Abstract
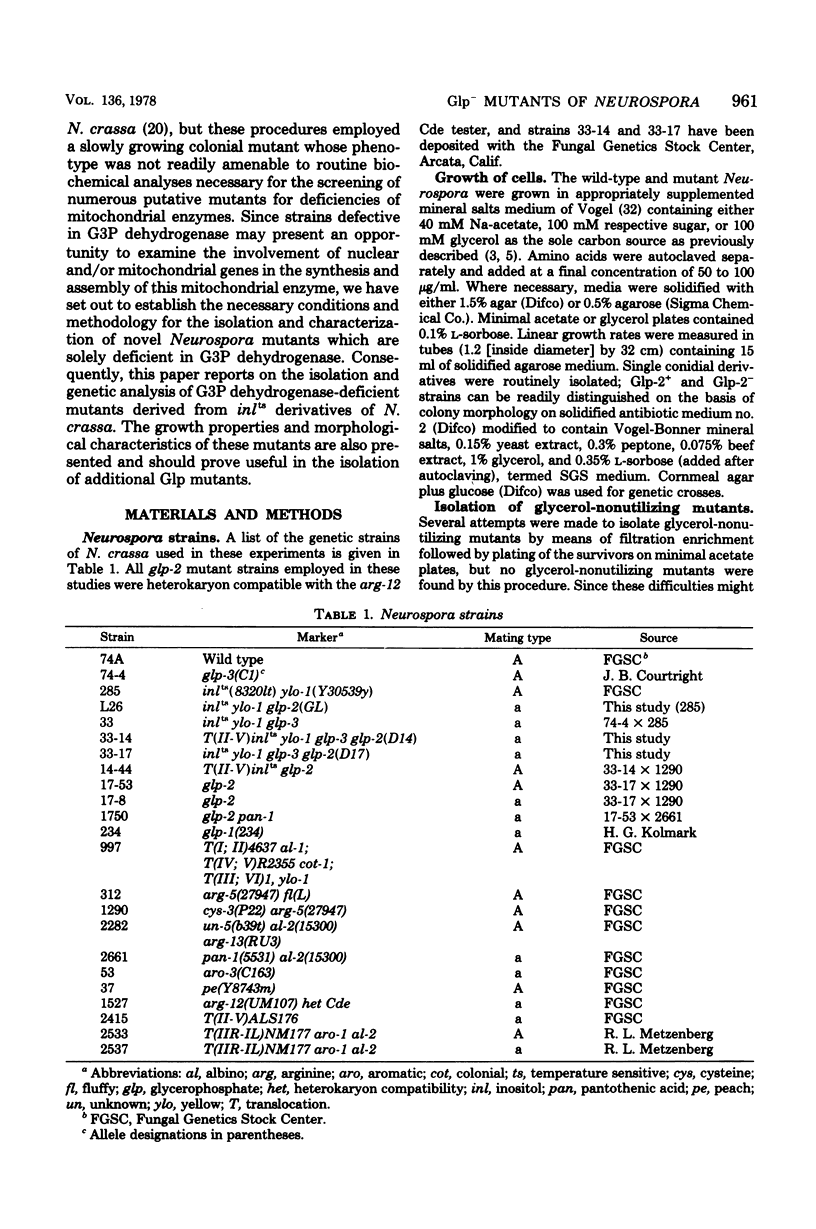
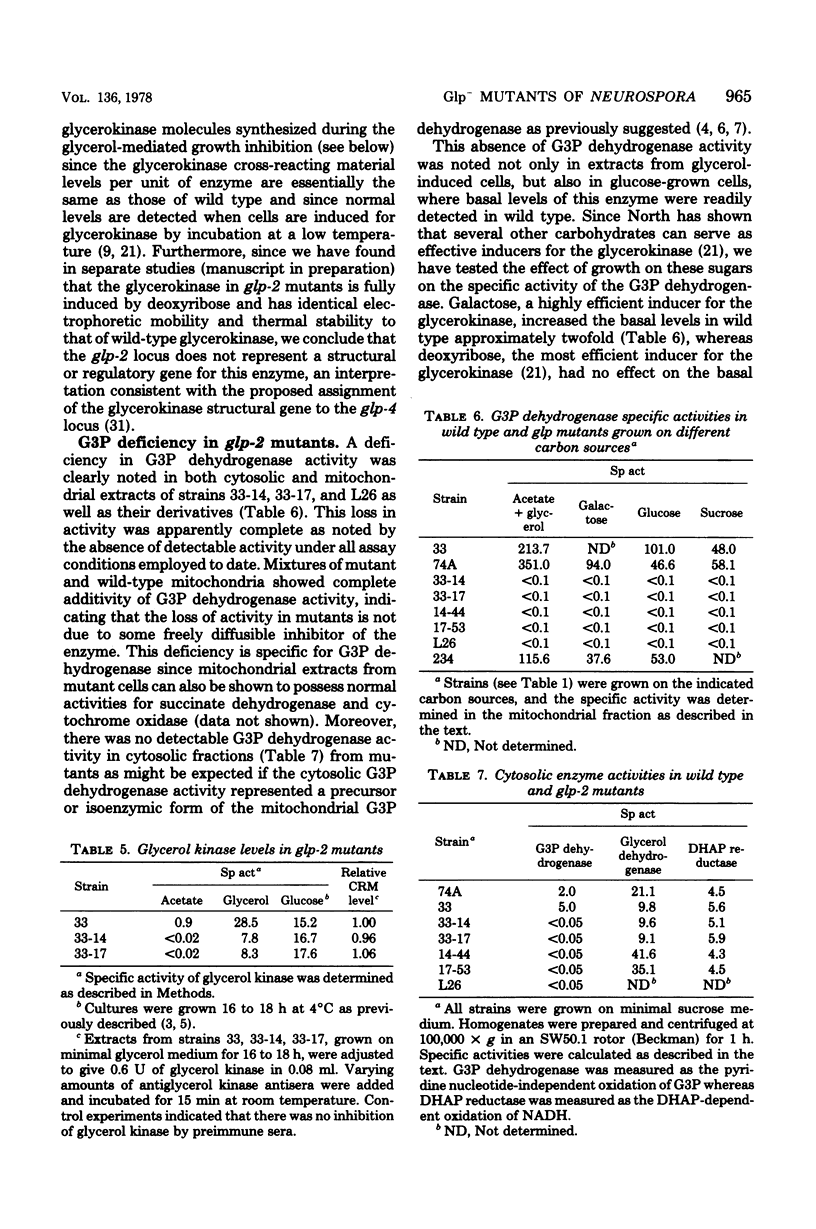
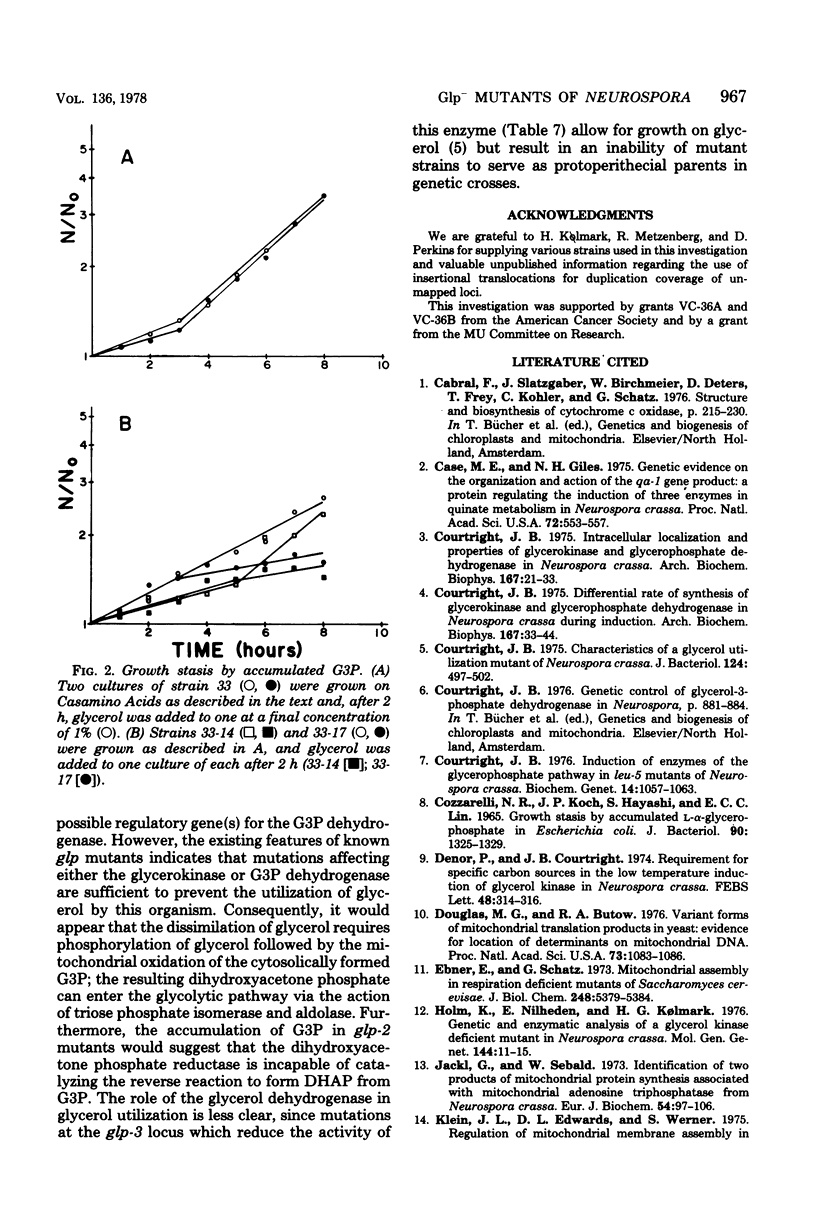
Three glycerol-nonutilizing mutants deficient in the mitochondrial glycerol-3-phosphate (G3P) dehydrogenase (EC 1.1.99.5) were isolated from inlts derivatives of Neurospora crassa following inositolless death at elevated temperatures on minimal glycerol medium. These mutants failed to grow on glycerol as a sole carbon source, but could grow on acetate, glucose, or mannitol media and were female fertile in genetic crosses, thereby distinguishing them from the previously reported polyol-protoperithecial defective Neurospora mutants. In addition, these glp mutants exhibited a distinct morphological alteration during vegetative growth on sucrose slants and colonial growth on sorbose-containing semicomplete medium. The glp-2 locus was assigned a location between arg-5 and nuc-2 on chromosome IIR on the basis of two-factor crosses and by duplication coverage by insertional translocation ALS176, but not NM177. All mutations were allelic as judged from the absence of both complementation in forced heterokaryons and genetic recombination among glp-2 mutations. The reversion frequency of all three mutations was less than 1010, indicating probable deletions in these strains. No G3P dehydrogenase activity could be detected in either cytosolic or mitochondrial extracts from mutant strains grown on glycerol, glucose, or galactose media. These results suggest that the glp-2 locus may be the structural gene for both the cytosolic and mitochondrial forms of G3P dehydrogenase or for a cytosolic precursor of the mitochondrial G3P dehydrogenase. The defect is specific for the G3P dehydrogenase since normal activities of the mitochondrial cytochrome oxidase and succinate dehydrogenase and the cytosolic glycerol dehydrogenase and dihydroxyacetone phosphate reductase are detected in mutant extracts. During attempted growth of glp-2 mutants on glycerol