Abstract
To study the physiological and molecular mechanisms of age-related memory loss, we assessed spatial memory in C57BL/B6 mice from different age cohorts and then measured in vitro the late phase of hippocampal long-term potentiation (L-LTP). Most young mice acquired the spatial task, whereas only a minority of aged mice did. Aged mice not only made significantly more errors but also exhibited greater individual differences. Slices from the hippocampus of aged mice exhibited significantly reduced L-LTP, and this was significantly and negatively correlated with errors in memory. Because L-LTP depends on cAMP activation, we examined whether drugs that enhanced cAMP would attenuate the L-LTP and memory defects. Both dopamine D1/D5 receptor agonists, which are positively coupled to adenylyl cyclase, and a cAMP phosphodiesterase inhibitor ameliorated the physiological as well as the memory defects, consistent with the idea that a cAMP–protein kinase A-dependent signaling pathway is defective in age-related spatial memory loss.
Animal models of aging have proven useful not only for understanding the aging process but also for understanding the functioning of the hippocampus. Age-related deficits in hippocampus-dependent memory have been observed across a wide variety of species (1–11) and have been explored most extensively in rodents, where a marked defect in the ability to store spatial memory has been observed (1, 3, 6–11). This defect is consistent with the spatial memory deficits observed in aged humans (4, 5). Interestingly, not all aged rodents exhibit these defects. A proportion age successfully and acquire spatial memory tasks similarly to young cohorts (1, 3, 6–11). Moreover, within an aged cohort, much greater individual differences are observed (1, 7, 8, 11). These individual differences indicate that age-related effects are not ubiquitous across all subjects and allows one to study the relationship between memory and a particular neurobiological marker both across age cohorts as well as within a particular age cohort.
Aging is associated with a variety of memory deficits. One prominent hippocampal component is a defect in the ability to consolidate short-term memory into long-term memory. Pharmacological studies of consolidation in experimental animals revealed that consolidation of short-term to long-term memory requires new protein and mRNA synthesis, a finding that implies that long-term memory requires the expression of specific genes (12). The consolidation of protein synthesis-dependent memory is thought to involve long-term changes in synaptic strength (13). Consistent with this idea, long-term potentiation (LTP) in hippocampus has been shown to have distinct phases that correspond to the stages of memory: an early phase (E-LTP) that can be induced by 1 or 2 trains, lasts 1–2 hours, and does not require new protein synthesis, and a late phase (L-LTP) that is induced by 3 or 4 trains, lasts longer than 3 hours, and does require protein and RNA synthesis (14–17). Furthermore, pharmacological and genetic studies have shown that the induction of L-LTP in the CA1 region of mouse hippocampal slices involves a molecular pathway that includes D1/D5 receptors, cAMP, cAMP-dependent protein kinase (PKA), and the transcriptional activator CREB (18–22).
We have therefore examined the relationship between L-LTP in mouse hippocampal slices and both memory and its defects during aging. In particular, we have addressed three questions: (i) Is there an age-related defect in L-LTP, and if so, does it correlate with age-related defects in spatial memory? (ii) Independent of aging, do aged mice that exhibit good memory have robust L-LTP, and conversely, do young mice that exhibit deficits in spatial memory have diminished L-LTP? (iii) Can pharmacological manipulations of the cAMP pathway reverse the age-related defects in L-LTP and memory?
Consistent with the idea that a cAMP–PKA-dependent signaling pathway is defective in age-related memory loss, we find that there is an age-related defect in both spatial memory and in the late phase of LTP that is reversed by agents that act to increase the level of cAMP.
MATERIALS AND METHODS
Animals.
All behavioral and electrophysiological studies were done with pathogen-free male C57BL/B6 mice acquired from the National Institute of Aging colony at Charles River Breeding Laboratories. Animal care was performed according to National Institutes of Health guidelines in a facility accredited by the Association for Accreditation of Laboratory Animal Care. The mice were singly housed on arrival to our animal care facility and acclimated to the new environment for at least 1 week before testing. The colony was maintained on a 12 hr light/dark schedule at 70°F. Food and water were available ad lib.
Behavioral Testing.
