Abstract
We report the existence and distribution of an unusual type of projection neuron, a large, spindle-shaped cell, in layer Vb of the anterior cingulate cortex of pongids and hominids. These spindle cells were not observed in any other primate species or any other mammalian taxa, and their volume was correlated with brain volume residuals, a measure of encephalization in higher primates. These observations are of particular interest when considering primate neocortical evolution, as they reveal possible adaptive changes and functional modifications over the last 15–20 million years in the anterior cingulate cortex, a region that plays a major role in the regulation of many aspects of autonomic function and of certain cognitive processes. That in humans these unique neurons have been shown previously to be severely affected in the degenerative process of Alzheimer’s disease suggests that some of the differential neuronal susceptibility that occurs in the human brain in the course of age-related dementing illnesses may have appeared only recently during primate evolution.
The evolution of the neocortex in primates long has been recognized to be the result of great expansion of cortical areas, with a several-hundredfold increase in cortical volume between prosimians and humans (1, 2). However, the neuronal types that populate the neocortex, i.e., pyramidal neurons and numerous classes of nonpyramidal neurons, have remained remarkably constant, being morphologically recognizable across primate species and even other orders. An exception is the spindle neuron, found in the anterior cingulate cortex. It has been described in the human (3–10) and reported in the common chimpanzee (6). In the human, spindle cells, so called for their distinctive morphology, are found in layer Vb in Brodmann’s subareas 24a, 24b, and 24c and are most abundant in the cortex forming the medial wall of the cingulate gyrus (area 24b; ref. 10). These neurons are characterized by a very elongate, gradually tapering, large-sized soma that is virtually symmetrical about its vertical and horizontal axes as well as a light staining pattern with the Nissl stain (8, 10). More recently, a study in humans demonstrated that the spindle cells represent a class of projection neurons that is especially vulnerable to degeneration in Alzheimer’s disease, with a loss of approximately 60% of these particular neurons (10). Here we show that this neuronal type is a feature of the anterior cingulate cortex of all great apes, but not of any other primate species.
MATERIALS AND METHODS
Specimens.
Samples of the anterior cingulate cortex (Brodmann’s area 24) were obtained from 28 primate species representing all superfamilies of prosimian and anthropoid primates (Table 1; ref. 11). Materials from additional neocortical regions were available for comparison from all of the anthropoid species. All specimens were obtained postmortem or from terminally ill adult animals sacrificed for humane reasons and were fixed by immersion in 10% neutral formalin. Specimens of macaque, owl, squirrel, and capuchin monkeys were obtained from animals perfused transcardially with 4% paraformaldehyde in the context of unrelated experiments. The great ape brains were from young and adult individuals (age range, 4–34 years). Human brain specimens were obtained at autopsy from neurologically normal individuals (65–78 years old) and were prepared as described previously (8, 10). In many cases, only a single specimen was obtained because of the scarcity of such tissue. The specimens were obtained from the following institutions and collections: Bioqual Inc., the California Institute of Technology, Cleveland Metrozoo, Coulston Foundation, Lincoln Park Zoo, Los Angeles Zoo, Mount Sinai School of Medicine, Oregon Regional Primate Research Center, Southwest Foundation for Biomedical Research, University of Texas M. D. Anderson Cancer Center Science Park, National Institute of Mental Health, and University of Geneva School of Medicine (Switzerland).
Table 1.
