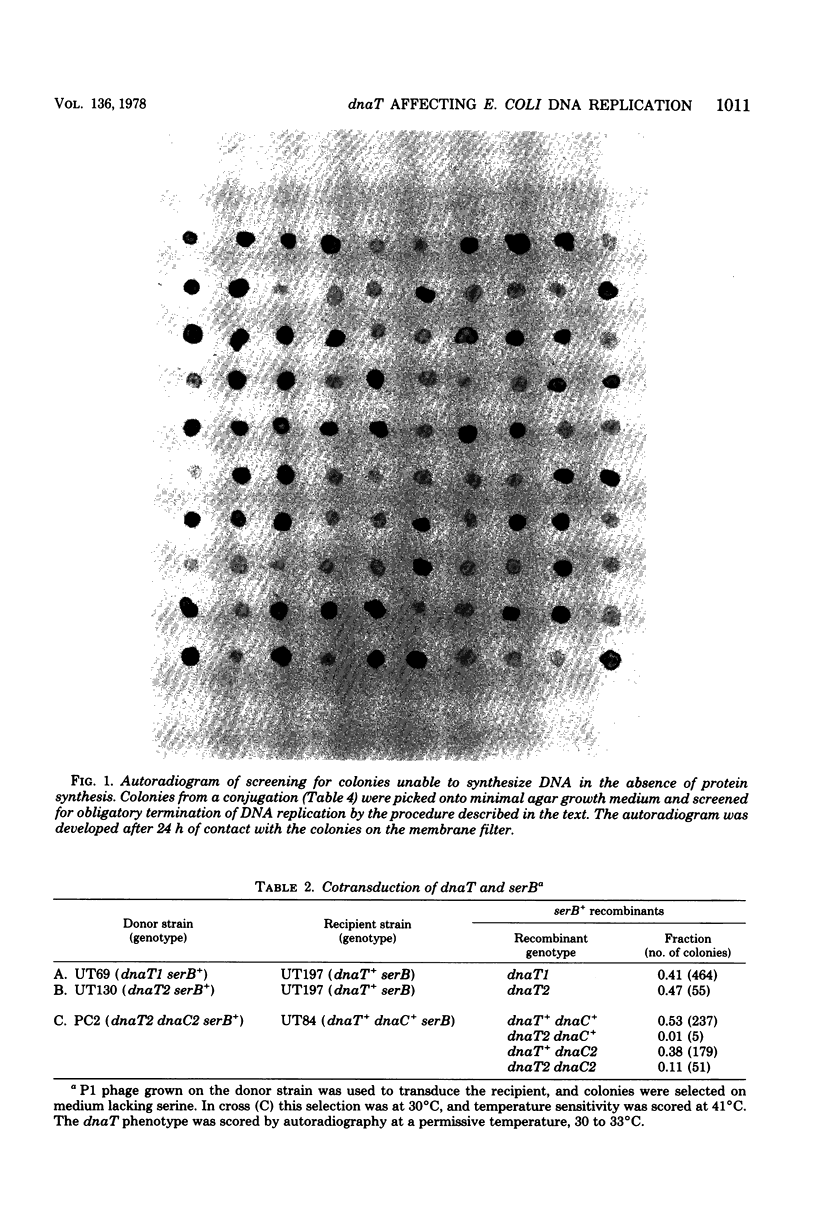
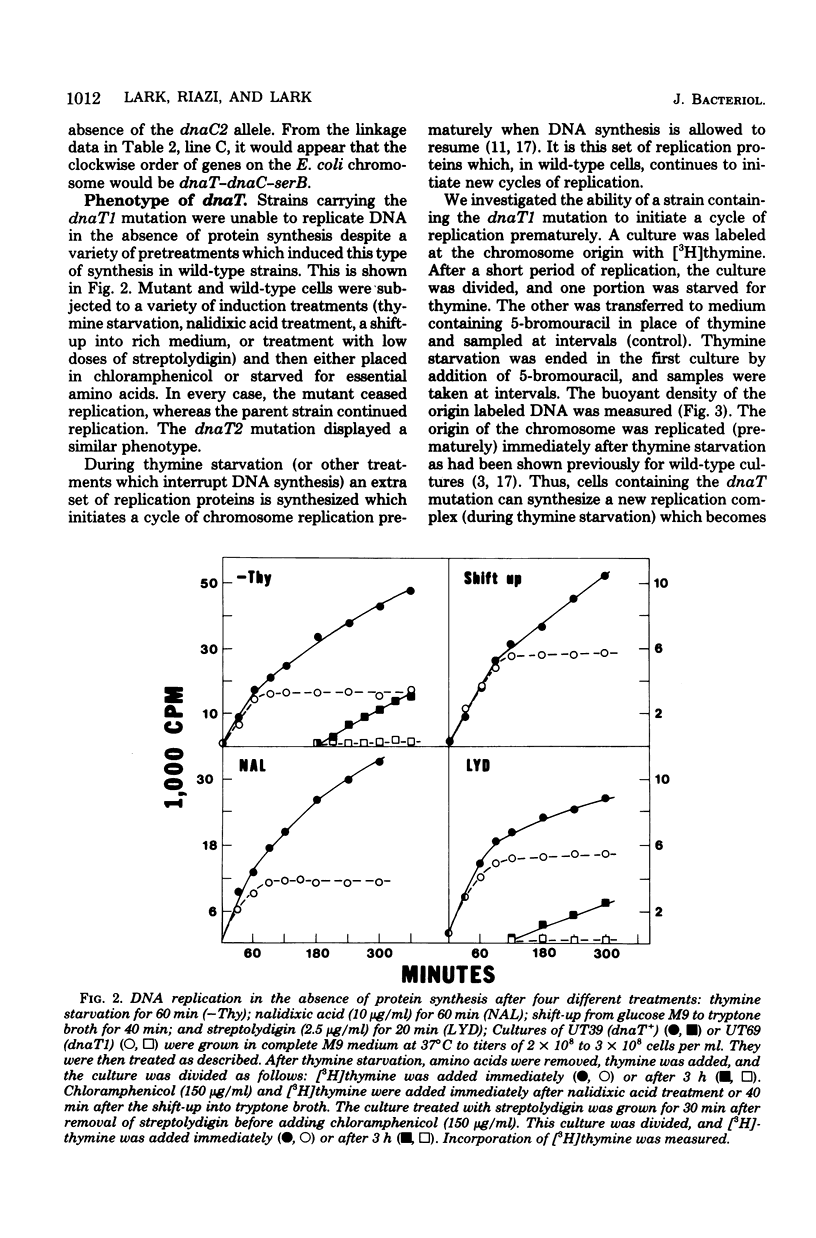
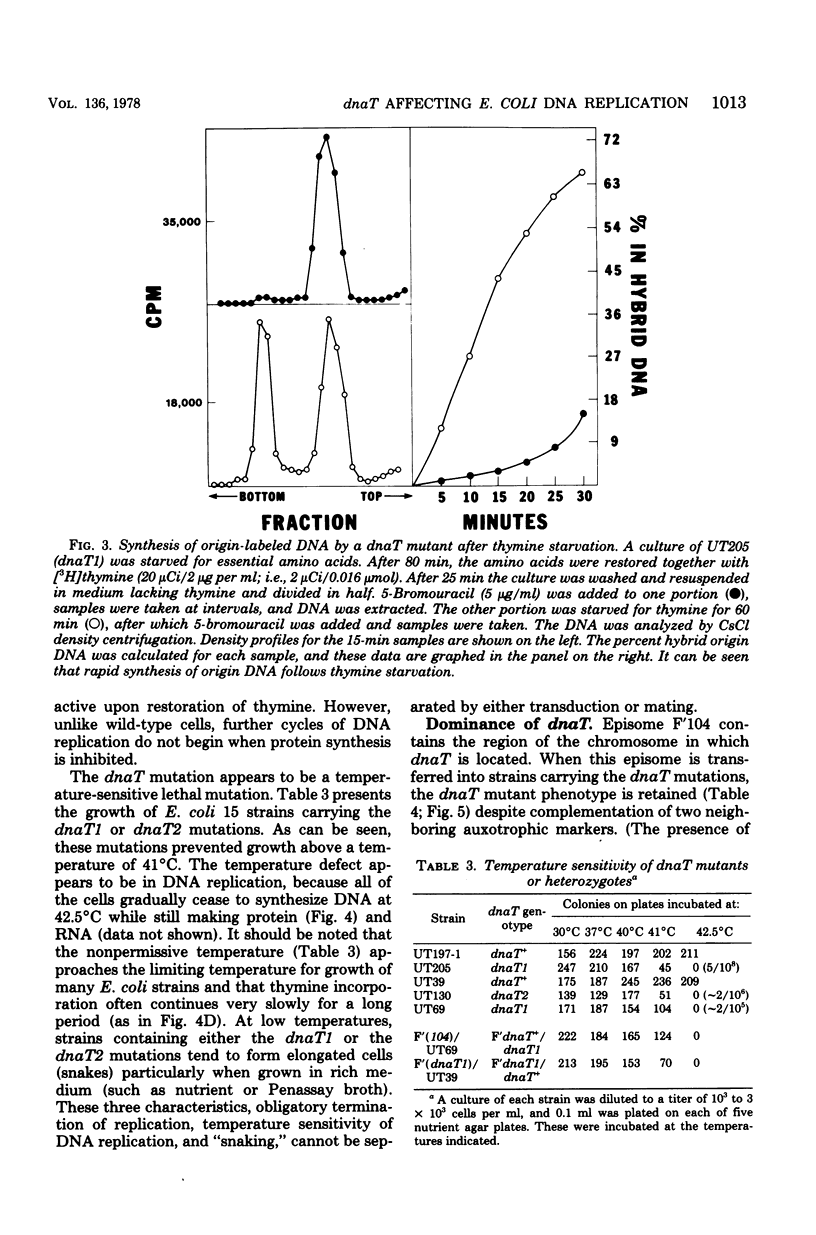
Abstract
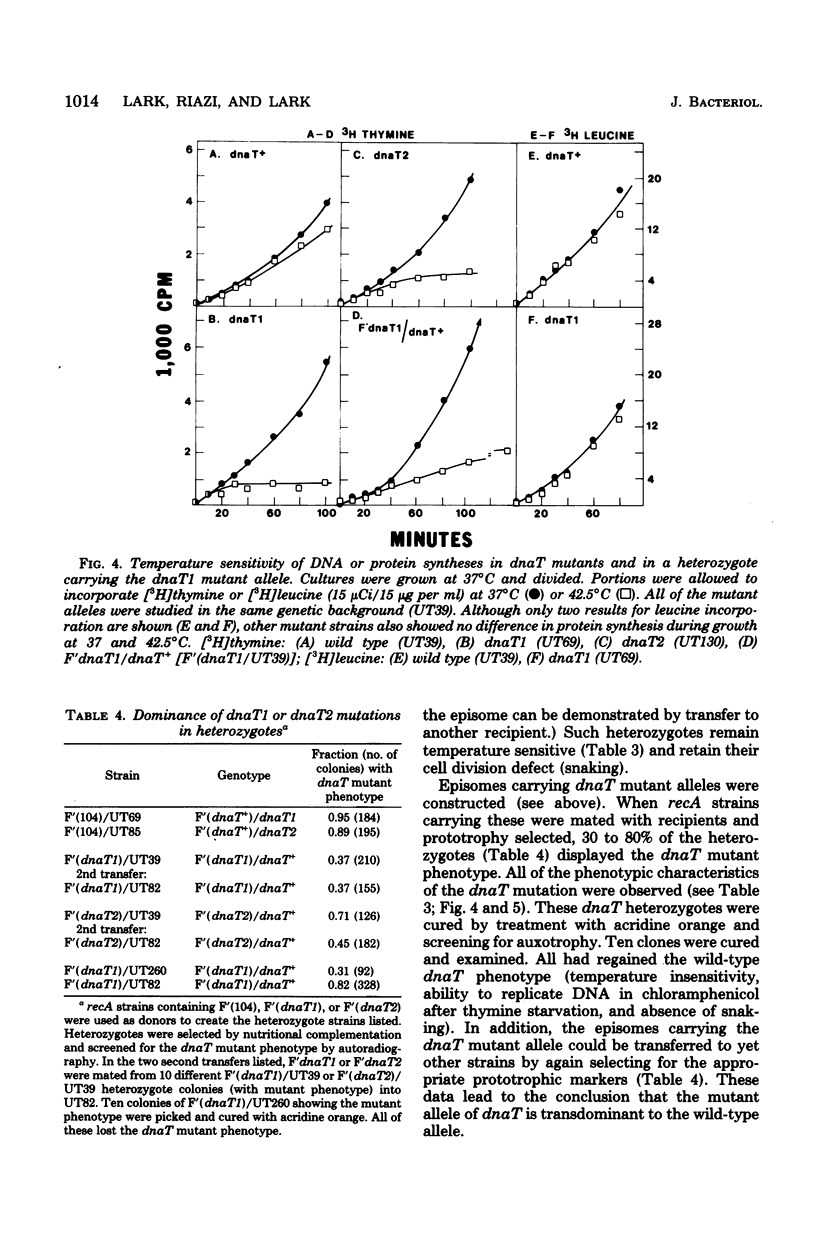
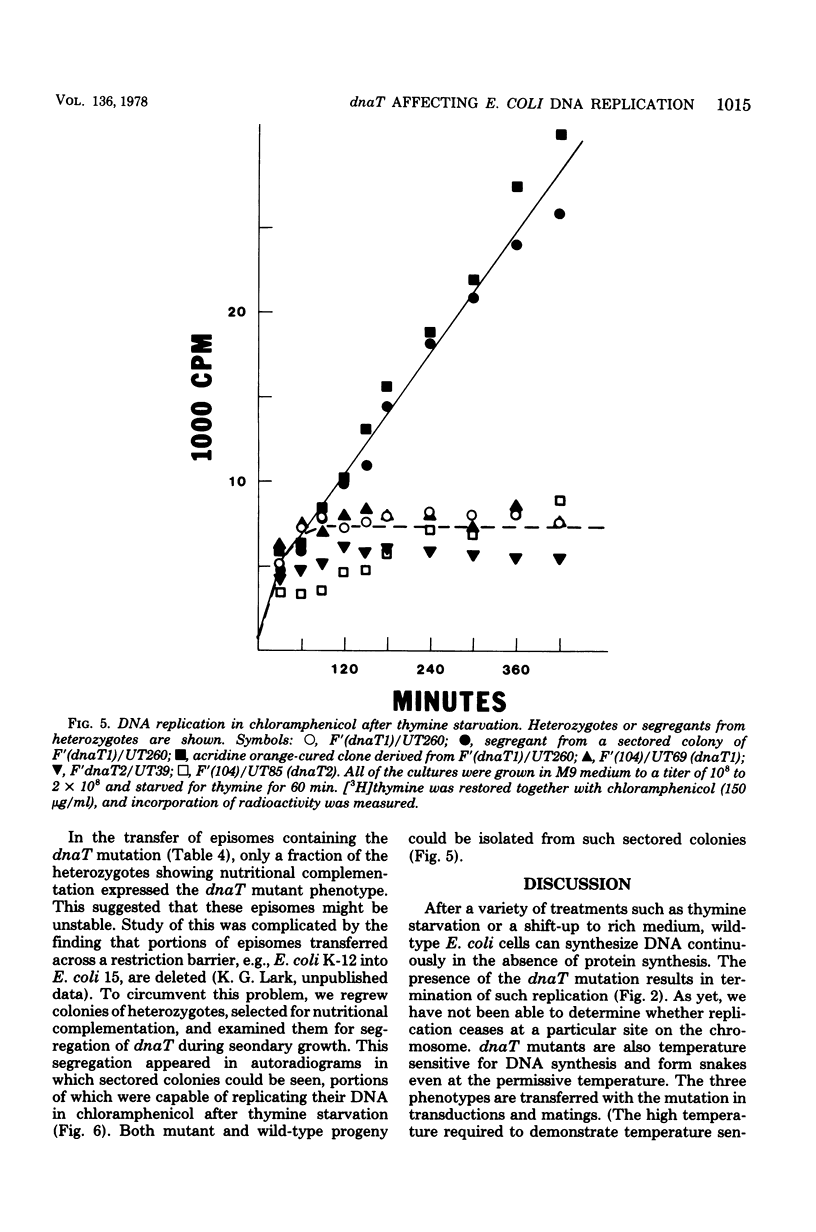
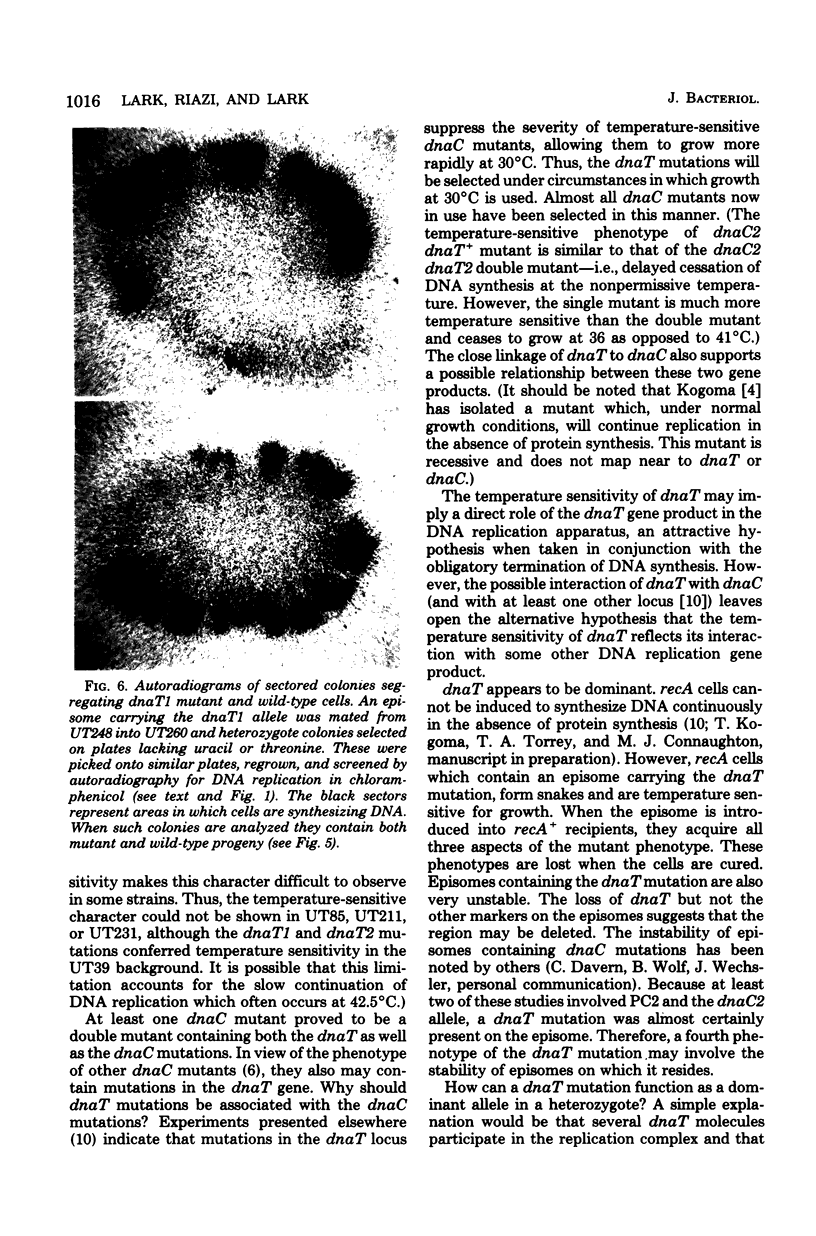
Normally, bacteria cease DNA replication in the absence of protein synthesis. A variety of treatments, such as thymine starvation or a shift-up to rich medium, lead to continued DNA replication in the absence of protein synthesis. Mutants are described which always terminate replication under these conditions. These conditional lethal mutants, dnaT1 and dnaT2, contransduce