The Barnes circular maze has been described (23). Testing occurred once a day until an acquisition criterion of 7 of 8 consecutive days with three or fewer errors was met or 40 consecutive days elapsed. Errors were defined as searching a hole that did not have the tunnel beneath it, and included nose pokes and head deflections. At the end of each session, the search strategy used was recorded. The random search strategy was operationally defined as localized searches of holes separated by maze center crosses. The serial search strategy was defined as a systematic search of consecutive holes in a clockwise or counterclockwise direction. The spatial search strategy was defined as navigating directly to the tunnel with error and distance scores ≤3. Distance was calculated by counting the number of holes between the first hole searched within a session and the escape tunnel. Analysis of errors (from both the spatial and cued versions) and search strategy data was done by using a two-factor ANOVA (age and session blocks) with one repeated measure. If a significant F value was obtained, a one-factor ANOVA (age) was calculated for each session block and the Scheffe post hoc test was employed. A Fisher’s r to z was employed to determine whether a correlation coefficient was statistically different from zero. The Kruskal–Wallis test was used to compare percentage of each age group that acquired the task.
Drug administration began on the 15th session and continued each day until acquisition occurred or 40 days elapsed. The D1/D5 agonist (±) SKF-38393 HCL (Research Biochemicals, Natick, MA) was dissolved in saline. Rolipram (Research Biochemicals, Natick, MA) was dissolved in 10% Cremophor in PBS. Neither SKF 38393 nor rolipram produced any observable side effects. i.p. injections were given 40 min before each daily session. The experimenter recording the data was blind to drug and dose. Because the 18-month-old mice were significantly different from the other age cohorts during the last session block, for the SKF 38393 study we compared the mean number of errors across dose groups during this session block by using a one-way ANOVA, and we used a Bonferroni–Dunn test to determine which groups were significantly different. For the rolipram study, a Student’s t test was used.
Electrophysiological Testing.
Slices of mouse hippocampus (400 μm thick) were prepared as described (24) and maintained at 29°C in an interface chamber containing artificial cerebrospinal fluid (124 mM NaCl/4.4 mM KCl/25 mM NaHCO3/1.0 mM Na2HPO4/2.0 mM CaCl2/2.0 mM MgSO4/10 mM glucose). The perfusion solution was bubbled with 95% O2 and 5% CO2 at a flow rate of 1.5–2 ml/min. Electrophysiological recording began 1.5 hours after the slice was placed in the chamber. A bipolar tungsten electrode was used to stimulate fibers in the Schaffer collateral-commissural pathway at 0.02 Hz. Field excitatory postsynaptic potentials were recorded in the stratum radiatum of CA1 with an extracellular glass microelectrode filled with artificial cerebrospinal fluid with a resistance of 5–10 MΩ. The strength of presynaptic fiber stimulation was adjusted to evoke excitatory postsynaptic potentials that were 25–30% of the maximal excitatory postsynaptic potential slope. After a stable baseline (20–30 min) was obtained, E-LTP was induced by 2 trains of 100 Hz, 1-sec stimulation separated by 20 sec and L-LTP was induced by 4 trains of 100 Hz, 1-sec stimulation separated by 5 min. The experimenter was blind to both age and learning ability. The D1/D5 agonist, 6-bromo-ApB-hydrobromide (200 nM) (Research Biochemicals, Natick, MA) was dissolved in DMSO and then diluted 1:1,000 in saline. SKF 38393 (1 μM) (Research Biochemicals, Natick, MA) was dissolved directly in saline.
RESULTS
Spatial Memory Is Impaired in Aged Mice Whereas Cued Performance Is Intact.
