To identify novel KLPs that may be involved in axonal and/or dendritic transport, a degenerate PCR based screen (see Yang et al., 1997) was performed using a retinal mouse cDNA library. Two novel KLPs, which we named KIF21A and KIF21B, as well as many previously reported KLPs sequences were identified. Based on the cDNA sequences, KIF21A protein is predicted to contain 1,573 amino acids, while KIF21B protein is predicted to contain 1,668 amino acids (Fig. 1 A).
Figure 1.
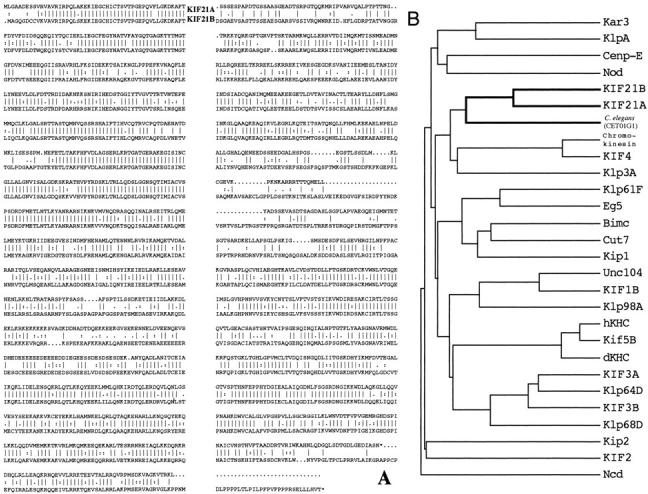
KIF21A and KIF21B identify a new family of KLPs. (A) Alignment of KIF21A and KIF21B amino acid sequences. Solid lines, amino acid identity; dots, amino acid similarities. (B) Dendrogram analysis comparing KIF21A and KIF21B motor domains to many previously identified KLPs.
To determine whether known mouse mutants or human diseases mapped to the same chromosomal regions as the KIF21A and KIF21B genes, the chromosomal location of each KIF21 gene was established using The Jackson Laboratories SBS backcross panel (see Materials and Methods). The KIF21A gene maps to 39.7 on mouse chromosome 15 (syntenic to human chromosome 8 at ∼8q24), and KIF21B maps to 64.7 on chromosome 1 (syntenic to human chromosome 2 at ∼2cen-q21). The unique chromosome locations establish KIF21A and KIF21B as independent genes, but no known mouse mutants or human diseases map close to these chromosomal locations.
KIF21A and KIF21B Define a Novel KLP Family that Contains WD-40 Repeats
Members of a protein family often share a high degree of amino acid similarity, as well as common protein motifs. A comparison of the core amino acids of the KIF21A and KIF21B motor domains to previously identified KLPs reveals that KIF21A and KIF21B are most similar to each other and a Caenorhabditis elegans KLP sequence (CET01G1) identified during the C. elegans genome sequencing project (Fig. 1 B).
KIF21A and KIF21B proteins share 61% amino acid sequence identity along their entire length (Fig. 1 A) with the highest identity in the NH2-terminal 25% and COOH-terminal 25% of the proteins. Like true kinesin, KIF21A and KIF21B proteins are comprised of three functional domains: an NH2-terminal head motor domain (1–400), a predicted coiled-coil stalk (data not shown; 400–1,000), and COOH tail (1,000 to end; Fig. 2 A). Both proteins have a cluster of negatively charged amino acids of unknown function within their stalk domain and seven consensus WD-40 repeats (van der Voorn and Ploegh, 1992; Neer et al., 1994) in their tails (Fig. 2, A and B). WD-40 repeats were first identified in β-transducin (Simon et al., 1991), and subsequently have been found in numerous, functionally unrelated proteins and are believed to be involved in protein–protein interactions (Fig. 2 B; see reviews van der Voorn and Ploegh, 1992; Neer et al., 1994). Thus, the KIF21 family of KLPs may mediate interactions with their cargoes through these WD-40 domains.
Figure 2.
