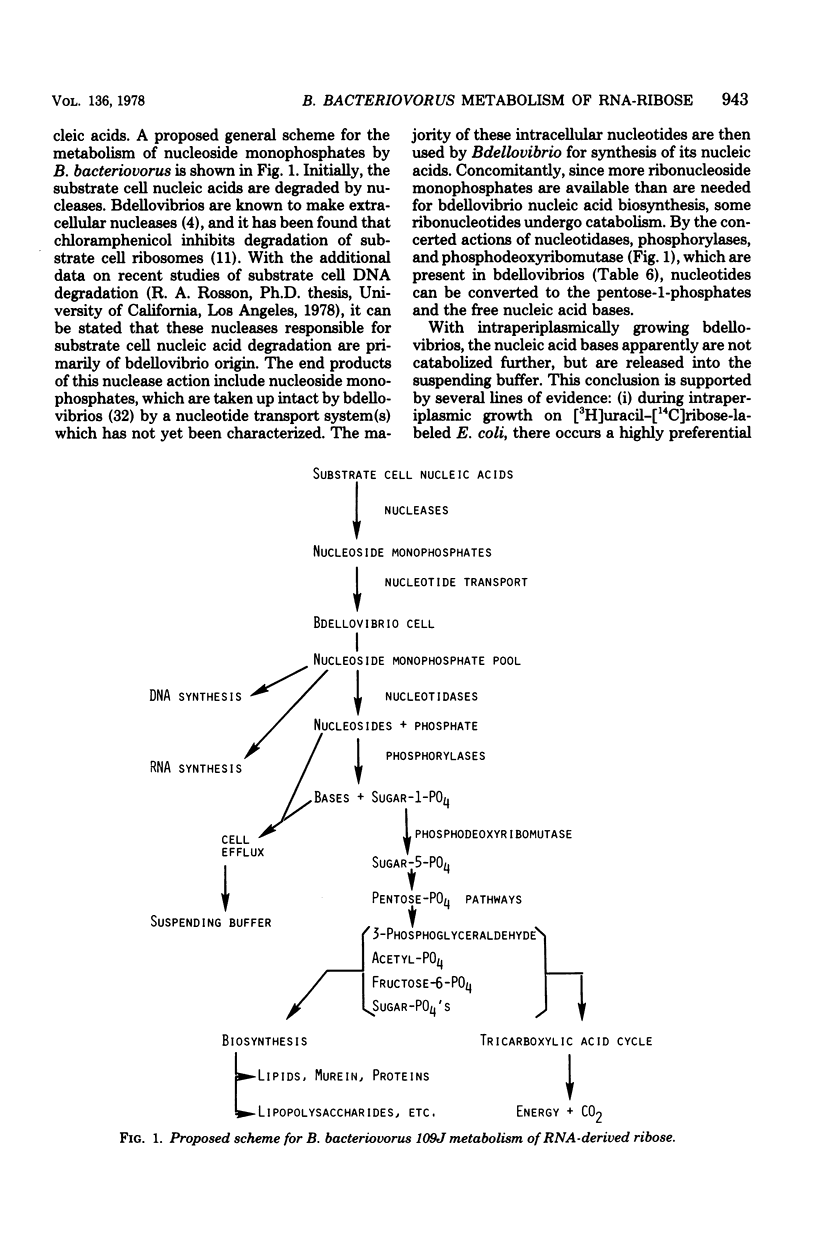
Abstract
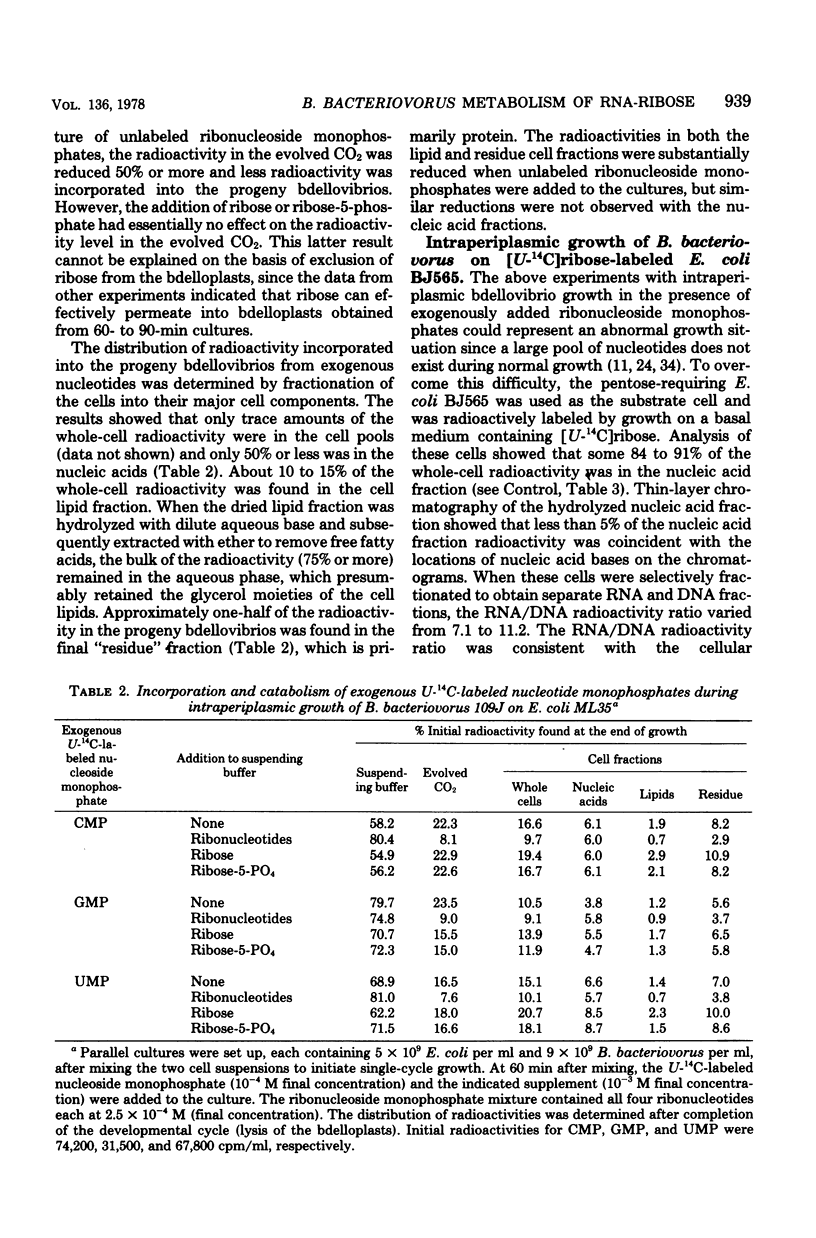
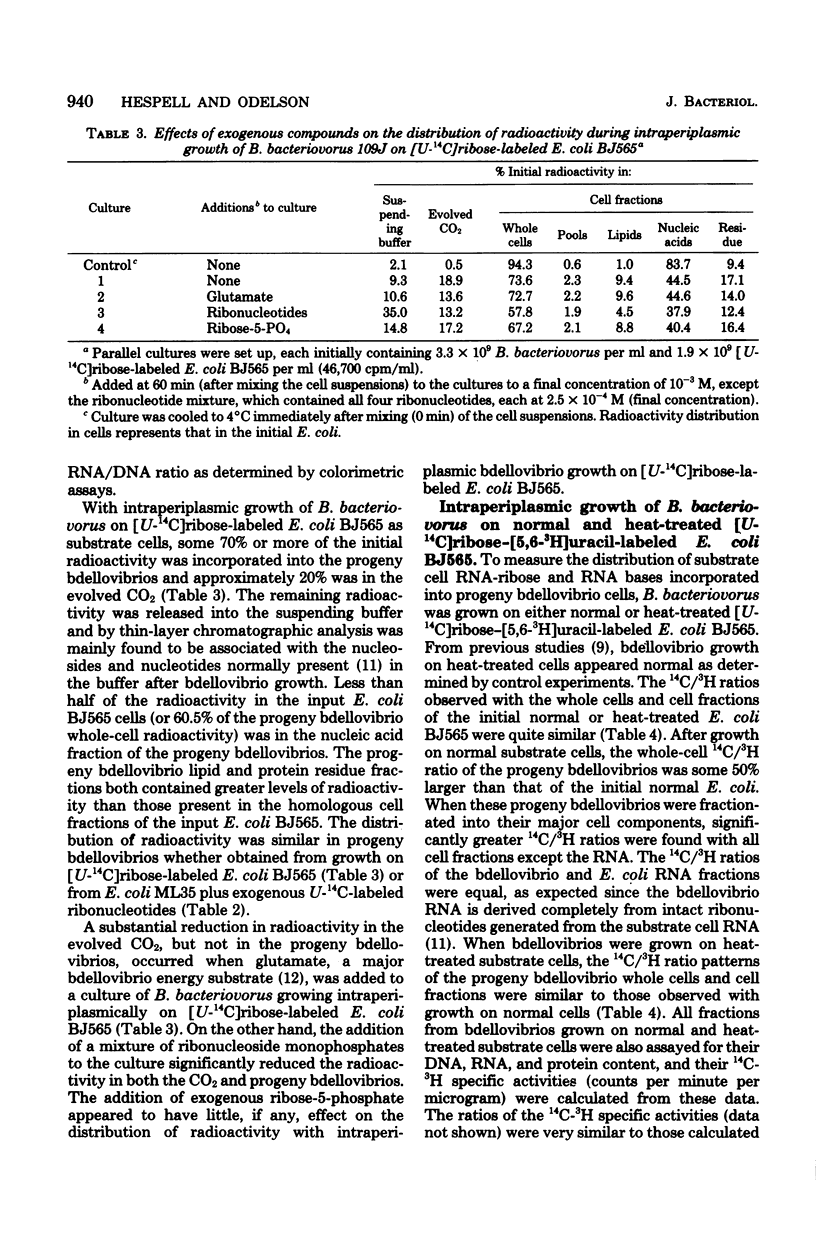
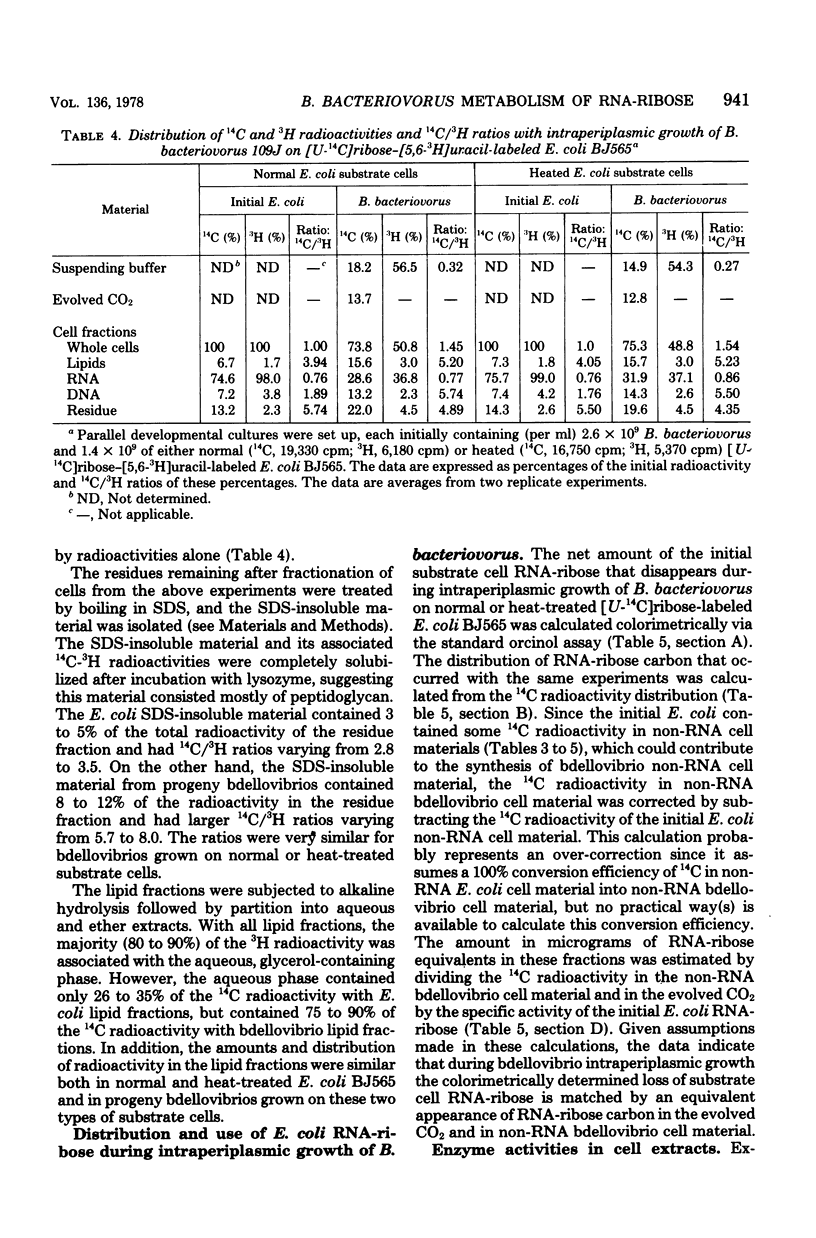
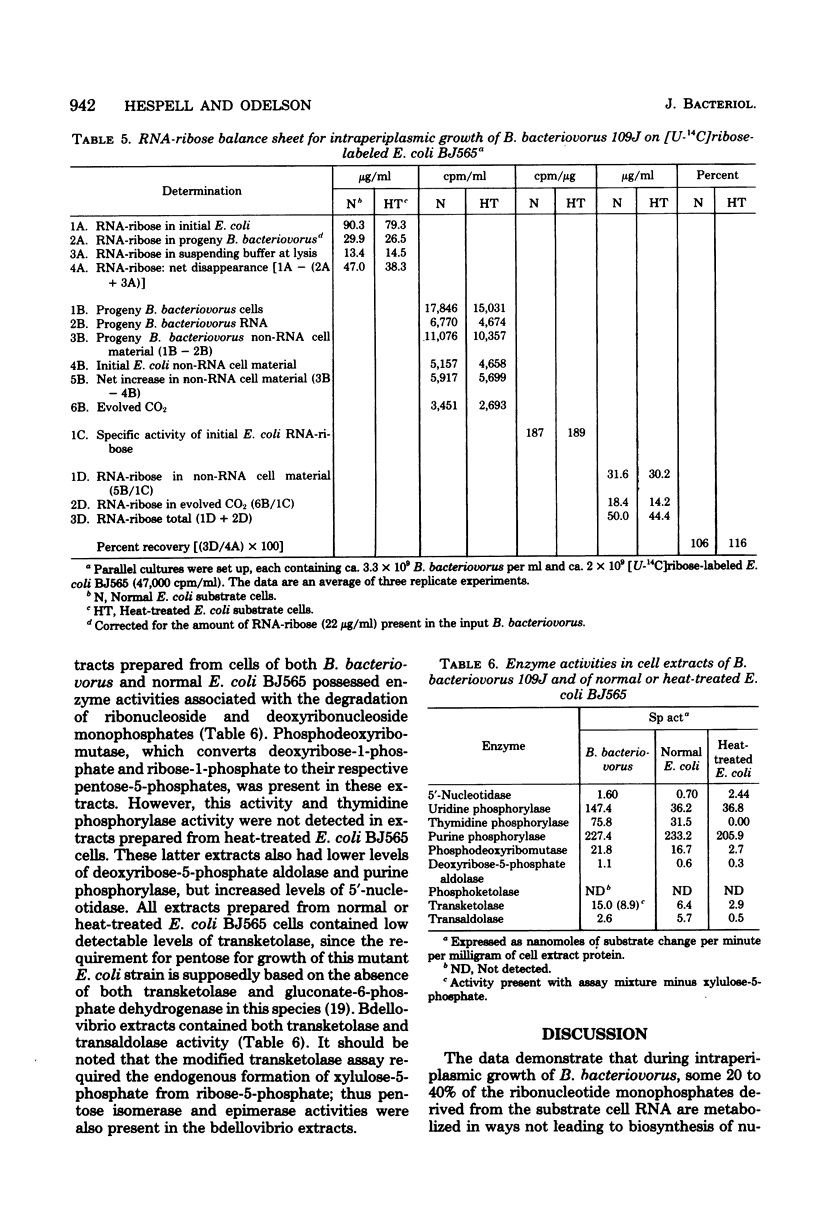
During intraperiplasmic growth of Bdellovibrio bacteriovorus 109J on Escherichia coli some 30 to 60% of the initial E. coli RNA-ribose disappeared as cell-associated orcinol-positive material. The levels of RNA-ribose in the suspending buffer after growth together with the RNA-ribose used for bdellovibrio DNA synthesis accounted for 50% or less of the missing RNA-ribose. With intraperiplasmic growth in the presence of added U-14C-labeled CMP, GMP, or UMP, radioactivity was found both in the respired CO2 and incorporated into the bdellovibrio cell components. The addition of exogenous unlabeled ribonucleotides markedly reduced the amounts of both the 14CO2 and 14C incorporated into the progeny bdellovibrios. During intraperiplasmic growth of B. bacteriovorus on [U-14C]ribose-labeled E. coli BJ565, ca. 74% and ca. 19% of the initial 14C was incorporated into the progeny bdellovibrios and respired CO2, respectively. Under similar growth conditions, the addition of