Summary of the primate species investigated
| Taxonomy | Spindle cells | N |
|---|---|---|
| Prosimii | ||
| Lemuroidea | ||
| Lemuridae | ||
| Eulemur fulvus | None | 1 |
| Lemur catta | None | 1 |
| Indridae | ||
| Propithecus verreauxi | None | 1 |
| Cheirogaleidae | ||
| Cheirogaleus medius | None | 1 |
| Microcebus murinus | None | 1 |
| Loroidea | ||
| Galagonidae | ||
| Galago senegalensis | None | 1 |
| Galagoides demidoff | None | 1 |
| Lorisidae | ||
| Loris tardigradus | None | 1 |
| Nycticebus coucang | None | 1 |
| Perodicticus potto | None | 1 |
| Tarsioidea | ||
| Tarsiidae | ||
| Tarsius syrichta | None | 1 |
| Anthropoidea | ||
| Ceboidea | ||
| Callithricidae | ||
| Callithrix jacchus | None | 2 |
| Cebidae | ||
| Aotus trivirgatus | None | 2 |
| Cebus apella | None | 4 |
| Saimiri sciureus | None | 1 |
| Cercopithecoidea | ||
| Cercopithecidae | ||
| Macaca fascicularis | None | 8 |
| Macaca fuscata | None | 4 |
| Macaca mulatta | None | 4 |
| Macaca nemestrina | None | 4 |
| Macaca nigra | None | 4 |
| Erythrocebus patas | None | 4 |
| Papio hamadryas cynocephalus | None | 1 |
| Hominoidea | ||
| Hylobatidae | ||
| Hylobates lar | None | 4 |
| Pongidae | ||
| Pongo pygmaeus | Rare | 1 |
| Hominidae | ||
| Gorilla gorilla gorilla | Frequent | 5 |
| Pan troglodytes | Abundant | 8 |
| Pan paniscus | Abundant/clusters | 1 |
| Homo sapiens | Abundant/clusters | 6 |
Taxonomic position of the primate species investigated within their families, superfamilies, and suborders (11). Spindle cells in layer Vb of anterior cingulate cortex area 24 are observed with certainty only among hominoids, in all extant pongid and hominid species (shown in bold). N, number of available specimens in each species.
Histological Processing and Analysis.
All samples were cut into 40-μm-thick sections on a cryostat, Nissl-stained, and analyzed by two independent observers (P.R.H., E.A.N.). The criteria for categorizing a neuron as a spindle cell were: an elongate, large soma in layer Vb, lighter staining than surrounding pyramidal neurons, and symmetrical morphology about the cell’s horizontal and vertical axes (10). When present, they were unambiguous. Areal boundaries were based on previous parcellations of the cingulate cortex in macaque monkey and human (8, 12). Distinctions from layer VI atypical pyramidal cells and small vertical fusiform neurons (10, 13) were made by analyzing the laminar boundaries at low magnification to determine the laminar position of the neurons. Although the nature of many of the specimens did not permit a quantitative analysis based on rigorous stereologic methods, the available tissue was prepared according to a serial sampling paradigm similar to that used in our previous analyses of the human and macaque monkey neocortex (8, 10, 14, 15). Reflections of the local density of spindle cells in great apes and human were obtained from counting the number of spindle cells in 10 sections 1 mm apart at the level of the genu of the corpus callosum, where they are more frequent than at more posterior levels (10). In these sections, the number of spindle cells also was expressed as a percentage of the total number of resident neurons in series of 1-mm-wide traverses in layer V. It is therefore unlikely that isolated spindle neurons were overlooked in prosimians, Old and New World monkeys, and lesser apes. Representative maps of spindle cell location were prepared from each sample by using a computer-assisted image-analysis system consisting of a Zeiss Axiophot photomicroscope equipped with a Zeiss MSP65 computer-controlled motorized stage, a Zeiss ZVS-47E video camera, a Macintosh 840AV microcomputer, and neurozoom, a custom-designed software for morphology and stereology (16). To produce these maps, the coordinates of each labeled element were recorded in each microscopic field, typically a fraction of a cortical layer at a ×20 magnification, relative to an origin, and the maps were assembled automatically. The degree of randomness (R) of the laminar distribution of spindle cells was assessed further by relating their mean nearest-neighbor distance to their mean density as described by Morrison et al. (17). With this method, if R = 1 the distribution is random, if R < 1 it is clustered, and if R > 1 it is nonrandom (17). Stereologic estimates of individual neuronal volumes were performed at ×100 by using the rotator protocol in neurozoom software (16, 18). These neuronal volumes were compared with relative brain volume by calculating the linear regression of estimated brain volume vs. body weight for a dataset including humans, apes, and monkeys. The brain residuals are a measure of encephalization within anthropoid primates (19, 20). Statistical analysis was done by using a one-way ANOVA and post-hoc t test and tests of correlation.
