Abstract
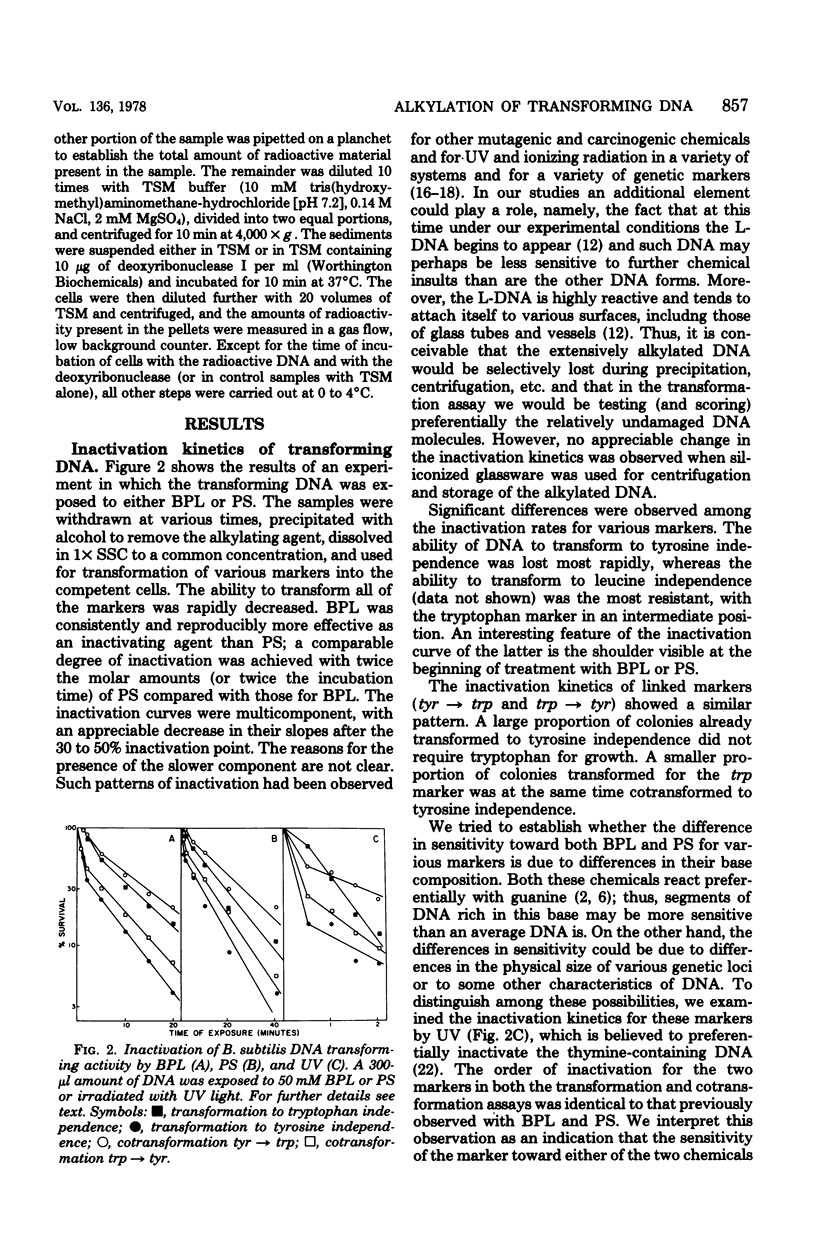
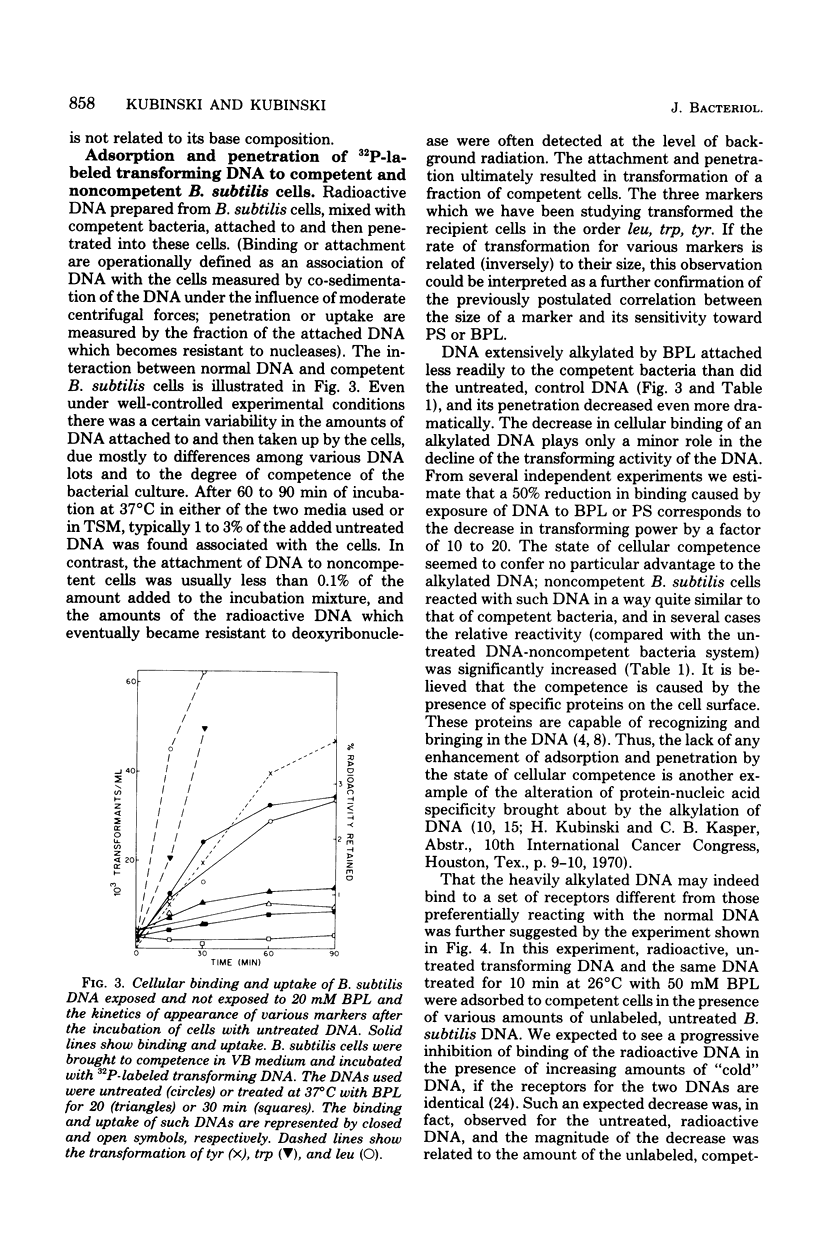
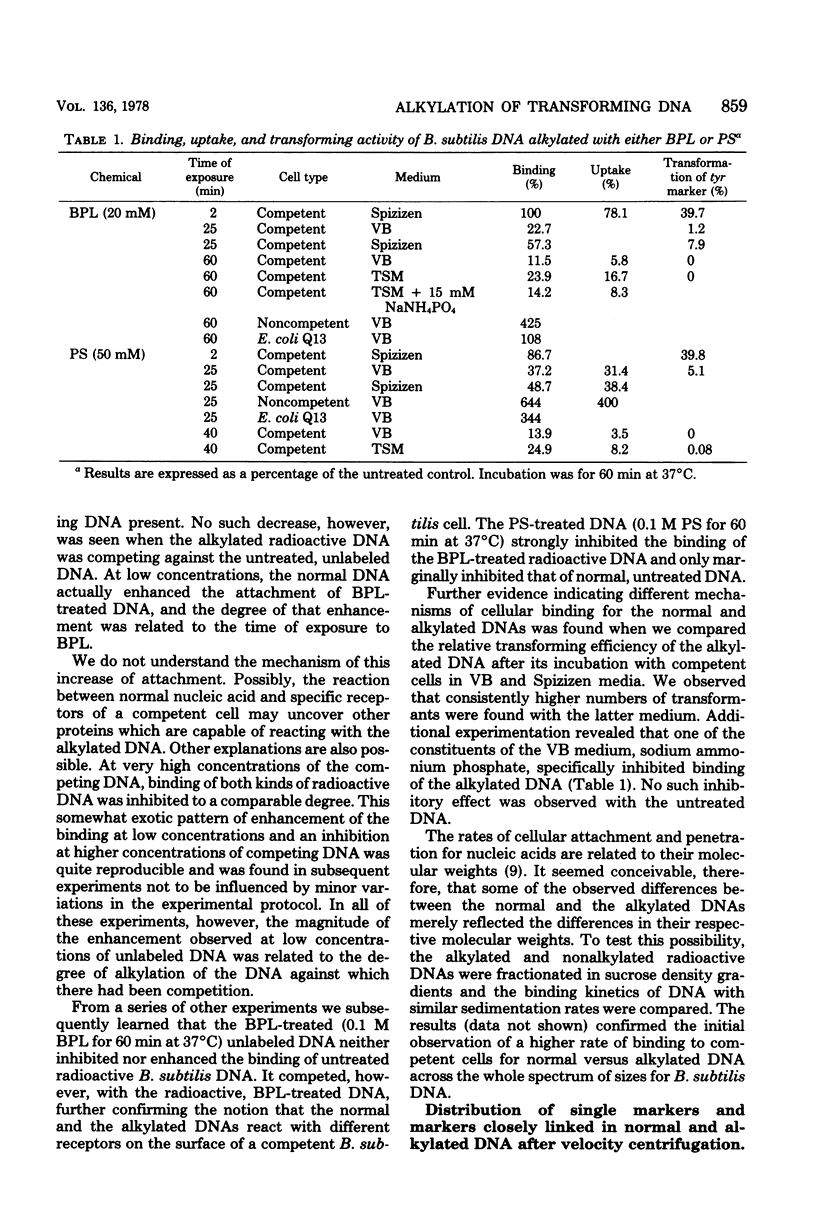
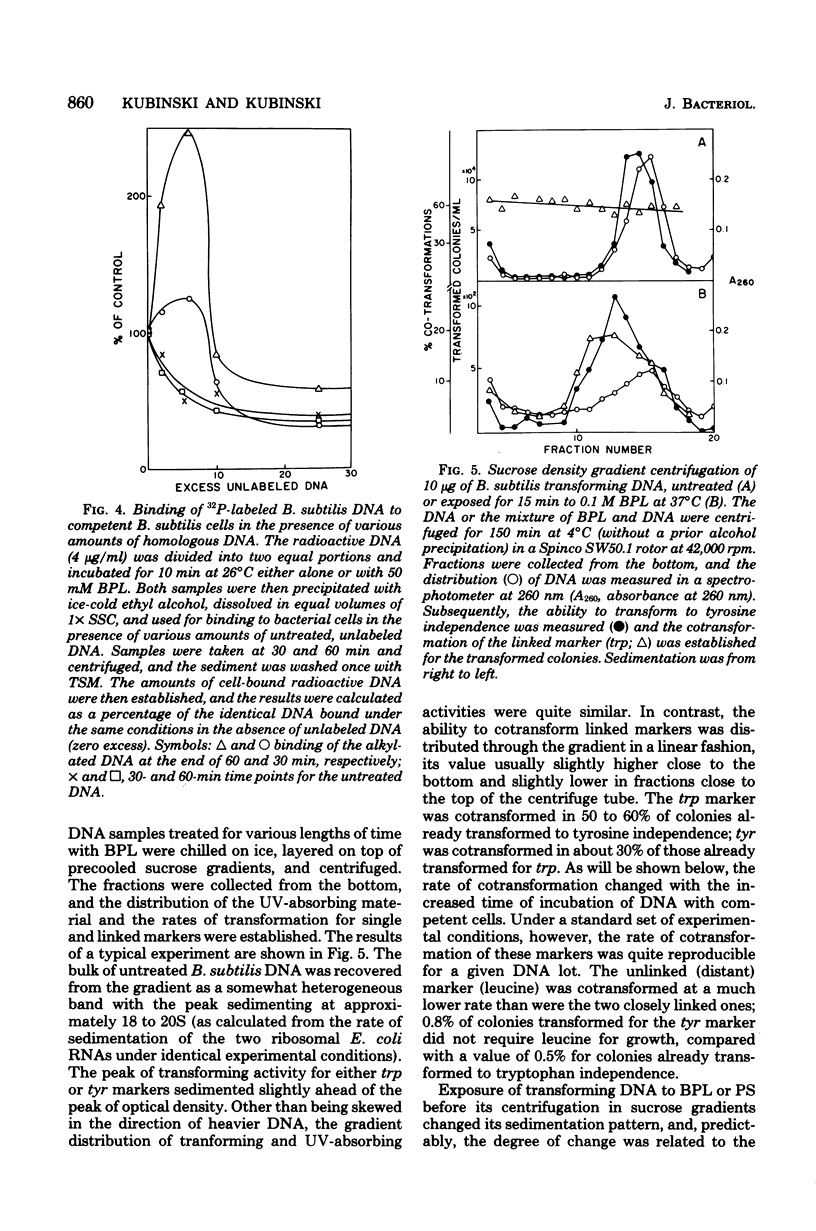
Transforming DNA was exposed to either beta-propiolactone or 1,3-propane sultone and then used for transformation of competent bacteria to nutritional independence from tyrosine and tryptophan (linked markers) and leucine (an unlinked marker). The ability to transform was progressively lost by the DNA during incubation with either of these two chemicals. For all three markers the inactivation curve was biphasic, with a short period of rapid inactivation followed by one characterized by a much slower rate. The overall rate of inactivation was different for all three markers and presumably was related to the size of the marker. The decrease in the transforming activity was in part due to the slower rate of penetration of alkylated DNA through the cellular membrane and its inability to enter the recipient bacteria. This decrease in the rate of cellular uptake, even for DNA eventually destined to enter the cell, began almost immediately after its exposure to the chemical and ended up with an almost complete lack of recognition of the heavily alkylated DNA