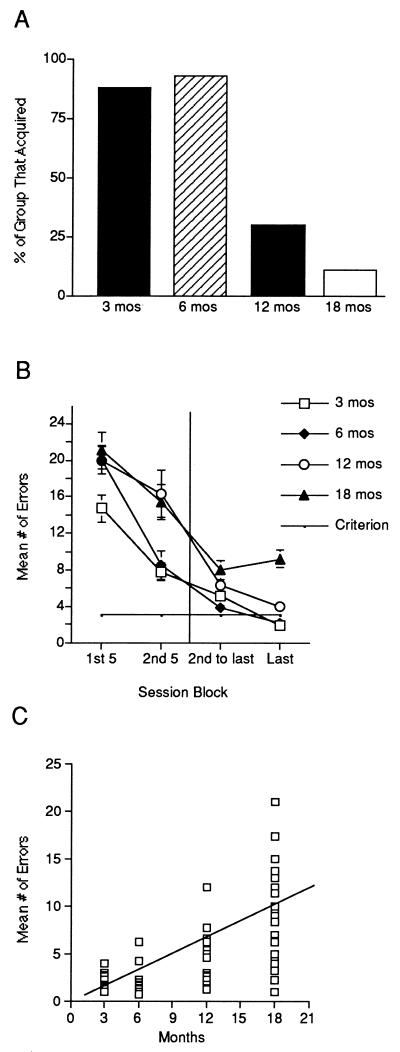
We first measured spatial memory in C57BL/B6 mice from four different age cohorts: 3, 6, 12, and 18 mo. After behavioral testing, we measured L-LTP in vitro with a blind procedure, using the same mice. This allowed us to analyze correlations between spatial memory and L-LTP. We measured spatial memory by using the spatial version of the Barnes circular maze, a task previously found to be sensitive to age-related defects in hippocampus-dependent memory (10). In that task, mice learn to navigate to an escape tunnel by using the relationships between distal cues in the environment. Consistent with previous studies, we found an age-related defect in spatial memory. A majority of the aged cohorts (12 and 18 mo) did not acquire the task, whereas the majority of younger cohorts (3 and 6 mo) did (Fig. 1A). A comparison of median trials to acquisition revealed a significant effect of age (Kruskal–Wallis: H = 30.22, P < 0.0001). We also compared the mean number of errors across blocks comprised of 5 sessions and found a significant age-related defect (Fig. 1B). Although the 18-mo-old mice showed an initial decrease in errors, this was followed by a leveling off in performance, after which no further improvement was observed. As a result, on the last session block, the 18-mo-old mice made significantly more errors than the younger mice. Furthermore, the number of errors on that block was positively and significantly correlated with age (Fig. 1C). In addition, the correlation scatterplot revealed a characteristic feature of aging described in other contexts. There was a progressive increase in individual differences across age cohorts, with the oldest cohort showing the greatest range of individual differences.
Figure 1.
Spatial memory is impaired in aged C57BL/B6 mice. (A) Percentage of 3- (n = 17), 6- (n = 16), 12- (n = 20), and 18- (n = 25) mo-old mice that acquired the spatial version of the Barnes maze. (B) A significant age-related defect was observed in mean number of errors made across 5 session blocks (age × session block interaction F9,175 = 3.14, P = 0.0016). Post hoc analysis revealed that 18-mo-old mice were significantly different from 3-, 6- and 12-mo-old mice during the last session block (P < 0.001 in each case). (C) Spatial memory errors significantly and positively correlated with age (r = 0.66, P < 0.0001). There were greater individual differences in the older cohorts.
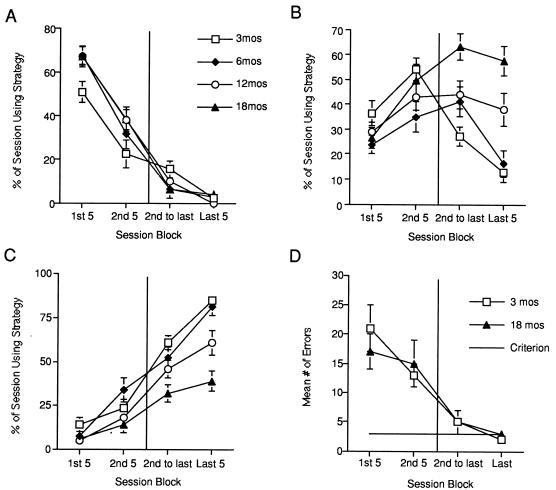
We next analyzed the performance differences between the age cohorts and found that they could be explained by differences in use of search strategies. Both young and aged cohorts similarly employed a random search strategy initially that produced a large number of errors in all groups (Fig. 2A). During the second session block, all cohorts began to use a serial search strategy. This became the primary strategy used by the aged cohorts at the end of testing (Fig. 2B). The shift from random to serial strategy by the 18-mo-old mice produced the initial decrease in errors followed by the leveling off. In contrast to the aged cohort, the majority of the young cohorts went on to use a spatial search strategy, which they then used significantly more during the last five sessions (Fig. 2C).