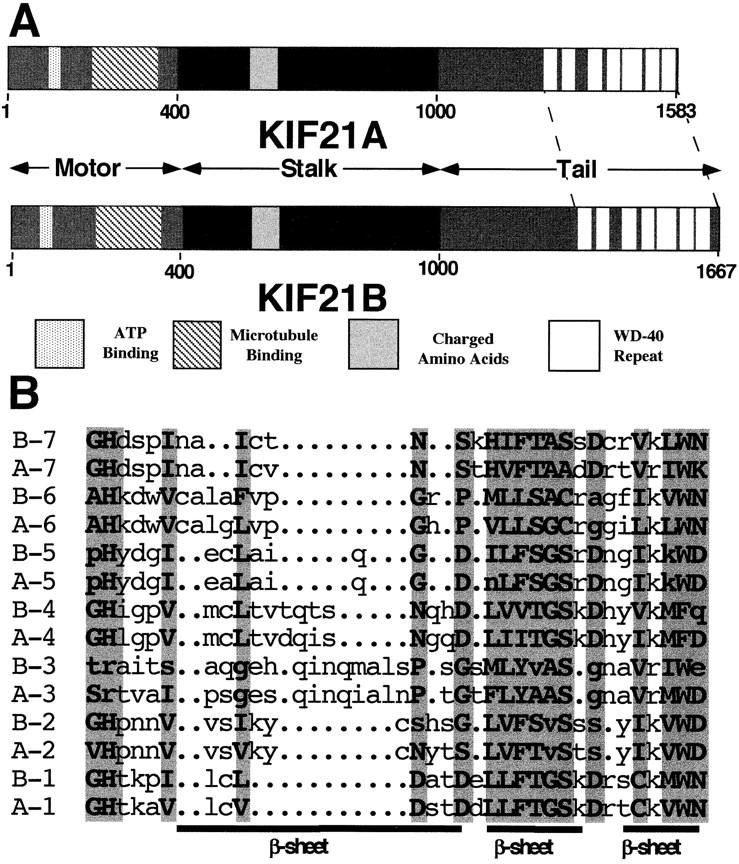
KIF21A and KIF21B protein structures and WD-40 repeat core amino acids. (A) Cartoon depicting sequence motifs and domains in KIF21A and KIF21B. (B) Alignment of WD-40 repeat amino acids of KIF21A and KIF21B. Shaded boxes, WD-40 consensus amino acids; CAPITAL letters, amino acids which fit consensus; lower case, amino acids which do not fit consensus. Predicted β-sheets are underlined.
KIF21 mRNA and Protein Are Enriched in Neural Tissues
To determine which tissues KIF21A and KIF21B proteins function in, Northern and Western analyses were performed using neural and nonneural tissues. A 6.2-kb KIF21A transcript was identified in all tissues examined except the spleen, and a 6.0-kb KIF21B transcript was identified in the brain, eye, and spleen (Fig. 3 A). Protein immunoblotting for KIF21A primarily identifies an ∼180-kD polypeptide in brain extract, with more moderate levels in the kidney, liver, and testes, and low levels in all other tissues except the spleen, where no protein is observed (Fig. 3 B, upper panel). KIF21B antibody recognizes an ∼178-kD band in the brain and spleen with lower levels in testes (Fig. 3 B, lower panel). The ∼6-kb mRNAs and ∼180-kD proteins sizes observed with mRNA and protein blots are consistent with sizes predicted from the isolated KIF21A and KIF21B cDNA sequences.
Figure 3.
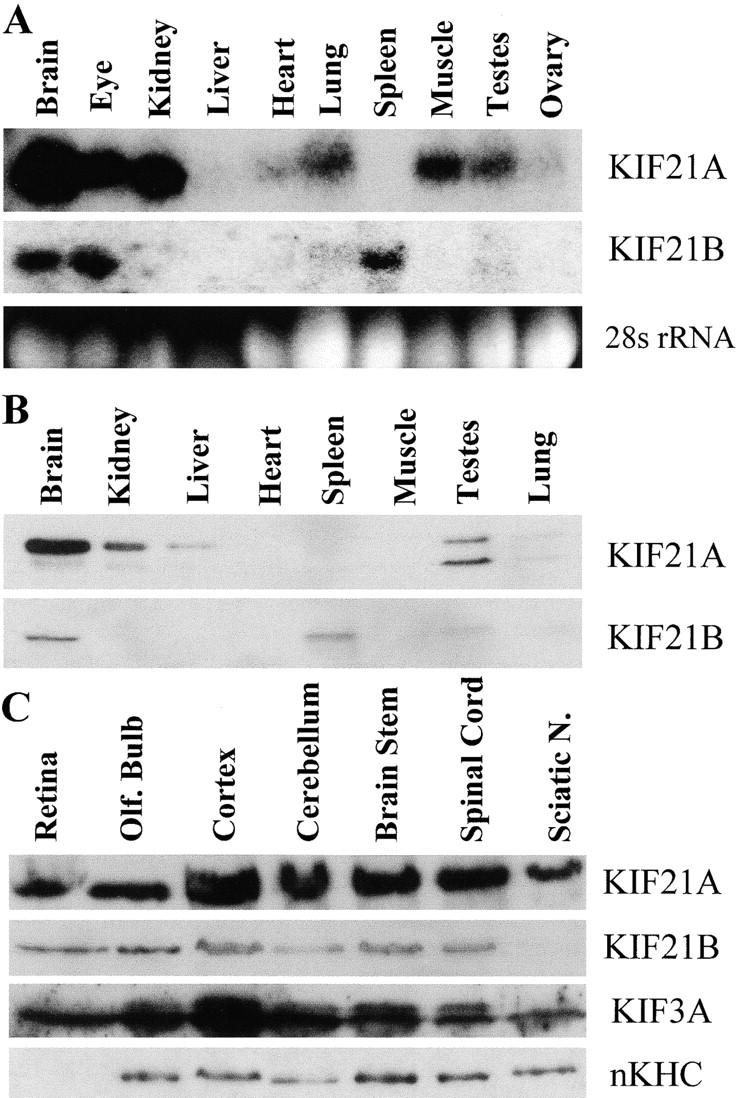
Distribution of KIF21A and KIF21B mRNAs and proteins in mouse neural and nonneural tissues. (A) Northern blots probed for KIF21A and KIF21B transcripts and ethidium bromide stained 28s rRNA (to show RNA levels). (B) Western analysis of mouse tissues showing enrichment of KIF21A and KIF21B proteins in neural tissue. Smaller band in KIF21A blot is most likely a degradation product. (C) Western analysis for KIF21A and KIF21B proteins in the murine nervous system. Note the minimal signal for KIF21B in the sciatic nerve. KIF3A and nKHC signals in the sciatic nerve indicate that this tissue is not underloaded.