media, there was an accumulation of G3P in culture filtrates, a reduction in the mycelial growth rate, and a decreased level of glycerokinase induction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case M. E., Giles N. H. Genetic evidence on the organization and action of the qa-1 gene product: a protein regulating the induction of three enzymes in quinate catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Feb;72(2):553–557. doi: 10.1073/pnas.72.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright J. B. Characteristics of a glycerol utilization mutant of Neurospora crassa. J Bacteriol. 1975 Oct;124(1):497–502. doi: 10.1128/jb.124.1.497-502.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright J. B. Differential rates of synthesis of glycerokinase and glycerophosphate dehydrogenase in Neurospora crassa during induction. Arch Biochem Biophys. 1975 Mar;167(1):34–44. doi: 10.1016/0003-9861(75)90438-5. [DOI] [PubMed] [Google Scholar]
- Courtright J. B. Induction of enzymes of the glycerophosphate pathway in leu-5 mutants of Neurospora crassa. Biochem Genet. 1976 Dec;14(11-12):1057–1063. doi: 10.1007/BF00485136. [DOI] [PubMed] [Google Scholar]
- Courtright J. B. Intracellular localization and properties of glycerokinase and glycerophosphate dehydrogenase in Neurospora crassa. Arch Biochem Biophys. 1975 Mar;167(1):21–33. doi: 10.1016/0003-9861(75)90437-3. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denor P., Courtright J. B. Requirement for specific carbon sources in the low temperature induction of glycerol kinase in Neurospora crassa. FEBS Lett. 1974 Nov 15;48(2):314–316. doi: 10.1016/0014-5793(74)80494-1. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner E., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. 3. A nuclear mutant lacking mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Aug 10;248(15):5379–5384. [PubMed] [Google Scholar]