Figure 2.
Age-related differences in spatial memory performance can be explained by differences in search strategy use, whereas cued performance is normal. (A) Aged and young cohorts use the random search strategy similarly across session blocks. (B) Aged mice used the serial search strategy significantly more often than younger cohorts (age × session block interaction F9,175 = 4.92, P < 0.0001). Post hoc analysis revealed that 18-mo-old mice were significantly different from 3- and 6-mo-old mice during the last 2 session blocks (P < 0.05 in each case). (C) Young mice used the spatial search strategy significantly more than aged cohorts (age × session block interaction F9,175 = 3.84, P < 0.0005). Post hoc analysis revealed that 18-mo-old mice were significantly different from 3- and 6-mo-old mice during the last session block (P < 0.001 in each case). (D) On the cued version, 3- (n = 6) and 18- (n = 11) mo-old mice made a similar number of errors across session blocks (main effect of age F1,15 = 0.0242, P > 0.05, age × session block interaction F3,13 = 0.3773, P > 0.05).
The defect observed on the spatial version of the Barnes maze may reflect an impairment in hippocampal memory functioning or a gross defect in sensory motor, motivational, or attentional ability. To distinguish between a performance defect and a memory defect, we tested a new group of 3- and 18-mo-old mice on the cued version of the Barnes maze, a task that does not require the hippocampus. The cued version has contingencies and response requirements that are similar to the spatial version except that the position of the tunnel is made visible to the mice by putting a cue behind the hole where the tunnel is placed. A similar percentage of young (87%) and aged (85%) mice acquired the task. Furthermore, a comparison of the mean number of errors across session blocks revealed no difference between age groups (Fig. 2D). This result suggests that the age-related impairment observed on the spatial version is probably related to a disruption of memory and not to a defect in performance.
L-LTP Is Impaired in Aged Mice and Correlates with Spatial Memory.
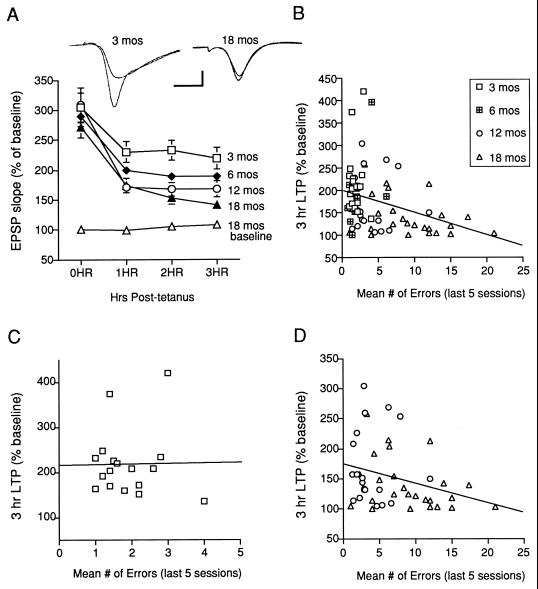
We next measured L-LTP induced in the CA1 region of hippocampal slices by 4 trains of high-frequency (100 Hz) stimulation of the Schaffer collateral pathway. Paralleling our behavioral data, there was an age-related defect in L-LTP (Fig. 3A). We found no defect immediately after tetanization. By 3 hr after tetanization, however, a significant difference between the age cohorts emerged. By contrast, the baseline EPSP in a second group of 18-mo-old mice was stable for 3 hr, suggesting the decline in L-LTP was not caused by an age-related deterioration of the slice. We also examined the effect of aging on synaptic transmission and other forms of synaptic plasticity in the CA1 region in a new group of 3- and 18-mo-old mice that did not undergo behavioral testing. In contrast to the significant age-related L-LTP defect, E-LTP measured 60 min after 2 trains of high-frequency (100 Hz) stimulation was not impaired in the 18-mo-old mice [3 mo (n = 4), 167 ± 13.0%; 18 mo (n = 4), 166 ± 8.0]. We also observed no difference between young and aged mice in the maximal excitatory postsynaptic potential slope [3 mo (n = 8), 1.77 ± 0.18 mV/msec; 18 mo (n = 7), 1.52 ± 0.13] and amplitude [3 mo (n = 8), 3.8 ± 0.33 mV; 18 mo (n = 7), 3.1 ± 0.29], or paired-pulse facilitation [3 mo (n = 8), 1.46 ± 0.05%; 18 mo (n = 7), 1.44 ± 0.06].