RESULTS
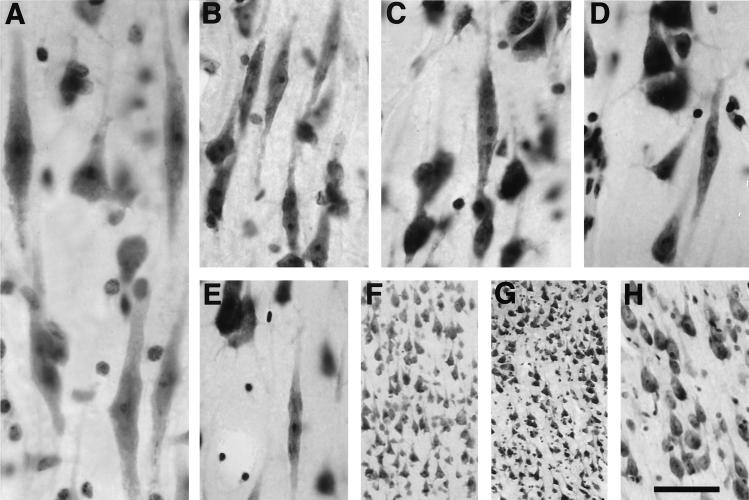
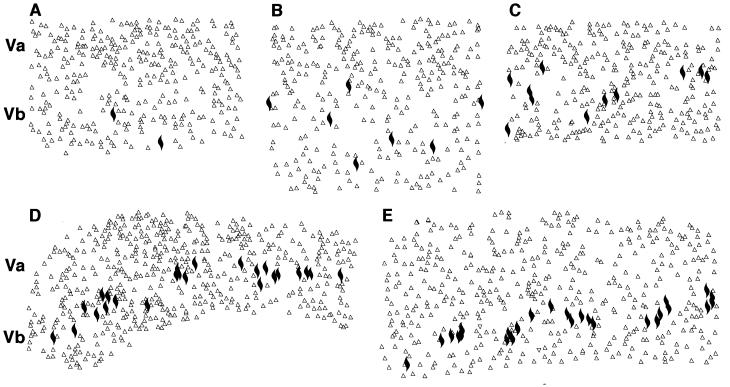
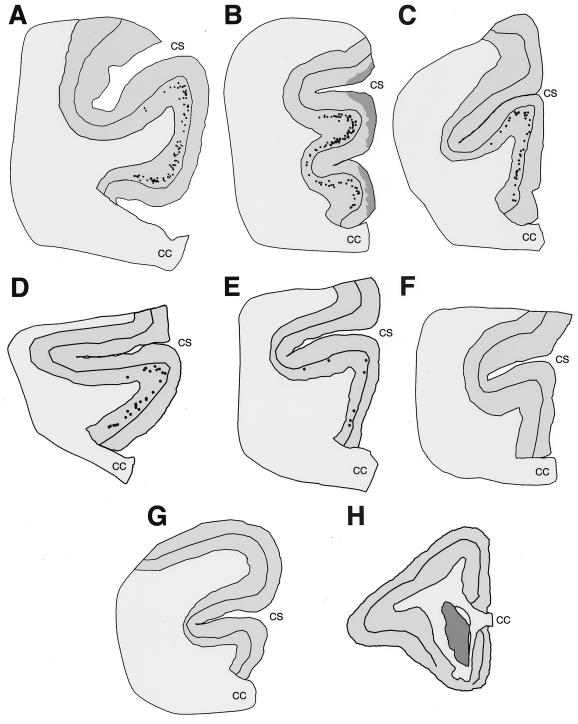
In the human anterior cingulate cortex, spindle cells occur most often in clusters of three to six neurons (Fig. 1A), are located exclusively in layer Vb, and are conspicuous because of the otherwise low cellular density of this layer (10). In the bonobo, the distribution of spindle cells most closely resembled that seen in humans, with clear clusters found throughout layer Vb of area 24 (Fig. 1B). The common chimpanzee had abundant spindle cells, but they were more likely to be found singly or in groups of two to three neurons (Fig. 1C). In the gorilla (Fig. 1D), the overall distribution resembled that in the common chimpanzee, but the spindle cells were considerably less abundant. In the orangutan (Fig. 1E), spindle cells were evident but observed only occasionally. Spindle cells were notably absent in the gibbon (Fig. 1F), as well as in New World monkeys (e.g., Callithrix, Aotus, Saimiri, and Cebus), Old World monkeys (e.g., Macaca, Erythrocebus, and Papio), and all of the prosimians (Fig. 1 G and H; Table 1). Nearest-neighbor analysis revealed that spindle cells are distributed regularly in layer Vb in orangutan, gorilla, and common chimpanzee with R values of 2.11, 1.74, and 1.28, respectively. Their distribution was markedly clustered in bonobo (R = 0.80) and humans (R = 0.40; Fig. 2). Semiquantitative estimates of local numbers of spindle cells revealed 7–10 spindle neurons in the sections from the orangutan specimen, 21.9 ± 5.4 (mean number per section ± SD) in gorillas, 37.1 ± 9.4 in common chimpanzees, 60–75 in the bonobo specimen, and 88.7 ± 14.6 in humans. Spindle cell numbers accounted for 0.6% in orangutan, 2.3% in gorilla, 3.8% in common chimpanzee, 4.8% in bonobo, and 5.6% in humans of the number of pyramidal cells in layer V traverses, further demonstrating their rarity (Figs. 2 and 3). These data were obtained from 4- to 34-year-old apes, so brain aging is very unlikely to have played a role in the low numbers of these neurons in these specimens, and normal brain aging is known to induce only a very marginal loss of neurons in human (21). Qualitative assessments of spindle cell densities and distribution on sections at levels rostral and caudal to the genu of the corpus callosum show a pattern comparable to that previously described in the human anterior cingulate cortex (10), indicating that it represents a valid sampling point for comparison across species (Fig. 3).
Figure 1.