- Holm K., Nilheden E., Kolmark H. G. Genetic and enzymatic analysis of a glycerol kinase deficient mutant in Neurospora crassa. Mol Gen Genet. 1976 Feb 27;144(1):11–15. doi: 10.1007/BF00277297. [DOI] [PubMed] [Google Scholar]
- Jackl G., Sebald W. Identification of two products of mitochondrial protein synthesis associated with mitochondrial adenosine triphosphatase from Neurospora crassa. Eur J Biochem. 1975 May;54(1):97–106. doi: 10.1111/j.1432-1033.1975.tb04118.x. [DOI] [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlewood B. S., Chia W., Metzenberg R. L. Genetic control of phosphate-metabolizing enzymes in Neurospora crassa: relationships among regulatory mutations. Genetics. 1975 Mar;79(3):419–434. doi: 10.1093/genetics/79.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Schatz G. Cytochrome c oxidase from bakers' yeast. II. Site of translation of the protein components. J Biol Chem. 1973 Feb 25;248(4):1355–1360. [PubMed] [Google Scholar]
- Mishra N. C. Genetics and biochemistry of morphogenesis in Neurospora. Adv Genet. 1977;19:341–405. doi: 10.1016/s0065-2660(08)60248-5. [DOI] [PubMed] [Google Scholar]
- Nilheden E., Holm K., Kolmark H. G. Glycerol non-utilizing mutants in neurospora crassa. Isolation by net replication. Hereditas. 1975;79(2):239–250. doi: 10.1111/j.1601-5223.1975.tb01480.x. [DOI] [PubMed] [Google Scholar]
- North M. J. Cold-induced increase of glycerol kinase in Neurospora crassa. FEBS Lett. 1973 Sep 1;35(1):67–70. doi: 10.1016/0014-5793(73)80578-2. [DOI] [PubMed] [Google Scholar]
- North M. J. Influence of the carbon source on glycerol kinase activity in Neurospora crassa. Biochim Biophys Acta. 1976 Feb 13;422(2):316–325. doi: 10.1016/0005-2744(76)90143-1. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Barry E. G. The cytogenetics of Neurospora. Adv Genet. 1977;19:133–285. doi: 10.1016/s0065-2660(08)60246-1. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Newmeyer D., Taylor C. W., Bennett D. C. New markers and map sequences in Neurospora crassa, with a description of mapping by duplication coverage, and of multiple translocation stocks for testing linkage. Genetica. 