Figure 3.
L-LTP is impaired in aged C57BL/B6 mice and correlates with spatial memory. (A) A significant age-related defect was observed in L−LTP (main effect of age F3,74 = 4.03, P < 0.01). Post hoc analysis revealed that 18-mo-old animals were significantly different from 3-mo-old mice 1 hr, 2 hr, and 3 hr after tetanization (P < 0.05 in each case). The baseline was stable for 3 hours in 18-mo-old mice (n = 4). (B) Across age cohorts, spatial memory errors and L-LTP are significantly and negatively correlated (r = −0.334, P < 0.005). (C) Within the 3-mo-old cohort, spatial memory errors during the last 5 sessions and L-LTP at 3 hrs are not correlated (r = −0.13, P > 0.05). (D) Within the aged cohorts (12- and 18-mo-old), spatial memory errors and L-LTP are significantly and negatively correlated (r = −0.294, P < 0.05).
To test whether L-LTP and spatial memory are significantly correlated independent of age, we used correlational statistics and focused on individual differences in the two variables—L-LTP at 3 hr and spatial memory during the last 5 sessions. We first examined the correlation between L-LTP and errors across all age cohorts and found a significant negative correlation (Fig. 3B). One concern with this analysis is that the correlation may not represent a causal relationship between the two variables but rather a main effect of age on both. This might be the case if there were little overlap between the data points from young and aged cohorts. For example, there could be a significant correlation if most young mice had robust L-LTP with few errors and most aged mice had diminished L-LTP with many errors. We therefore analyzed the relationship within age cohorts. We found no correlation in the 3-mo-old cohort (Fig. 3C). By contrast, we found a significant negative correlation between L-LTP and errors in the aged cohorts (Fig. 3D). Together these results suggest the significant correlation observed across the cohorts (Fig. 3B) is the result of a significant relationship between L-LTP and errors in the aged cohorts and not simply because of a lack of overlap in data between the young and aged mice.
D1/D5 Receptor Agonists Attenuate the L-LTP and Spatial Memory Defects in Aged Mice.
Our results suggest that L-LTP provides a cellular assay of an age-related hippocampal deficit in memory. Because we are now beginning to understand the molecular signaling pathway that underlie L-LTP, this assay can be used to design and test therapeutic strategies that might attenuate the age-related defects in memory consolidation. We have previously found that drugs that affect the cAMP-dependent intracellular signaling pathway such as analogs of cAMP and agonists of the dopamine D1/D5 receptors, which are positively coupled to adenylate cyclase, modulate L-LTP in young mice (18–20).
