Abstract
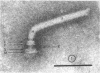
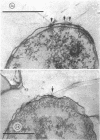
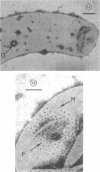
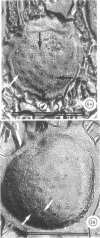
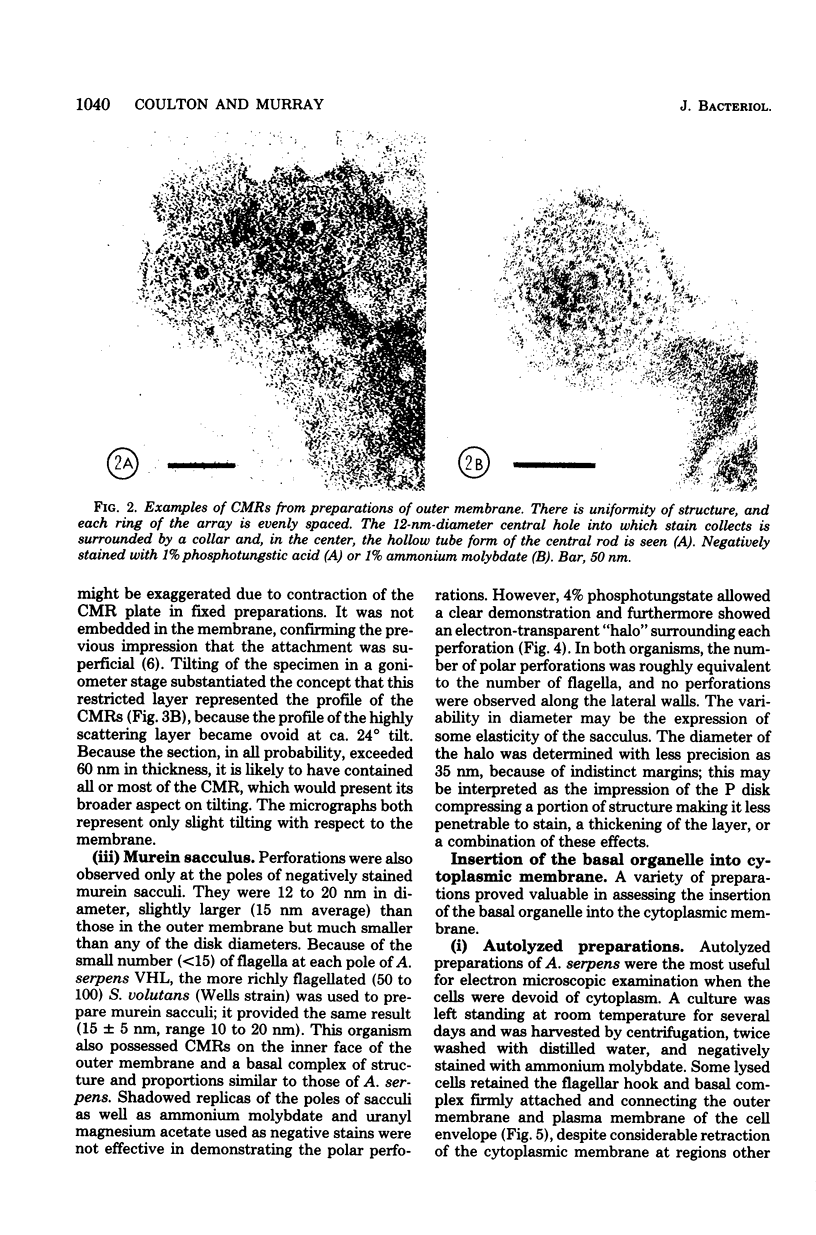
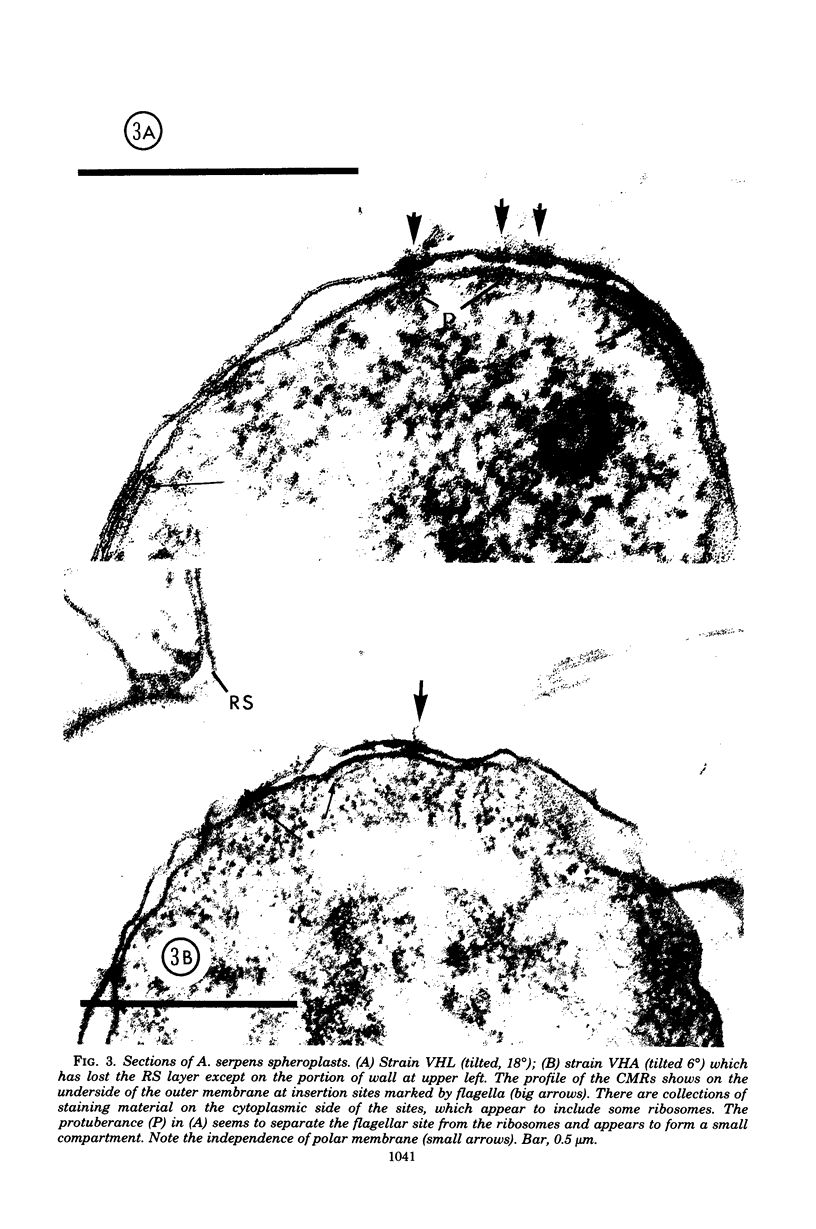
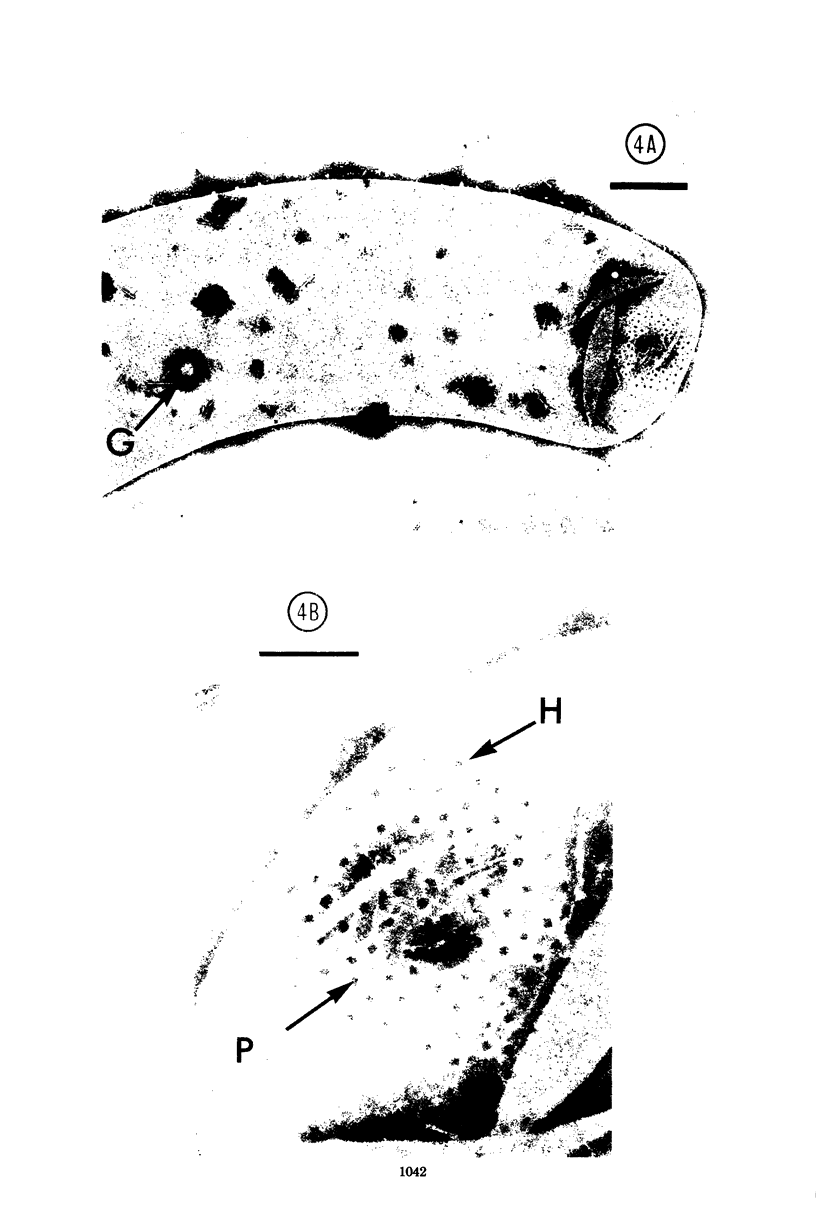
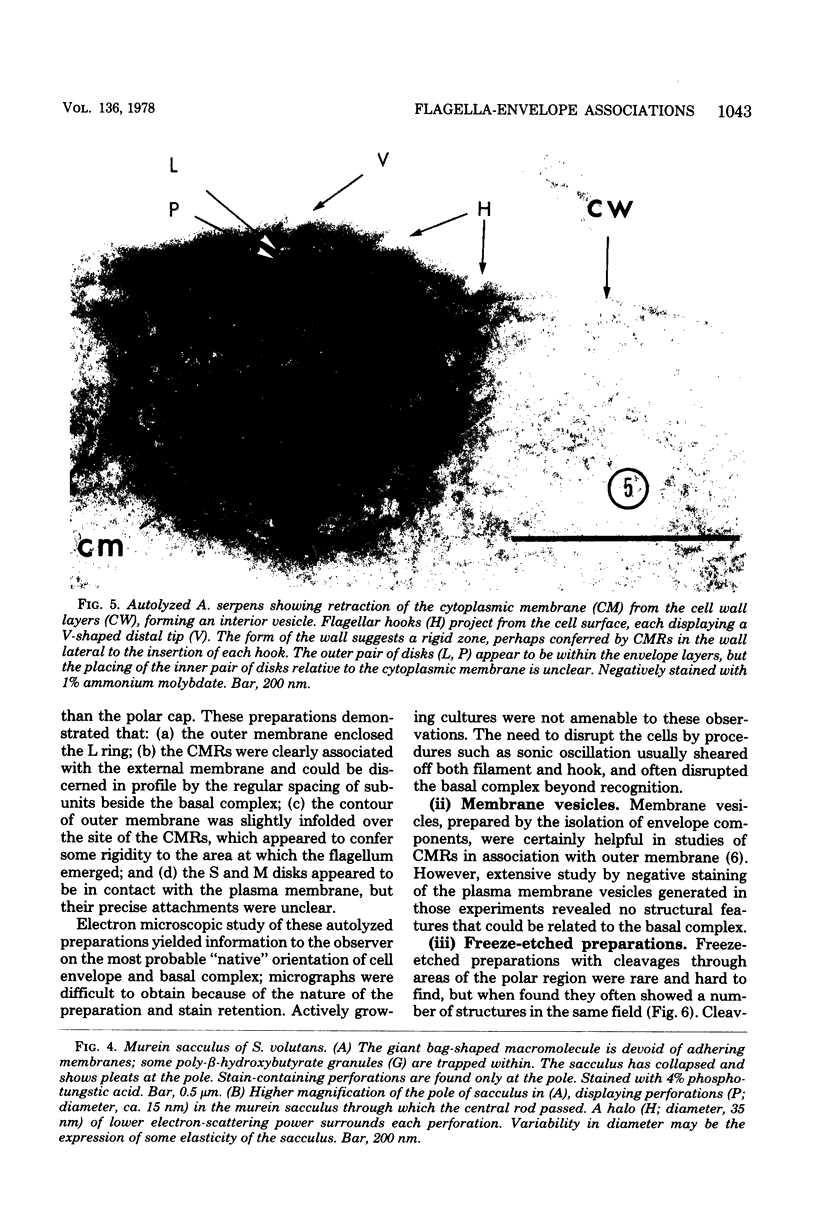
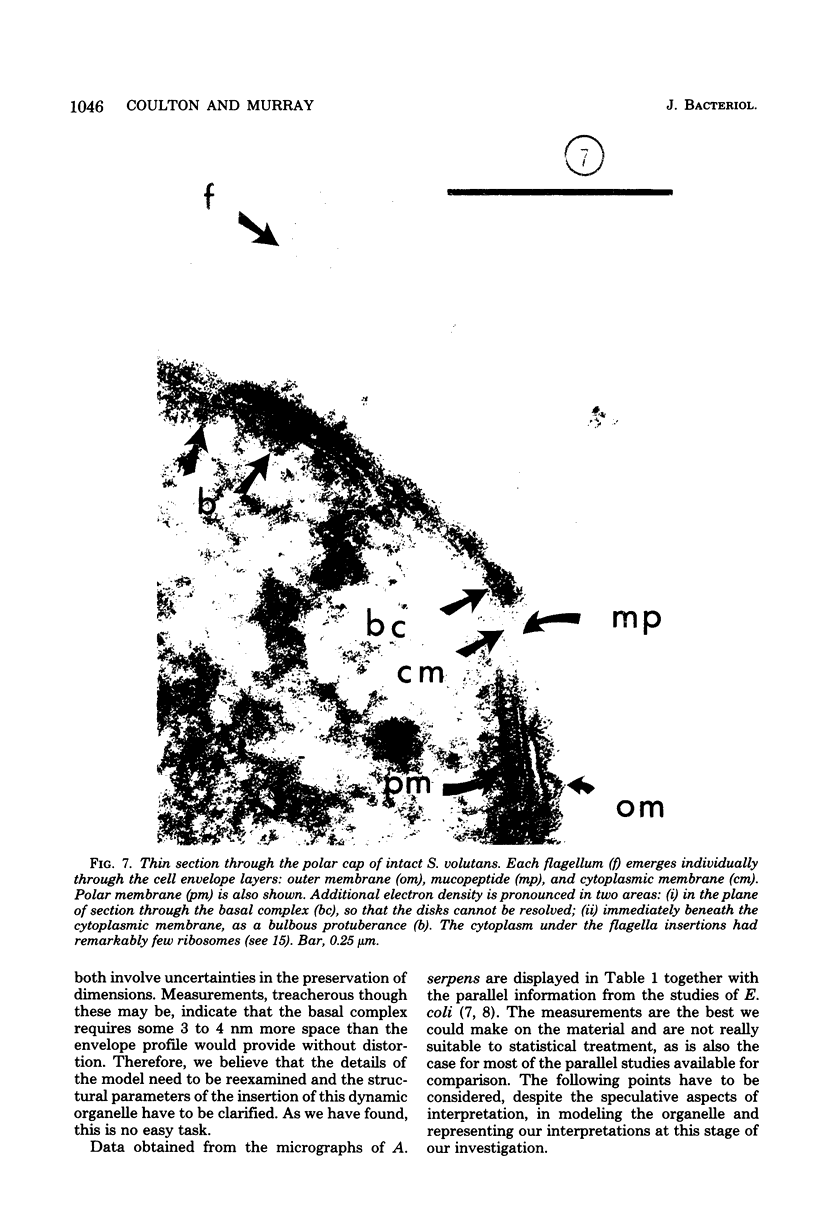
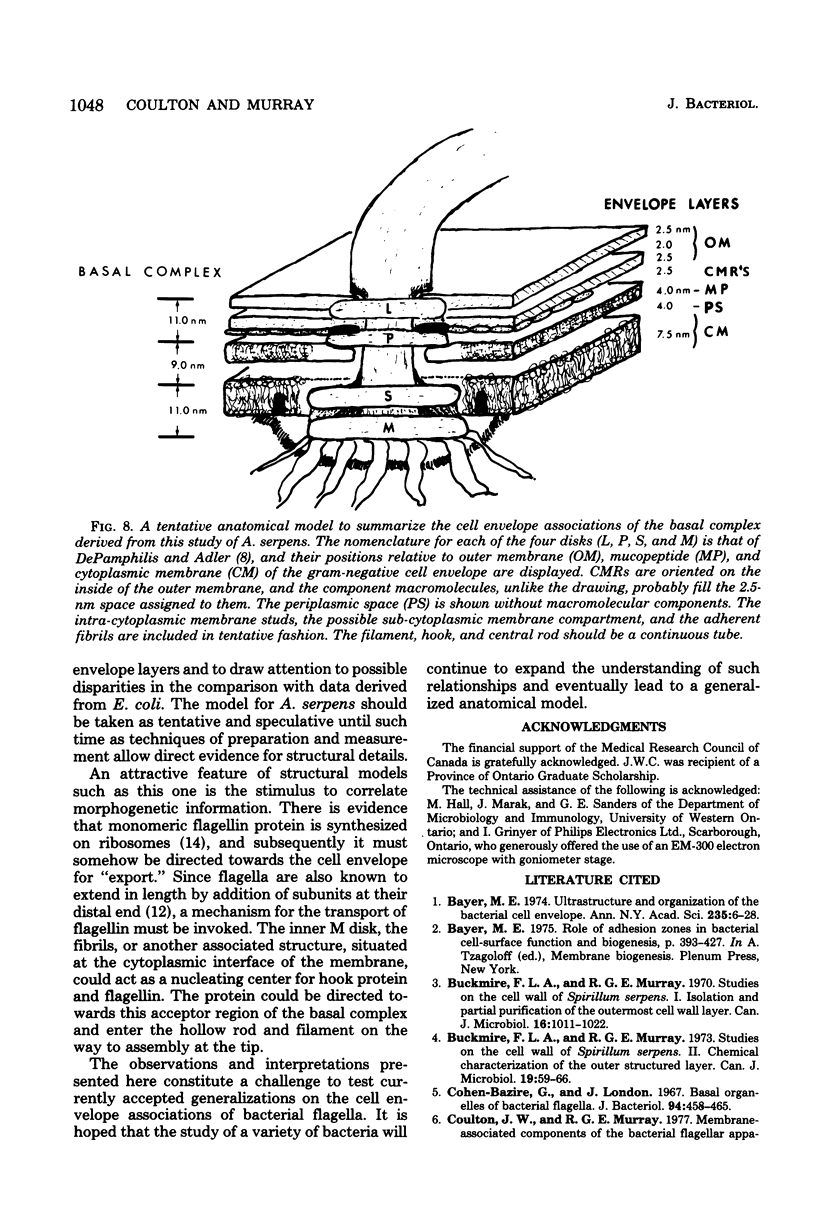
Specific regions of the cell envelope associated with the flagellar basal complex of the gram-negative bacterium Aquaspirillum (Spirillum) serpens were identified by studying each of the envelope layers: outer