by the specific surface receptors of competent cells. Such DNA attached to sites on the surface of competent bacteria which were different from receptors specific for the untreated nucleic acid. This attachment was not followed by uptake of the altered DNA. Presence of albumin during the incubation with a carcinogen further increased the degree of inactivation, indicating that the artificial nucleoproteins produced under such conditions were less efficient in the transformation assay than was the naked DNA. Cotransfomration of close markers progressively decreased, beginning immediately after the start of incubation of DNA with the chemicals. Extensively alkylated DNA fractionated by sedimentation through sucrose density gradients showed a peculiar distribution of cotransforming activity for such markers; namely, molecules larger than the bulk of DNA ("megamolecules") showed less ability to transform the second marker than did some of the apparently smaller molecules which sedimented more slowly through the gradient. An increase in cotransformation of distant markers was evident in DNA molecules after a short exposure to an alkylating agent, but cotransformation of such markers was absent in DNA treated for longer periods. The observed changes in the transforming and cotransforming activities of the alkylated DNA can be explained by what is known about the physicochemistry of such DNA and in particular about the propensity of the alkylated and broken molecules to form complexes with themselves and with other macromolecules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusick D. J. The genetic properties of beta-propiolactone. Mutat Res. 1977;39(3-4):241–255. doi: 10.1016/0165-1110(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Buitenwerf J., Venema G. Transformation in bacillus subtilis: fate of transforming DNA in transformation deficient mutants. Mol Gen Genet. 1977 Mar 7;151(2):203–213. doi: 10.1007/BF00338696. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D., Gabor M. Bacterial transformation, with special reference to recombination process. Annu Rev Genet. 1970;4:193–224. doi: 10.1146/annurev.ge.04.120170.001205. [DOI] [PubMed] [Google Scholar]
- Koch G. Interaction of poliovirus-specific RNAs with HeLa cells and E. coli. Curr Top Microbiol Immunol. 1973;62:89–138. doi: 10.1007/978-3-642-65772-6_4. [DOI] [PubMed] [Google Scholar]