Morphology of spindle cells in layer Vb of the anterior cingulate cortex in human (A), bonobo (B), common chimpanzee (C), gorilla (D), and orangutan (E). In all of these species the spindle cells display similar morphology and apparent somatic size. Note the clusters of spindle cells in the through-focus photomontage from the human and in the bonobo, whereas isolated neurons are observed in the three other great apes. (F–H) No spindle cells are present in the anterior cingulate cortex of the white-handed gibbon (F), Patas monkey (G), or ring-tailed lemur (H). [Bar = 50 μm (A), 80 μm (B–E and H), and 120 μm (F and G).]
Figure 2.
High-magnification computer-generated maps of the localization of spindle neurons in layers V of the anterior cingulate cortex in orangutan (A), gorilla (B), common chimpanzee (C), bonobo (D), and human (E). Spindle cells are represented by solid marks, and the neighboring pyramidal neurons are represented by open triangles. Note the differences in the densities of these neurons among species and the clustered pattern in bonobo and human. A–C are 1-mm wide and D and E are 1.5-mm wide.
Figure 3.
Computer-generated maps of the distribution of spindle neurons in the anterior cingulate cortex. All maps show a level situated at the genu of the corpus callosum in the human (A), bonobo (B), common chimpanzee (C), gorilla (D), and orangutan (E). The spindle cells are restricted to layer Vb and show much higher numbers in human and the two chimpanzee species, whereas fewer are seen in gorilla and orangutan. No spindle cells are observed in the white-handed gibbon (F), long-tailed macaque monkey (G), and owl monkey (H). The maps are approximately to scale. The darkly shaded areas in B reflect local damage to the superficial layers of the specimen. The caudate nucleus is shaded darkly in H. CC, corpus callosum; CS, cingulate sulcus.
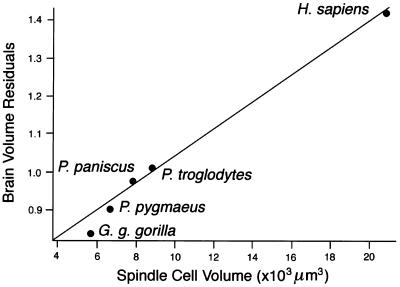
Stereologic volume estimates indicate that spindle cells were, on average, larger than neighboring layer V pyramidal cells and considerably larger than the small fusiform neurons of layer VI (Table 2). These volumetric data also demonstrate that spindle cells were larger in chimpanzees and humans than in gorillas and orangutans, whereas the two other cell types had comparable volumes across species (Table 2). Furthermore, when comparing neuronal volumes with relative brain volumes (19, 20), we observed that the volume of spindle cells is correlated strongly to encephalization, assessed by calculating brain residuals (r2 = 0.98, P = 0.001; Fig. 4), whereas the volumes of layer V pyramidal neurons and layer VI small fusiform cells are not (r2 = 0.49, P = 0.196 and r2 = 0.31, P = 0.33, respectively).
Table 2.
