Abstract
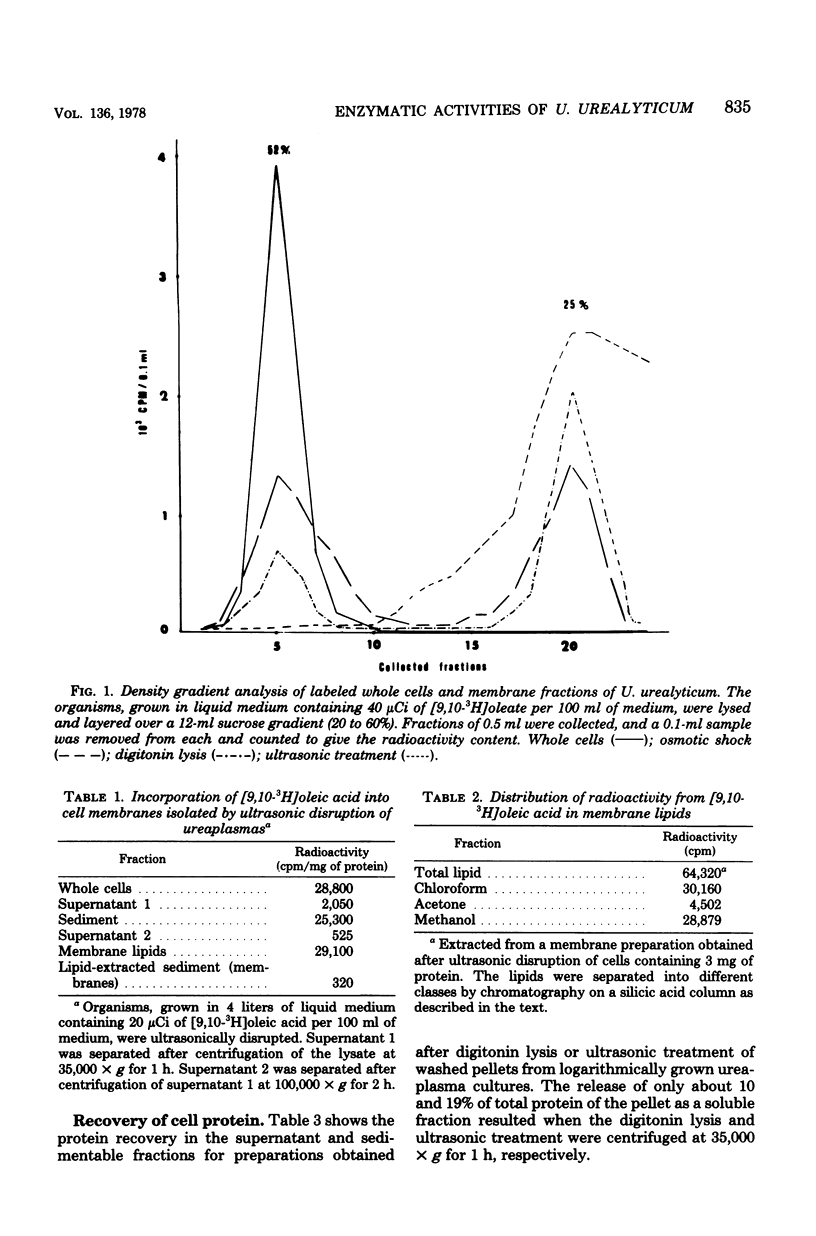
The localization of some enzymic activities in cell fractions of Ureaplasma urealyticum was studied. A quantitative evaluation of the effectiveness of several cell lysis procedures was obtained by using labeled membranes and sucrose density gradient centrifugation. Ultrasonic treatment was found to be the most effective procedure for lysing the cells, whereas digitonin and osmotic shock caused the lysis of only 70 and 50% of the cells, respectively. The localization of selected enzymes in Ureaplasma cells resembled that found in other Mycoplasma species. Adenosine triphosphatase, ribonuclease, deoxyribonuclease, and p-nitrophenylphosphatase activities were located exclusively in the membrane fraction, whereas urease and L-histidine ammonia-lyase were located in the cytoplasm.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajello F., Romano N., Massenti M. F. L-histidine ammonia-lyase from a T-strain Mycoplasma (Ureaplasma urealyticum). Boll Ist Sieroter Milan. 1977 Sep;56(4):343–350. [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MOLECULAR BASIS OF HISTIDASE INDUCTION IN BACILLUS SUBTILIS. J Mol Biol. 1963 Oct;7:401–420. doi: 10.1016/s0022-2836(63)80033-9. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Lund P., Neidhardt F. C., Schwartz D. T. Induction and repression of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4320–4324. [PubMed] [Google Scholar]
- Masover G. K., Razin S., Hayflick L. Localization of enzymes in Ureaplasma urealyticum (T-strain mycoplasma). J Bacteriol. 1977 Apr;130(1):297–302. doi: 10.1128/jb.130.1.297-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman Z., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. II. Solubilization and enzymic activities of Acholeplasma laidlawii membrane proteins. Biochim Biophys Acta. 1971 Oct 12;249(1):169–176. doi: 10.1016/0005-2736(71)90093-9. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Romano N., La Licata R., Scarlata G. Immunological analysis of plasma membranes of a T-strain of mycoplasma (Ureaplasma urealyticum). Infect Immun. 1977 Jun;16(3):734–737. doi: 10.1128/iai.16.3.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N., Rottem S., Razin S. Biosynthesis of saturated and unsaturated fatty acids by a T-strain mycoplasma (Ureaplasma). J Bacteriol. 1976 Oct;128(1):170–173. doi: 10.1128/jb.128.1.170-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Whitescarver J., Castillo F., Furness G. The preparation of membranes of some human T-mycoplasmas and the analysis of their carbohydrate content. Proc Soc Exp Biol Med. 1975 Oct;150(1):20–22. doi: 10.3181/00379727-150-38965. [DOI] [PubMed] [Google Scholar]