- Kubinski H., Andersen P. R., Kellicutt L. M. Association of nucleic acids and cellular membranes induced by a carcinogen, -propiolactone. Chem Biol Interact. 1972 Sep;5(4):279–283. doi: 10.1016/0009-2797(72)90031-2. [DOI] [PubMed] [Google Scholar]
- Kubinski H., Opara-Kubinska Z., Szybalski W. Patterns of interaction between polyribonucleotides and individual DNA strands derived from several vertebrates, bacteria and bacteriophages. J Mol Biol. 1966 Sep;20(2):313–329. doi: 10.1016/0022-2836(66)90067-2. [DOI] [PubMed] [Google Scholar]
- Kubinski H., Szybalski E. H. Intermolecular linking and fragmentation of DNA by beta-propiolactone, a monoalkylating carcinogen. Chem Biol Interact. 1975 Jan;10(1):41–55. doi: 10.1016/0009-2797(75)90045-9. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr, Robberson D. L. A sensitive endonuclease probe for lesions in deoxyribonucleic acid helix structure produced by carcinogenic or mutagenic agents. J Biol Chem. 1977 Dec 10;252(23):8740–8746. [PubMed] [Google Scholar]
- Maher V. M., Miller E. C., Miller J. A., Szybalski W. Mutations and decreases in density of transforming DNA produced by derivatives of the carcinogens 2-acetyl-aminofluorene and N-methyl-4-aminoazobenzene. Mol Pharmacol. 1968 Sep;4(5):411–426. [PubMed] [Google Scholar]