Volumes of layer V spindle and pyramidal cells and small-layer VI fusiform cells
| Species | Pyramidal cells | Spindle cells | Fusiform cells |
|---|---|---|---|
| P. pygmaeus | 1,951 ± 913 | 6,648 ± 2,667*† | 1,425 ± 445 |
| G. g. gorilla | 3,298 ± 1,295 | 5,684 ± 3,740† | 1,402 ± 648 |
| P. troglodytes | 2,786 ± 1,198 | 8,796 ± 5,069*† | 1,061 ± 462 |
| P. paniscus | 3,891 ± 1,859 | 7,743 ± 4,476† | 1,306 ± 476 |
| H. sapiens | 4,553 ± 2,295 | 20,822 ± 8,731*† | 1,677 ± 777 |
Volumetric data (μm3) were estimated in a sample of 50 neurons in each layer from each case by using the rotator (18) and are expressed as means ± SD. Note that, in spite of high variability, the volume of spindle cells tends to be generally higher than that of layer V pyramidal cells (∗, P < 0.05) and small-layer VI fusiform neurons (†, P < 0.01), and that spindle cells are larger in Pan and, particularly, in Homo compared with Gorilla and Pongo.
Figure 4.
Comparison of spindle cell volume with relative brain volume (see refs. 19 and 20). There is a strong correlation between the volumes of these neurons and brain volume residuals. No such correlation existed for pyramidal and small fusiform neurons of layers V and VI.
The present series spans all of the primate superfamilies, although it lacks representatives of several families such as large ceboids, colobine monkeys, guenons, and the siamang. However, given the lack of spindle cells in 23 primate families, as well in all of the other mammals we studied (22), the parallel emergence of this cell type in another primate seems unlikely. In fact, samples from the anterior cingulate (or anterior medial) cortex and several other neocortical regions of more than 30 other mammalian species, including monotremes, marsupials, insectivores, micro- and megachiropterans, rodents, carnivores, artiodactyls, and cetaceans, were analyzed (22), and spindle cells were not found in any of them. Spindle cells are also found, albeit in much lower numbers, in the anterior, agranular, and dysgranular portions of the insular cortex of the human, but not the macaque monkey (5, 8–10, 23). Unfortunately, samples of insular cortex were not available for the present study from most of the species studied, although from the limited number of samples we did obtain, it appears that some spindle cells also are found in the agranular insula of great apes. Spindle cells were never observed in any other neocortical areas in all of the available primate species.
DISCUSSION
The foregoing suggests the very rare, if not unprecedented, emergence of a unique, morphologic type of projection neuron, made all the more remarkable by its restriction to a very discrete cortical region and a very small but highly significant group of species. From a phylogenetic standpoint, the observation that among humans and great apes, chimpanzees have spindle cell densities comparable to humans—and it is the bonobo whose spindle cell distribution most closely matches that of the human—underscores the relatedness of Homo and Pan and offers a neuroanatomical correlate to the grouping of these taxa in the same clade, a contention that is supported by molecular biological and morphological data (24–27). Moreover, in view of data indicating that neurons forming defined projections tend to be clustered in the cortex (28), the more pronounced grouping of spindle cells in Pan and Homo is suggestive of the emergence and progressive anatomical refinement of a highly specific pathway in hominids, which began to emerge in the common ancestor of pongids and hominids and is particularly well developed in Pan and Homo.
The nature of the available material precludes the physiological and connectional studies that could help elucidate the role of spindle cells. However, existing data on the cortical area to which they are restricted might provide clues to their function. It is also worth remarking that the density of spindle cells is markedly reduced precisely in the transition area between anterior and posterior cingulate cortex (a region termed area 24′ in humans; refs. 8 and 29), which corresponds to the regions that contain the cingulate motor areas in macaque monkeys (30–33), suggesting that the distribution of spindle cells does not overlap with regions of the cingulate cortex involved in somatic motor function. In fact, the cingulate motor areas in humans have well defined cyto- and chemoarchitectural patterns (8, 29, 34) and are located caudally and dorsally to the region in which spindle cells are distributed. Although exhaustive samples seldom are available, preventing proper stereologic estimates of the number of these neurons, they constitute a small fraction of the cortical pyramidal neuron population (10). They could, however, represent a discrete projection, reminiscent of the Meynert neurons of the primary visual cortex (35, 36) or the Betz cells in the primary motor cortex (37), of primates. Alternatively, the emergence of a morphologic type could be merely an incidental consequence of vigorous adaptive changes. Either interpretation, however, would suggest that the anterior cingulate cortex may have been subjected to unusual adaptive pressure over the last 15–20 million years.