To consider potential neuronal functions of KIF21A and KIF21B, the distribution of these proteins in various neural regions was examined by protein immunoblotting. KIF21A and KIF21B proteins were present in all regions of the murine nervous system examined, except the sciatic nerve, where expression varied between the two proteins (Fig. 3 C). In this axon-rich tissue, KIF21A protein is readily detected while KIF21B protein is barely detectable. This low expression of KIF21B in the sciatic nerve is not expected for a protein predicted to be involved in axonal transport. Like KIF21A, KIF3A and nKHC, two plus end-directed KLPs believed to participate in axonal transport (Kondo et al., 1994; Niclas et al., 1994; Yamazaki et al., 1995), are expressed at similar levels in the sciatic nerve compared with the rest of the nervous system (Fig. 3 C).
KIF21A and KIF21B Have Different Affinities for Insoluble Complexes
To determine if KIF21A and KIF21B share similar functional characteristics, the ability to bind microtubules, to form heterodimers, and to interact with different cellular components were examined. Like most KLPs, KIF21A and KIF21B bind strongly to microtubules in the presence of the nonhydrolyzable ATP analogue AMP-PNP (Fig. 4 A). Although KIF21A and KIF21B proteins share amino acid identity along their lengths, they do not form heterodimers with each other, since neither antibody was able to immunoprecipitate both proteins (Fig. 4 B).
Figure 4.
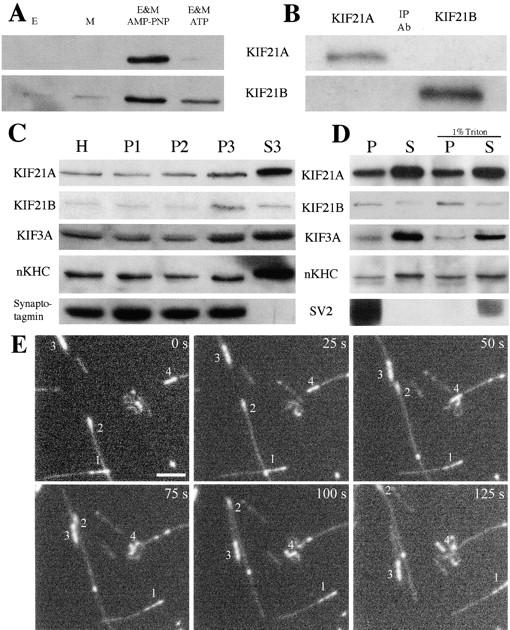
KIF21A and KIF21B interact with microtubules and have different fractionation characteristics. (A) Microtubule binding assay. Note that both KIF21A and KIF21B microtubule binding is enhanced by addition of AMP-PNP. E, extract only; M, microtubules only. (B) Western immunoblots of immunoprecipitations from mouse brain extract. Note that neither antibody is able to immunoprecipitate both KIF21A and KIF21B. (C) Cell fractionation of mouse brain extract. 50 μg of total protein is loaded in each lane: H, homogenate; P1, 3,000 g pellet; P2, 9,000 g pellet; P3, 100,000 g pellet; S3, final supernatant. (D) Extraction of the P3 pellet with buffer only and buffer with 1% Triton X-100. Note that an equal volume of this pellet and supernatant was loaded. P, pellet; S, supernatant. (E) Time lapse images of KIF21B in vitro motility assay using polarity marked fluorescent microtubules. Notice that microtubules 1–4 all move with the bright seed leading indicating movement by a plus end-directed motor. Microtubules 1–3, the whole microtubule can be seen and only one end is brightly labeled. Bar, 5 μM.