with serB and dnaC. The mutation also affects cell division. All aspects of the mutant phenotype (obligatory termination of replication, temperature sensitivity of DNA replication and growth, and aberrant cell division at permissive growth temperatures) were transdominant to the wild-type phenotype. Episomes carrying the dnaT mutation appeared to be unstable. The existence of such a dominant mutation was predicted by a model of chromosome termination proposed by Kogoma and Lark (J. Mol. Biol. 94:243-256, 1975).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977 Oct 20;269(5630):655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Fralick J. A., Lark K. G. Evidence for the involvement of unsaturated fatty acids in initiating chromosome replication in Escherichia coli. J Mol Biol. 1973 Nov 5;80(3):459–475. doi: 10.1016/0022-2836(73)90416-6. [DOI] [PubMed] [Google Scholar]
- Kogoma T. A novel Escherichia coli mutant capable of DNA replication in the absence of protein synthesis. J Mol Biol. 1978 May 5;121(1):55–69. doi: 10.1016/0022-2836(78)90262-0. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J Mol Biol. 1975 May 15;94(2):243–256. doi: 10.1016/0022-2836(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. DNA replication in Escherihia coli: replication in absence of protein synthesis after replication inhibition. J Mol Biol. 1970 Sep 14;52(2):143–164. doi: 10.1016/0022-2836(70)90022-7. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Multiple stages in the enzymic replication of DNA. Biochem Soc Trans. 1977;5(2):359–374. doi: 10.1042/bst0050359. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Renger H. Initiation of DNA replication in Escherichia coli 15T-: chronological dissection of three physiological processes required for initiation. J Mol Biol. 1969 Jun 14;42(2):221–235. doi: 10.1016/0022-2836(69)90039-4. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Wechsler J. A. DNA replication in dnaB mutants of Escherichia coli: gene product interaction and synthesis of 4 S pieces. J Mol Biol. 1975 Feb 15;92(1):145–163. doi: 10.1016/0022-2836(75)90095-9. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Nüsslein V., Otto B., Klein A., Bonhoeffer F., Herrmann R., Gloger L., Schaller H. Isolation and characterization of thermosensitive Escherichia coli mutants defective in deoxyribonucleic acid replication. J Bacteriol. 1973 Mar;113(3):1381–1388. doi: 10.1128/jb.113.3.1381-1388.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]