The anterior cingulate cortex is regarded as a phylogenetically ancient area (38). It subserves many functions that may vary across species, although its role in cortical control of autonomic functions, such as heart rate, blood pressure, and digestive functions (39–43), appears to be well conserved. Along these lines, area 24 in macaques is interconnected with the amygdala and has been shown to have layer V projections to the hypothalamus and the periaqueductal gray (43–45). From this perspective, the spindle cells of the anterior cingulate cortex might represent a population of specialized neurons that could integrate inputs with emotional overtones and project to highly specific motor centers controlling vocalization, facial expression, or autonomic function. In addition, the presence of at least some spindle cells in the anterior portion of the insula supports the notion of their role in autonomic control, because this region also is known to be involved in the regulation of visceral, olfactory, and gustatory functions, as well as complex alimentary behaviors (46–49). However, in humans the anterior cingulate cortex also appears to be involved in higher-level processes that are responsible for more than merely sensory input or motor output. Recent functional imaging studies have demonstrated an important role for the dorsal human anterior cingulate cortex in attention, with its degree of activation increasing with task difficulty, and the more ventral portion in the experience of the “unpleasantness” of pain and in the recognition of the emotional content of faces (43, 50, 51). Thus, in humans, at least, the anterior cingulate cortex is involved in complex processes that assist in integration and interpretation of sensory information. In this context, the unique correlation among the resident neurons of layers V and VI of the cellular volume of spindle cells with encephalization in human and great apes lends further support to the possible association of spindle cells with higher cortical functioning.
Interestingly, lesions in the anterior cingulate cortex in human are associated with a form of mutism (43, 52, 53), and the area to which the spindle cells are restricted is one of the only cortical areas known to elicit meaningful vocalizations (and not merely sounds) in squirrel monkeys when stimulated (54, 55). It has also been shown to participate in voluntary phonation in macaque monkeys (56). Thus, this region may be involved in some aspects of communication in primates, and it is possible that the appearance of these modified pyramidal neurons might signal the further anatomic and possibly functional elaboration of this cortical area in the only mammalian lineage known to have evolved speech and its emotional implications. It is also worth noting that the emergence of this unique neuronal type in a neocortical area involved in vocalization in primates coincides with the evolution as a definable anatomic structure of the planum temporale, a region that is important for language comprehension (57, 58). In view of the language comprehension abilities of great apes (59), it is therefore possible that several cortical structures involved in the production of specific vocalizations and in communicative skills sustained simultaneous, considerable, adaptive modifications during brain evolution in hominoids.
Finally, taken together with our earlier finding that spindle cells in the human apparently are more vulnerable to neurodegeneration in Alzheimer’s disease than other pyramidal neurons, possibly owing to their high content of neurofilament protein (10, 21), the present study points out some of the possible limitations of making comparisons between humans and more distantly related nonhuman primates and emphasizes the importance of the study of great apes in the context of aging and of age-related diseases affecting the cerebral cortex. Such knowledge might assist in our understanding of the possibly phylogenetic basis of differential neuronal vulnerability (60) and of some of the most devastating neurologic and psychiatric illnesses from which our own species suffers.
Acknowledgments
We thank Drs. C. Bouras, I. I. Glezer, S. G. Kohama, J. Marcus, E. J. Mufson, and L. G. Ungerleider for providing some of the specimens; Dr. T. Insel for providing a dataset on bonobo brain volume and A. Hakeem for help with data analysis; Drs. P. J. Gannon, C. V. Mobbs, J. H. Morrison, P. R. Rapp, and B. A. Vogt for helpful discussions; Dr. W. G. Young for software development; and S. Bruns, A. P. Leonard, E. Lugo, F. Robenzadeh, and R. Vertesi for technical assistance. Most of the great ape brains were supplied by the Great Ape Aging Project, a comparative neurobiology of aging resource at Bioqual Inc. (J.M.E.). This work was supported by National Institutes of Health Grants AG14308 (J.M.E.), CA/MH08944, and EY11759 (J.M.A.), the Del Webb Foundation (E.G.), and Mount Sinai School of Medicine (P.R.H.).