To determine whether KIF21A and KIF21B proteins associate with vesicles or protein complexes, differential centrifugation was performed. KIF21A and KIF21B, like previously characterized neuronally expressed KLPs, KIF3A and nKHC, were observed in soluble (S3) and insoluble (P1, P2, and P3) pools, while synaptotagmin, an integral membrane protein (Matthew et al., 1981), was found only in the insoluble pool (Fig. 4 C). The KLP/cargo interaction was examined directly by either extracting the P3 pellet with buffer only or with buffer plus detergent and then recentrifuging. Much less KIF21B protein was released from the P3 pellet than KIF21A, nKHC, or KIF3A, suggesting that KIF21B has a higher affinity for its cargo than axonally localized kinesins (Fig. 4 D). Extraction of the P3 pellet with Triton X-100, which solubilizes membranes (i.e., SV2; Feany et al., 1992), was performed to determine whether the insoluble fraction of KLPs is associated with membranous vesicles. Triton X-100 did not increase further the dissociation of any of these KLPs from the P3 pellet, suggesting that the KLP pools isolated in the P3 pellet are not associated with membranous vesicles.
KIF21 Kinesin Family Are Plus End-directed Motors
To directly determine whether the KIF21 family members are plus end-directed microtubule motors, the direction of movement of polarity marked microtubules by a KIF21B truncated motor fusion protein was determined. We found that all microtubules for which we could unambiguously identify a single bright seed moved with the bright seed leading, indicating movement by a plus end-directed microtubule motor (Fig. 4 E). In this assay, microtubules moved at a rate between 0.1 and 0.3 μm/s, independent of the length of the microtubules.
Sciatic nerve ligations were also performed and stained for the presence of KIF21A and KIF21B protein (data not shown). Previous work demonstrates that plus end-directed motors accumulate only on the proximal side of a ligation, while minus end-directed motors accumulate on both sides of a ligation (see Hirokawa et al., 1990, 1991). KIF21A and KIF21B accumulate only on the proximal side of the ligation, consistent with both proteins being plus end-directed motors. However, the amount of signal detected for KIF21B was greatly reduced compared with KIF21A and required 10-fold more laser power to see comparable levels of staining as KIF21A, which is consistent with the low protein level observed by protein immunoblotting (Fig. 3 C).
KIF21A Localizes throughout Neurons and KIF21B Localizes to Dendrites
To determine in which neurons, and where within neurons KIF21A and KIF21B function, an immunofluorescent survey of the mouse nervous system was performed (Fig. 5). Protein immunoblotting (Fig. 3 C) indicated that KIF21A and KIF21B are present throughout the nervous system. Although KIF21A and KIF21B are sometimes expressed in the same cells (compare Fig. 5, G, very weak, but reproducible cell body staining to H, pyramidal cell of the cerebral cortex; and I to J, motor neurons, arrows and arrowheads), their intracellular staining patterns are markedly different from each other. Strong KIF21A protein staining was observed in cell bodies (arrows in Fig. 5, A, retinal ganglion cells; C, mitral cells; G, pyramidal cells; and I, motor neurons) and processes extending from it (arrowheads in Fig. 5, C, mitral cells; and I, motor neurons). KIF21B signal, on the other hand, much lower in the cell bodies (arrows) than in the processes (arrowheads in Fig. 5, B, retinal ganglion cells; D, mitral cells; F, Purkinje cells; H, pyramidal neurons of the cerebral cortex; J, motor neurons; and L, hippocampal pyramidal neurons).
Figure 5.