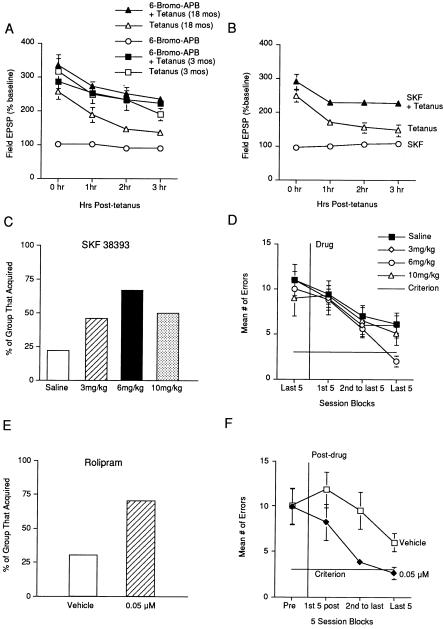
We therefore next explored whether the deficit in hippocampal-dependent memory and L-LTP observed in aged C57BL/B6 mice could be attenuated by dopamine D1/D5 receptor agonists. We first assessed whether 200 nM full agonist 6-bromo-ApB hydrobromide or 1 μM partial agonist SKF 38393 could attenuate the age-related decrease in L-LTP that we observed in slices from 18-mo-old mice. When these concentrations of 6-bromo-ApB and SKF 38393 were applied alone (without tetanic stimulation), they did not induce any change from baseline in slices from aged mice (Fig. 4 A and B). When the same concentrations were applied to the bath 30 min before and during tetanic stimulation, both compounds significantly attenuated the age-related decline in potentiation (Fig. 4 A and B). In contrast to the significant facilitation of potentiation observed in slices from aged mice, we found that 6-bromo-ApB did not enhance L-LTP in slices from 3-mo-old mice (Fig. 4A).
Figure 4.
Drugs that enhance the cAMP signaling pathway attenuate both the L-LTP and spatial memory defects in aged C57BL/B6 mice. (A) 6-Bromo-ApB application paired with four train stimulation (n = 5) significantly enhanced L-LTP in slices from 18-mo-old mice compared with tetanus only (n = 5) (main effect drug F1,8 = 25.1, P < 0.001). 6-Bromo-ApB applied alone to slices from 18-mo-old mice had no effect on baseline values. 6-Bromo-ApB application paired with four train stimulation (n = 5) had no effect on L-LTP in slices from 3-mo-old mice compared with tetanus only (n = 5) (main effect of drug F1,8 = 0.0054, P > 0.05). (B) SKF 38393 application paired with four train stimulation (n = 6) significantly enhanced L-LTP in slices from 18-mo-old mice compared with tetanus only (n = 5) (main effect of drug F1,9 = 10.9, P = 0.0092). (C) Percentage of 18-mo-old mice receiving saline (n = 31), 3 (n = 13), 6 (n = 18) or 10 (n = 8) mg/kg SKF 38393 that acquired the spatial version of the Barnes maze. (D) SKF 38393 significantly decreased the number of errors made by 18-mo-old mice (main effect drug F4,58 = 2.788, P = 0.0471). Mice receiving 6 mg/kg SKF 38393 were significantly different from mice receiving saline. (E) Percentage of 18-mo-old mice receiving vehicle (n = 10) or 0.05 μM (n = 10) rolipram that acquired the spatial version of the Barnes maze. (F) Rolipram significantly decreased the number of errors made by 18-mo-old mice (t = 2.204, P = 0.041).
We next sought to assess the effect of a D1/D5 agonist on the age-related defect in spatial memory by using the Barnes maze. Because 6-bromo-ApB cannot be dissolved in saline, we could only use SKF 38393 for behavioral experiments. During the first 2 weeks of testing on the Barnes maze, no drugs were administered, because during this time, both hippocampus-dependent and nonhippocampus-dependent learning take place. The 18-mo-old mice were placed in the different dose groups based on mean number of errors to assure the groups were composed of mice with similar impairments (Fig. 4D). We administered i.p. doses of 3, 6, and 10 mg/kg SKF 38393.
We observed a dose-related improvement in the percent of aged mice that acquired the task (Fig. 4C). A comparison of median number of trials to acquisition also revealed a significant effect of dose (Kruskal–Wallis: H = 9.67, P = 0.022). Furthermore, a significant dose-related attenuation of the age-related defect in the mean number of errors was observed (Fig. 4D). During the last block of 5 sessions, the aged mice that received 6 mg/kg of SKF 38393 made significantly fewer errors than the group that received saline.
To further test whether modulation of the cAMP signaling pathway facilitates memory in aged mice, we administered the cAMP phosphodiesterase type IV inhibitor rolipram to 18-mo-old mice. The dose we used (0.05 μM) was calculated to produce brain concentrations shown to increase stimulated levels of cAMP without affecting basal levels (19). The mice were matched to dose groups based on performance after 2 wk of training (Fig. 4F). Rolipram was then administered i.p. 35 min before testing each day until the learning criterion was met or a total of 40 testing days elapsed. We observed that rolipram increased the percentage of aged mice that acquired the task (Fig. 4E). Furthermore, a significant decrease in the mean number of errors was observed in the group that received rolipram (Fig. 4F).