References
- 1.Frahm H D, Stephan H, Stephan M. J Hirnforsch. 1982;23:375–389. [PubMed] [Google Scholar]
- 2.Dunbar R I M. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 3.Betz W. Zentralbl Med Wiss. 1881;19:193–195. , 209–213, 231–234. [Google Scholar]
- 4.Hammarberg C. Studien über Klinik und Pathologie der Idiotie nebst Untersuchungen über die normale Anatomie des Hirnrinde. Uppsala: Berling; 1895. [Google Scholar]
- 5.Ramón y Cajal S. Textura del Sistema Nervioso del Hombre y de los Vertebrados, Tomo II. Madrid: Nicolás Moya; 1899. [Google Scholar]
- 6.Rose M. J Psychol Neurol. 1927;35:5–217. [Google Scholar]
- 7.De Crinis M. J Psychol Neurol. 1933;45:439–449. [Google Scholar]
- 8.Vogt B A, Nimchinsky E A, Vogt L J, Hof P R. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 9.Von Economo C. Zschr Ges Neurol Psychiat. 1926;100:706–712. [Google Scholar]
- 10.Nimchinsky E A, Vogt B A, Morrison J H, Hof P R. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- 11.Rowe N. The Pictorial Guide to the Living Primates. East Hampton, NY: Pogonias Press; 1996. [Google Scholar]
- 12.Vogt B A, Pandya D N, Rosene D L. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- 13.Tömböl T. In: Cerebral Cortex, Cellular Components of the Cerebral Cortex. Peters A, Jones E G, editors. Vol. 1. New York: Plenum; 1984. pp. 479–519. [Google Scholar]
- 14.Hof P R, Morrison J H. J Comp Neurol. 1995;352:161–186. doi: 10.1002/cne.903520202. [DOI] [PubMed] [Google Scholar]
- 15.Hof P R, Mufson E J, Morrison J H. J Comp Neurol. 1995;359:48–68. doi: 10.1002/cne.903590105. [DOI] [PubMed] [Google Scholar]
- 16.Bloom F E, Young W G, Nimchinsky E A, Hof P R, Morrison J H. In: Neuroinformatics—An Overview of the Human Brain Project. Koslow S H, Huerta M F, editors. Mahwah, NJ: Lawrence Erlbaum; 1997. pp. 83–123. [Google Scholar]
- 17.Morrison J H, Magistretti P J, Bloom F E. Brain Res. 1984;292:269–282. doi: 10.1016/0006-8993(84)90763-7. [DOI] [PubMed] [Google Scholar]
- 18.Vedel-Jensen E B, Gundersen H J G. J Microsc. 1993;170:35–44. [Google Scholar]
- 19.Allman J M, McLaughlin T, Hakeem A. Proc Natl Acad Sci USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allman J M, McLaughlin T, Hakeem A. Proc Natl Acad Sci USA. 1993;90:3359–3363. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison J H, Hof P R. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 22.Hof P R, Glezer I I, Condé F, Flagg R A, Rubin M B, Nimchinsky E A, Vogt Weisenhorn D M. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 23.Ngowyang G. J Psychol Neurol. 1932;44:671–674. [Google Scholar]
- 24.Arnason U, Xu X, Gullberg A, Graur D. J Mol Evol. 1996;43:41–45. doi: 10.1007/BF02352298. [DOI] [PubMed] [Google Scholar]
- 25.Barriel V. Folia Primatol. 1997;68:50–56. doi: 10.1159/000157232. [DOI] [PubMed] [Google Scholar]
- 26.Ruvolo M. Mol Biol Evol. 1997;14:248–265. doi: 10.1093/oxfordjournals.molbev.a025761. [DOI] [PubMed] [Google Scholar]
- 27.Begun D R. Science. 1992;257:1929–1933. doi: 10.1126/science.1411507. [DOI] [PubMed] [Google Scholar]
- 28.Hiorns W, Neal J W, Pearson R C A, Powell T P S. Proc R Soc London Ser B. 1991;246:1–9. doi: 10.1098/rspb.1991.0117. [DOI] [PubMed] [Google Scholar]
- 29.Nimchinsky E A, Vogt B A, Morrison J H, Hof P R. J Comp Neurol. 1997;384:597–620. doi: 10.1002/(sici)1096-9861(19970811)384:4<597::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Muakkassa K F, Strick P L. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 31.Dum R P, Strick P L. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Basel: Birkhauser; 1993. pp. 415–441. [Google Scholar]