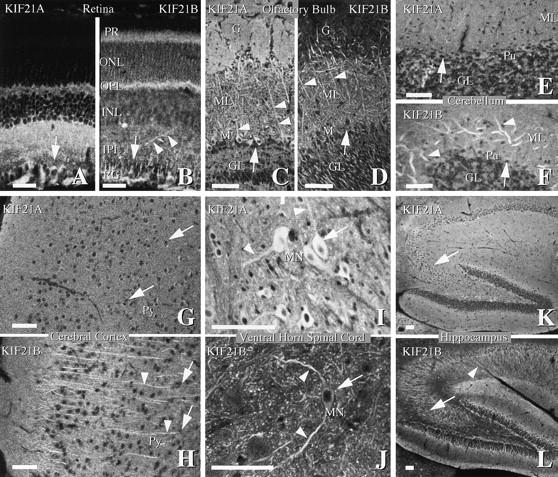
Cellular localization of KIF21A and KIF21B protein throughout the nervous system. (A and B) Neuroretina. (C and D) Olfactory bulb. (E and F) Cerebellum. (G and H) Cerebral cortex. (I and J) Ventral horn of the spinal cord. (K and L) Hippocampus immunostained for KIF21A or KIF21B protein. Note that KIF21A staining is apparent in many cell bodies (arrows) and processes (arrowheads), while KIF21B staining is weak in cell bodies (arrows) and very strong in the processes (arrowheads). PR, photoreceptors; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; RG, retinal ganglion cell bodies; GL, granular layer; M, mitral cell; ML, molecular layer; Pu, Purkinje cell; MN, motor neuron; Py, pyramidal cell. Bars, 100 μm.
To determine whether KIF21A or KIF21B protein is present in axons and/or dendrites, the spinal cord was double immunostained for each KIF21 protein and either phosphorylated neurofilament H (pNF-H), an axonal marker (Sternberger and Sternberger, 1983), or MAP2, a dendrite specific marker (Bernhardt and Matus, 1984). KIF21A protein colocalized with pNF-H and MAP2 positive processes, indicating KIF21A presence in dendrites (Fig. 6, A and B, arrowheads) and axons (Fig. 6, E–G, arrows). KIF21B protein only colocalized with MAP2 positive processes (Fig. 6, C and D, arrowheads), but not with most pNF-H positive processes (Fig. 6, H–J, arrows), indicating that KIF21B protein is primarily a somatodendritic protein that is highly enriched within dendrites. This localization pattern is consistent with the protein level observed by protein immunoblotting (i.e., absence from the sciatic nerve; Fig. 3 C), and the low levels of accumulation observed in a sciatic nerve ligation experiment (data not shown).
Figure 6.
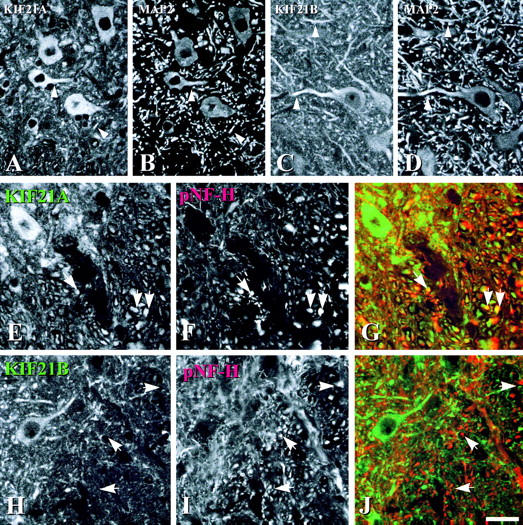
KIF21A protein localizes to axons and dendrites while KIF21B protein is detected in dendrites but not axons. (A and B) Double staining of the spinal cord ventral horn for KIF21A and MAP2. Note the high degree of overlap of staining (arrowheads). (C and D) Double staining of the spinal cord ventral horn for KIF21B and MAP2. Note that most MAP2 positive processes also stain for KIF21B. (E and F) Ventral horn stained for KIF21A and pNF-H protein. (G) Merge of E and F. Note that many pNF-H positive axons are also positive for KIF21A (yellow signal and arrows). (H and I) Ventral horn stained for KIF21B and pNF-H protein. (J) Merge of H and I. Note that pNF-H positive processes do not stain for KIF21B (arrows). Bar, 100 μm.
KIF21A and KIF21B mRNAs Localize to Cell Bodies
In situ hybridization analysis was performed on mouse hippocampus to determine if KIF21B protein enrichment within dendrites might result from its mRNA being localized to the dendrites. KIF21A and KIF21B mRNA signal was detected only in the cell bodies of the CA pyramidal neurons (Fig. 7, A, B, E, F, and H). When this same tissue was probed for MAP2 mRNA, which localizes partially to dendrites, signal was detected mainly in the cell bodies and in the molecular layer where the pyramidal neuron dendrites reside (Fig. 7, C, G, and I), consistent with previous reports (Garner et al., 1988). The restriction of the majority, if not all of the KIF21B mRNA to the cell body suggests that KIF21B protein is enriched within dendrites via a protein sorting mechanism rather than by local dendritic synthesis following mRNA localization.
Figure 7.