glutamate substantially reduced only the 14CO2; however, added ribonucleotides reduced both the 14CO2 and the 14C incorporated into the progeny bdellovibrios. No similar effects were found with added ribose-5-phosphate. The distribution of 14C in the major cell components was similar in progeny bdellovibrios whether obtained from growth on [U-14C]ribose-labeled E. coli BJ565 or from E. coli plus added U-14C-labeled ribonucleotides. After intraperiplasmic growth of B. bacteriovorus on [5,6-3H-]uracil-[U-14C]ribose-labeled E. coli BJ565 (normal or heat treated), the whole-cell 14C/3H ratio of the progeny bdellovibrios was some 50% greater and reflected the higher 14C/3H ratios found in the cell fractions. B. bacteriovorus and E. coli cell extracts both contained 5'-nucleotidase, uridine phosphorylase, purine phosphorylase, deoxyribose-5-phosphate aldolase, transketolase, thymidine phosphorylase, phosphodeoxyribomutase, and transaldolase enzyme activities. The latter three enzyme activities were either absent or very low in cell extracts prepared from heat-treated E. coli cells. It is concluded that during intraperiplasmic growth B. bacteriovorus degrades some 20 to 40% of the ribonucleotides derived from the initial E. coli RNA into the base and ribose-1-phosphate moieties. The ribose-1-phosphate is further metabolized by B. bacteriovorus both for energy production and for biosynthesis, of non-nucleic acid cell material. In addition, the data indicate that during intraperiplasmic growth B. bacteriovorus can metabolize ribose only if this compound is available to it as the ribonucleoside monophosphate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Weinfeld H. Regulation of thymidine metabolism in Escherichia coli K-12: optimal conditions for the assay of 1,5-phosphodeoxyribomutase in ultrasonic extracts. J Bacteriol. 1970 Sep;103(3):650–655. doi: 10.1128/jb.103.3.650-655.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelking H. M., Seidler R. J. The involvement of extracellular enzymes in the metabolism of Bdellovibrio. Arch Mikrobiol. 1974 Feb 13;95(4):293–304. doi: 10.1007/BF02451770. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C. E., LAMPEN J. O. Products of desoxyribose degradation by Escherichia coli. J Biol Chem. 1952 Oct;198(2):885–893. [PubMed] [Google Scholar]