- 32.Morecraft R J, Van Hoesen G W. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- 33.Nimchinsky E A, Hof P R, Young W G, Morrison J H. J Comp Neurol. 1996;374:136–160. doi: 10.1002/(SICI)1096-9861(19961007)374:1<136::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Braak H. Brain Res. 1976;109:219–233. doi: 10.1016/0006-8993(76)90526-6. [DOI] [PubMed] [Google Scholar]
- 35.Spatz W B. Brain Res. 1975;92:450–455. doi: 10.1016/0006-8993(75)90329-7. [DOI] [PubMed] [Google Scholar]
- 36.Fries W, Keizer K, Kuypers H G J M. Exp Brain Res. 1985;58:613–616. doi: 10.1007/BF00235878. [DOI] [PubMed] [Google Scholar]
- 37.Scheibel M E, Scheibel A B. In: Architectonics of the Cerebral Cortex. Brazier M A B, Petsche H, editors. New York: Raven; 1978. pp. 43–57. [Google Scholar]
- 38.Sanides F. Ann N Y Acad Sci. 1969;167:404–423. [Google Scholar]
- 39.Kaada B, Pribram K H, Epstein J. J Neurophysiol. 1949;12:347–356. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- 40.Pool J L, Ransohoff J. J Neurophysiol. 1949;12:385–392. doi: 10.1152/jn.1949.12.6.385. [DOI] [PubMed] [Google Scholar]
- 41.Showers M J C, Crosby E C. Neurology. 1958;8:561–565. doi: 10.1212/wnl.8.7.561. [DOI] [PubMed] [Google Scholar]
- 42.Showers M J. J Comp Neurol. 1959;112:231–302. doi: 10.1002/cne.901120118. [DOI] [PubMed] [Google Scholar]
- 43.Devinsky O, Morrell M J, Vogt B A. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 44.An X, Bandler R, Öngür D, Price J L. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 45.Öngür D, An X, Price J L. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 46.Penfield W, Faulk M E. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- 47.Showers M J C, Lauer E W. J Comp Neurol. 1961;117:107–116. doi: 10.1002/cne.901170109. [DOI] [PubMed] [Google Scholar]
- 48.Mesulam M M, Mufson E J. In: Cerebral Cortex, Association and Auditory Cortices. Peters A, Jones E G, editors. Vol. 4. New York: Plenum; 1985. pp. 179–226. [Google Scholar]
- 49.Oppenheimer S M, Gelb A, Girvin J P, Hachinski V C. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 50.Rainville P, Duncan G H, Price D D, Carrier B, Bushnell M C. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 51.Paus T, Koski L, Caramanos Z, Westbury C. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 52.Barris R W, Schuman H R. Neurology. 1953;3:44–52. doi: 10.1212/wnl.3.1.44. [DOI] [PubMed] [Google Scholar]
- 53.Nemeth G, Hegedus K, Molnar L. Eur Arch Psychiatry Neurol Sci. 1988;237:218–222. doi: 10.1007/BF00449910. [DOI] [PubMed] [Google Scholar]
- 54.Jürgens U, Ploog D. Exp Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- 55.Jürgens U. Naturwissenschaften. 1998;85:376–388. doi: 10.1007/s001140050519. [DOI] [PubMed] [Google Scholar]
- 56.Trachy R E, Sutton D, Lindeman R C. Am J Primatol. 1981;1:43–55. doi: 10.1002/ajp.1350010106. [DOI] [PubMed] [Google Scholar]
- 57.Gannon P J, Holloway R L, Broadfield D C, Braun A R. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 58.Hopkins W D, Marino L, Rilling J K, McGregor L A. NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- 59.Savage-Rumbaugh E S. Ape Language: From Conditioned Response to Symbol. New York: Columbia Univ. Press; 1986. [Google Scholar]
- 60.Rapoport S I. Brain Res Rev. 1990;15:267–294. doi: 10.1016/0165-0173(90)90004-8. [DOI] [PubMed] [Google Scholar]