- Maher V. M., Reuter M. A. Mutations and loss of transforming activity of DNA caused by the O-glucuronide conjugate of the carcinogen, N-hydroxy-2-aminofluorene. Mutat Res. 1973 Apr;21(2):63–71. [PubMed] [Google Scholar]
- Morin N. R., Zeldin P. E., Kubinski Z. O., Bhattacharya P. K., Kubinski H. Macromolecular complexes produced by chemical carcinogens and ultraviolet radiation. Cancer Res. 1977 Oct;37(10):3802–3814. [PubMed] [Google Scholar]
- Nietert W. C., Kellicutt L. M., Kubinski H. DNA-protein complexes produced by a carcinogen, beta-propiolactone. Cancer Res. 1974 Apr;34(4):859–864. [PubMed] [Google Scholar]
- Rownd R. Physical chemistry of transforming deoxyribonucleic acid. Br Med Bull. 1965 Sep;21(3):187–194. doi: 10.1093/oxfordjournals.bmb.a070394. [DOI] [PubMed] [Google Scholar]
- Soltyk A., Shugar D., Piechowska M. Heterologous deoxyribonucleic acid uptake and complexing with cellular constituents in competent Bacillus subtilis. J Bacteriol. 1975 Dec;124(3):1429–1438. doi: 10.1128/jb.124.3.1429-1438.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Zeldin P. E., Bhattacharya P. K., Kubinski H., Nietert W. C. Macromolecular complexes produced by 1,3-propanesultone. Cancer Res. 1975 Jun;35(6):1445–1452. [PubMed] [Google Scholar]