- Hammer-Jespersen K., Nygaard P. Multiple regulation of nucleoside catabolizing enzymes in Escherichia coli: effects of 3:5' cyclic AMP and CRP protein. Mol Gen Genet. 1976 Oct 18;148(1):49–55. doi: 10.1007/BF00268545. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B. Glycolytic and tricarboxylic acid cycle enzyme activities during intraperiplasmic growth of Bdellovibrio bacteriovorus on Escherichia coli. J Bacteriol. 1976 Nov;128(2):677–680. doi: 10.1128/jb.128.2.677-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B. Intraperiplasmic growth of Bdellovibrio bacteriovorus on heat-treated Escherichia coli. J Bacteriol. 1978 Mar;133(3):1156–1162. doi: 10.1128/jb.133.3.1156-1162.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Mertens M. Effects of nuclei acid compounds on viability and cell composition of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1978 Feb;116(2):151–159. doi: 10.1007/BF00406030. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Miozzari G. F., Rittenberg S. C. Ribonucleic acid destruction and synthesis during intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Aug;123(2):481–491. doi: 10.1128/jb.123.2.481-491.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Rosson R. A., Thomashow M. F., Rittenberg S. C. Respiration of Bdellovibrio bacteriovorus strain 109J and its energy substrates for intraperiplasmic growth. J Bacteriol. 1973 Mar;113(3):1280–1288. doi: 10.1128/jb.113.3.1280-1288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Imada A., Igarasi S. Ribosyl and deoxyribosyl transfer by bacterial enzyme systems. J Bacteriol. 1967 Nov;94(5):1551–1559. doi: 10.1128/jb.94.5.1551-1559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Nygaard P. Purine nucleoside phosphorylase from Escherichia coli and Salmonella typhimurium. Purification and some properties. Eur J Biochem. 1975 Feb 3;51(1):253–265. doi: 10.1111/j.1432-1033.1975.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Josephson B. L., Fraenkel D. G. Sugar metabolism in transketolase mutants of Escherichia coli. J Bacteriol. 1974 Jun;118(3):1082–1089. doi: 10.1128/jb.118.3.1082-1089.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson B. L., Fraenkel D. G. Transketolase mutants of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1289–1295. doi: 10.1128/jb.100.3.1289-1295.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlström O. Mutants of Escherichia coli defective in ribonucleoside and deoxyribonucleoside catabolism. J Bacteriol. 1968 Mar;95(3):1069–1077. doi: 10.1128/jb.95.3.1069-1077.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANSON L. A., LAMPEN J. O. The metabolism of desoxyribose nucleosides in Escherichia coli. J Biol Chem. 1951 Dec;193(2):539–547. [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Kinetics of deoxyribonucleic acid destruction and synthesis during growth of Bdellovibrio bacteriovorus strain 109D on pseudomonas putida and escherichia coli. J Bacteriol. 1972 Sep;111(3):664–673. doi: 10.1128/jb.111.3.664-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A. On the catabolism of deoxyribonucleosides in cells and cell extracts of Escherichia coli. Eur J Biochem. 1968 Nov;6(3):432–442. doi: 10.1111/j.1432-1033.1968.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Shilo M. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Apr;102(1):149–160. doi: 10.1128/jb.102.1.149-160.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. G., BERNSTEIN I. A. Studies on phosphodeoxyribomutase. Biochim Biophys Acta. 1961 Sep 2;52:184–193. doi: 10.1016/0006-3002(61)90916-7. [DOI] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]