membrane, mucopeptide, and plasma membrane. The outer membrane around the flagella insertion site was differentiated by concentric membrane rings and central perforations surrounded by a closely set collar. The perforations in both the outer membrane and the isolated mucopeptide layer were of a size accomodating the central rod of the basal complex but smaller than either the L or the P disks. The P disk of the complex may lie between the mucopeptide and the outer membrane. Electron microscopy of intact, spheroplasted, or autolyzed preparations did not adequately resolve the location of the inner pair of disks of the basal complex. Freeze-etching, however, revealed differentiation within the plasma membrane that appeared to be related to the basal complex. The convex fracture face showed depressions which are interpreted as impressions of a disk surrounded by a set of evenly spaced macromolecular studs and containing a central "plug" interpreted as the central rod. In thin sections, blebs, which appear to be associated with the flagellar apparatus, were seen on the cytoplasmic side of the plasma membrane. Superimposing the dimensions of the flagellar basal complex and the spacings of the cell envelope layers and using the position of the L disk within the outer membrane for reference, showed that the S disk might be within and the M disk beneath the plasma membrane. A tentative model was developed for comparison with that based on the structure of the Escherichia coli basal complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Ultrastructure and organization of the bacterial envelope. Ann N Y Acad Sci. 1974 May 10;235(0):6–28. doi: 10.1111/j.1749-6632.1974.tb43254.x. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., London J. Basal organelles of bacterial flagella. J Bacteriol. 1967 Aug;94(2):458–465. doi: 10.1128/jb.94.2.458-465.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Polarity of flagellar growth in salmonella. J Gen Microbiol. 1969 May;56(2):227–239. doi: 10.1099/00221287-56-2-227. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Gordee E. Z. Formation of bacterial flagella. I. Demonstration of a functional flagellin pool in spirillum serpens and bacillus subtilis. J Bacteriol. 1966 Feb;91(2):870–875. doi: 10.1128/jb.91.2.870-875.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor H. Use of freeze-etching in the study of biological ultrastructure. Int Rev Exp Pathol. 1966;5:179–216. [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellen J. E., Starr M. P. Alterations in the cell wall of Spirillum serpens VHL early in its association with Bdellovibrio bacteriovorus 109D. Arch Microbiol. 1976 May 3;108(1):55–64. doi: 10.1007/BF00425093. [DOI] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Relationship between cell wall, cytoplasmic membrane, and bacterial motility. J Bacteriol. 1969 Oct;100(1):512–521. doi: 10.1128/jb.100.1.512-521.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- Wells J. S., Krieg N. R. Cultivation of Spirillum volutans in a Bacteria-Free Environment. J Bacteriol. 1965 Sep;90(3):817–818. doi: 10.1128/jb.90.3.817-818.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]