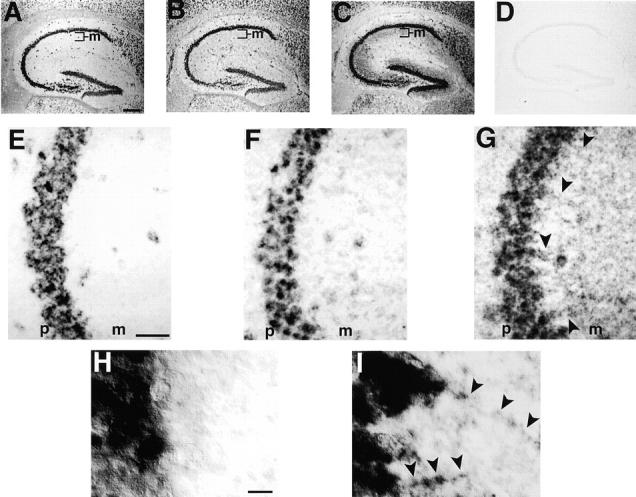
KIF21B mRNA is predominantly localized to the cell body. In situ hybridization was performed on adjacent sagittal sections through the adult hippocampus using antisense riboprobes for KIF21A (A and E), KIF21B (B, F, and H), or MAP2 (C, G, and I), or a sense riboprobe for KIF21B (D; this and all other sense probes produced no specific signal). At low magnification (A–D), KIF21A (A) and KIF21B (B) hybridization signal can be observed primarily in the pyramidal cell bodies of the CA fields and in the granule cell bodies of the dentate gyrus. In contrast, MAP2 mRNA, which is known to be transported to the dendrite, is detected both in the cell bodies and in the adjacent molecular layer (m) that contains pyramidal cell dendrites. This difference in localization is confirmed at higher magnification (E–I). While radial dendrite-like labeled structures are clearly visible using the MAP2 riboprobe (G and I, arrowheads), no such labeled structures are observed using either KIF21A (E) or KIF21B (F and H) riboprobes. Labeling is confined predominantly to the pyramidal cell body layer (p). Bars: 700 μM (A–D); 150 μM (E–G); and 10 μM (H and I).
Discussion
KIF21B Is the First Kinesin that Is Enriched in the Dendrites Compared with the Cell Body
We report here the identification and initial characterization of two KLPs, KIF21A and KIF21B. These proteins share extensive amino acid and motif similarity to each other throughout their lengths, but not to any previously characterized kinesins outside of the motor domain, suggesting that they are the founding members of a new kinesin family. Not only do KIF21A and KIF21B share amino acid sequence similarity, but each also contains seven analogous WD-40 repeats. WD-40 repeat domains have been shown in other proteins to interact with pleckstrin homology (PH; Wang et al., 1994, 1995) and tetratrico peptide repeats (TPR) domains (Keleher et al., 1992). Modular protein–protein interaction domains have also been identified in other kinesins, for example, TPR domains in kinesin light chain (Gindhart and Goldstein, 1996) and PH domains in KIF1A (Okada et al., 1995). Data from detergent extraction of high speed cell fractionation pellets suggest that the KIF21 proteins may interact with the insoluble cytoskeleton, a large protein complex, or the detergent insoluble vesicle cytoskeleton, possibly through their WD-40 repeat domains.
Northern and Western data indicate that KIF21A and KIF21B mRNAs and proteins are not expressed in the same tissues except for the nervous system. Differential tissue expression among family members is not uncommon and may represent specialized tissue or unique intracellular functions for each member. While KIF21A protein was found throughout the neuron in a pattern similar to other plus end-directed kinesins that are believed to function in axonal transport (Kondo et al., 1994; Nangaku et al., 1994; Niclas et al., 1994; Noda et al., 1995; Okada et al., 1995; Yamazaki et al., 1995; Yang and Goldstein, 1998), KIF21B protein was found to localize primarily to the somatodendritic compartment of neurons, being highly enriched in the dendritic processes compared with the cell body. KIF21B protein distribution is unique from the ubiquitous localization observed for plus end-directed motors and distinct from the somatodendritic distribution observed for the putative minus end-directed kinesin, KIFC2 (Saito et al., 1997).