1969;40(3):247–278. doi: 10.1007/BF01787357. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. The manifestation of chromosome rearrangements in unordered asci of Neurospora. Genetics. 1974 Jul;77(3):459–489. doi: 10.1093/genetics/77.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Partial resolution of enzymes containing seven or six subunits, from yeast and beef heart, respectively. J Biol Chem. 1976 Jan 25;251(2):257–269. [PubMed] [Google Scholar]
- Sebald W., Weiss H., Jackl G. Inhibition of the assembly of cytochrome oxidase in Neurospora crassa by chloramphenicol. Eur J Biochem. 1972 Nov 7;30(3):413–417. doi: 10.1111/j.1432-1033.1972.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Cronan J. E. Isolation and characterization of Saccharomyces cerevisiae mutants defective in glycerol catabolism. J Bacteriol. 1977 Mar;129(3):1335–1342. doi: 10.1128/jb.129.3.1335-1342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system. Characterization of nuclear mutants of Saccharomyces cerevisiae with defects in mitochondrial ATPase and respiratory enzymes. J Biol Chem. 1975 Oct 25;250(20):8228–8235. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B., Zulch G. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem. 1975 Oct 25;250(20):8236–8242. [PubMed] [Google Scholar]
- Viswanath-Reddy M., Bennett S. N., Howe H. B., Jr Characterization of glycerol nonutilizing and protoperithecial mutants of Neurospora. Mol Gen Genet. 1977 May 20;153(1):29–38. doi: 10.1007/BF01035993. [DOI] [PubMed] [Google Scholar]
- Werner S., Schwab A. J., Neupert W. Precursors of cytochrome oxidase in cytochrome-oxidase-deficient cells of Neurospora crassa. Comparison of the nuclear mutant cni-1, the cytoplasmic mutant mi-1, and copper-depleted wild type. Eur J Biochem. 1974 Dec 2;49(3):607–617. doi: 10.1111/j.1432-1033.1974.tb03864.x. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]