KIF21B and Current Models for Protein Sorting in Neurons
Current proposals to explain how cargoes are differentially targeted to either a dendrite or the axon have focused on the differences in microtubule polarity between the processes and the directionality of the motors that move along the microtubules (Black and Baas, 1989; Saito et al., 1997). It has been suggested that plus end-directed motors may perform most of the axon specific anterograde transport, while minus end-directed motors may perform most of the dendrite specific transport. This model is based on the premise that orientation of axonal microtubules would not allow minus end-directed motors to enter the axon from the cell body under their own power. The mixed microtubule polarity in the proximal portion of dendrites should allow all kinesin motors to cycle between the cell body and the dendrite. Since active minus end-directed motors should only enter the dendrite and not the axon, they are ideal for sorting dendrite specific cargo to the dendrites. Plus end-directed kinesins should eventually enter the axon from the cell body and then unidirectionally translocate down the axon where they should then be capable of releasing their cargo. So far, the intracellular distribution observed for all characterized neuronally expressed kinesins, including KIF21A, conform to the prediction made by this model. The recent identification of the first putative minus end-directed neuronal kinesin, KIFC2, and its predominantly somatodendritic localization provided further support for this kinesin-dependent sorting mechanism.
Our identification of a plus end-directed kinesin that exhibits somatodendritic localization and enrichment within dendrites, KIF21B, is inconsistent with the current kinesin sorting model, indicating that this model is too simple to account for all aspects of neuronal process transport. We have shown that KIF21B mRNA is confined almost exclusively to the cell body and that KIF21B protein levels are very low in the cell body and axon, compared with the dendrites.
How Might KIF21B Become Enriched in the Dendrites and What Function Might KIF21B Perform?
Several different mechanisms could account for the dendritic enrichment observed for KIF21B protein within neurons. There is some evidence that differences in protein stability between the axonal and dendritic processes can account for enrichment of protein within one of the processes (i.e., MAP2; Okabe and Hirokawa, 1989; Kanai and Hirokawa, 1995). It is possible that active KIF21B moves into both axons and dendrites, but becomes stabilized only within the dendrite while it is actively degraded within the axon.
Another way to enrich KIF21B protein within the dendrite would be to inactivate the motor function within the cell body and then use a minus end motor, such as KIFC2, to transport KIF21B specifically into dendrites where it then can be sequestered. Pellet extraction data showed minimal KIF21B release from the pellet, suggesting that there may be a strong interaction between KIF21B protein and an insoluble cargo. This insoluble cargo may be the dendritic cytoskeleton and is being used by the neuron to sequester inactive KIF21B motor until it is needed.
What function might KIF21B perform within the dendrites? It has been shown that the mixture of microtubule polarity in the dendrites of cultured hippocampal neurons is not constant along its proximal to distal length (Baas et al., 1988). In the proximal portion of the dendrite, there is a 50/50 mixture of oppositely oriented microtubules. However, in the most distal portion of the dendrites, as many as 95% of the plus ends of microtubules are oriented towards the synapse. It is unclear whether the distal portion of dendrites in vivo possess similar microtubule distributions as cultured neurons. If this distribution does occur in vivo, it produces an environment that would favor movement of a plus end-directed kinesin toward the synapse. KIF21B may function within distal dendrites to deliver cargoes to the distal regions of dendrites.
To create and maintain enrichment within the dendrite when KIF21B becomes active, it must be continuously returned to the dendrite from the cell body. KIF21B may be targeted to dendrites via a dendritic sorting signal sequence that is possessed by KIF21B, but not KIF21A. Recently, it has been shown that membrane proteins, which target to the basolateral surface within polarized epithelial cells (e.g., transferrin receptor), also target to dendrites when they are expressed in neurons. When the targeting signal is disrupted the protein is no longer restricted to the somatodendritic compartment, but becomes dispersed throughout the neuron (West et al., 1997; Jareb and Banker, 1998). Future generation of KIF21A/KIF21B chimeric proteins will be useful in separating the different functional domains within each protein, and identifying the sequence differences that produce the markedly different localizations and characteristics observed for the two proteins.
Acknowledgments
We would like to thank current and past members of the Goldstein and Cleveland laboratories, especially Janet Marszalek, Roman Sakowicz, Andreas Merdes, and Toni Williamson for helpful comments and useful discussions during the generation of this manuscript. We would also like to thank James Frazier for help with motility assays and Jason Kahana for help with image capturing in the fluorescent motility assays.
Abbreviations used in this paper
- KLPs
kinesin-like proteins
- nt
nucleotide
- pNF-H
phosphorylated neurofilament H
Footnotes
A pharmacology training grant has supported J.R. Marszalek. L.S.B. Goldstein is an Investigator of the Howard Hughes Medical Institute.
References
- Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M, Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 1992;119:1287–1296. doi: 10.1083/jcb.119.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984;226:203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Black MM, Baas PW. The basis of polarity in neurons. Trends Neurosci. 1989;12:211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Burton PR. Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res. 1988;473:107–115. doi: 10.1016/0006-8993(88)90321-6. [DOI] [PubMed] [Google Scholar]
- Burton PR, Paige JL. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci USA. 1981;78:3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- DeBry RW, Seldin MF. Human/mouse homology relationships. Genomics. 1996;33:337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Garner CC, Tucker RP, Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988;336:674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gindhart JG, Jr, Goldstein LS. Tetratrico peptide repeats are present in the kinesin light chain. Trends Biochem Sci. 1996;21:52–53. [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. . Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hanlon DW, Yang Z, Goldstein LS. Characterization of KIFC2, a neuronal kinesin superfamily member in mouse. Neuron. 1997;18:439–451. doi: 10.1016/s0896-6273(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Hyman AA. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence microscopy. Methods Cell Biol. 1993;39:105–113. doi: 10.1016/s0091-679x(08)60164-8. [DOI] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Jareb M, Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hirokawa N. Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron. 1995;14:421–432. doi: 10.1016/0896-6273(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Keleher CA, Redd MJ, Schultz J, Carlson M, Johnson AD. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992;68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- Kishino A, Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew WD, Tsavaler L, Reichardt LF. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Quiroga S, Rosa A, Kosik K, Caceres A. Suppression of KIF2 in PC12 cells alters the distribution of a growth cone nonsynaptic membrane receptor and inhibits neurite extension. J Cell Biol. 1997;138:657–669. doi: 10.1083/jcb.138.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Tanaka Y, Matsuoka E, Kondo S, Okada Y, Noda Y, Kanai Y, Hirokawa N. Identification and classification of 16 new kinesin superfamily (KIF) proteins in mouse genome. Proc Natl Acad Sci USA. 1997;94:9654–9659. doi: 10.1073/pnas.94.18.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins [published erratum appears in Nature.1994. 371:812] Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Niclas J, Navone F, Hom-Booher N, Vale RD. Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron. 1994;12:1059–1072. doi: 10.1016/0896-6273(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Noda Y, Sato-Yoshitake R, Kondo S, Nangaku M, Hirokawa N. KIF2 is a new microtubule-based anterograde motor that transports membranous organelles distinct from those carried by kinesin heavy chain or KIF3A/B. J Cell Biol. 1995;129:157–167. doi: 10.1083/jcb.129.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Hirokawa N. Rapid turnover of microtubule-associated protein MAP2 in the axon revealed by microinjection of biotinylated MAP2 into cultured neurons. Proc Natl Acad Sci USA. 1989;86:4127–4131. doi: 10.1073/pnas.86.11.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe LB, Nadeau JH, Turner R, Frankel WN, Letts VA, Eppig JT, Ko MS, Thurston SJ, Birkenmeier EH. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource [published erratum appears in Mamm. Genome.1994. 5:463] Mamm Genome. 1994;5:253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM, Porter ME, Grissom PM, McIntosh JR. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985;318:483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voorn L, Ploegh HL. The WD-40 repeat. FEBS Lett. 1992;307:131–134. doi: 10.1016/0014-5793(92)80751-2. [DOI] [PubMed] [Google Scholar]
- Wang DS, Shaw R, Winkelmann JC, Shaw G. Binding of PH domains of beta-adrenergic receptor kinase and beta-spectrin to WD40/beta-transducin repeat containing regions of the beta-subunit of trimeric G-proteins. Biochem Biophys Res Commun. 1994;203:29–35. doi: 10.1006/bbrc.1994.2144. [DOI] [PubMed] [Google Scholar]
- Wang DS, Shaw R, Hattori M, Arai H, Inoue K, Shaw G. Binding of pleckstrin homology domains to WD40/beta-transducin repeat containing segments of the protein product of the Lis-1 gene. Biochem Biophys Res Commun. 1995;209:622–629. doi: 10.1006/bbrc.1995.1545. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Chun J. Png-1, a nervous system-specific zinc finger gene, identifies regions containing postmitotic neurons during mammalian embryonic development. J Comp Neurol. 1997;381:130–142. doi: 10.1002/(sici)1096-9861(19970505)381:2<130::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- West AE, Neve RL, Buckley KM. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci. 1997;17:6038–6047. doi: 10.1523/JNEUROSCI.17-16-06038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Goldstein LS. Characterization of the KIF3C neural kinesin-like motor from mouse. Mol Biol Cell. 1998;9:249–261. doi: 10.1091/mbc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Hanlon DW, Marszalek JR, Goldstein LS. Identification, partial characterization, and genetic mapping of kinesin-like protein genes in mouse. Genomics. 1997;45:123–131. doi: 10.1006/geno.1997.4901. [DOI] [PubMed] [Google Scholar]