DISCUSSION
We have delineated several major findings in this study: (i) Spatial memory is selectively impaired in aged C57BL/B6 mice, whereas cued performance is normal. (ii) The defect in spatial memory is associated with a defect in L-LTP in CA1. (iii) L-LTP correlates with spatial memory within age cohorts. (iv) Basal synaptic physiology and E-LTP in CA1 are normal in aged mice. (v) D1/D5 agonists attenuated the age-related defects in L-LTP and spatial memory. (vi) Likewise, a cAMP phosphodiesterase inhibitor facilitated spatial memory in aged mice.
Consistent with previous studies, we found a significant age-related defect in spatial memory, whereas cued performance, which is not hippocampus-dependent, remained intact. Moreover, consistent with the observation that aged humans and rodents often use less efficient strategies to solve tasks (3, 25), we found that the aged cohorts were more apt to use a serial as opposed to a spatial search strategy at the end of training. Thus, aged mice were capable of learning and modifying their behavior in response to task requirements even though they were not able to perform the task with a hippocampus-based spatial strategy. These results are consistent with the results from studies showing abnormalities in the firing properties of hippocampal place cells in aged animals (26, 27).
We did not observe an effect of age on E-LTP induced by a two-train tetanus. This result is in agreement with previous studies (9, 28), although some other studies have found an age-related decrease in E-LTP with particular tetanus protocols (29, 30). Because E-LTP depends on N-methyl-d-aspartate receptor activation and several serine-threonine and tyrosine kinases, our results suggest these components of the E-LTP cascade are not severely affected by aging. By contrast, we did see a significant age-related impairment in L-LTP induced by four trains of tetanization. Although potentiation immediately after the tetani was normal in the aged cohorts, a significant decrease in potentiation was evident 3 hr after tetanization. This again suggests that basic LTP induction mechanisms are relatively intact in aging but that mechanisms specifically involved in L-LTP are not. We also found that L-LTP was significantly correlated with spatial memory within the aged cohorts, suggesting that L-LTP may be directly involved in a component of long-term memory consolidation.
Similar results have been observed in aged animals in vivo (1, 10). Our studies of L-LTP in vitro demonstrate that the aging defect is intrinsic to the hippocampus itself and does not depend on input from other brain areas. In addition, we used the same experimental protocols as previous pharmacological and genetic studies that have shown that L-LTP in the CA1 region of hippocampus in vitro is mediated in part by D1/D5 receptors, cAMP, PKA activity, CREB phosphorylation, and mRNA and protein synthesis (14–22). Interestingly, there is biochemical evidence that suggests that there are age-related changes in the hippocampus that involve the same dopamine–cAMP–PKA–CREB signaling pathway (31–33). Thus, the poor maintenance of both memory and L-LTP observed in aged mice may reflect alterations in this molecular pathway.
Initially, it was thought that the hippocampus underwent dramatic neuronal degeneration during aging. With the advent of new stereologic techniques, it became clear that if neuronal loss occurs at all, it may be modest (10, 11, 34). Thus, observed impairments in hippocampus-dependent memory and synaptic plasticity may arise more from functional vs. gross structural abnormalities and may therefore emerge more from specific reversible versus widespread irreversible defects. Consistent with this idea, we found that D1/D5 agonists reversed both the age-related L-LTP and spatial memory defects. Furthermore, rolipram, a cAMP phosphodiesterase inhibitor, also reversed the spatial memory defect. The fact that the L-LTP defect could be reversed by D1/D5 agonists suggests that most components in the cAMP cascade necessary for L-LTP in aging are intact. Furthermore, the fact that spatial memory similar to that measured in young mice could be produced in some aged mice after SKF 38393 or rolipram administration suggests that the old mice have the basic capacity for memory consolidation but are deficient in some key molecules.
A role for the D1/D5 receptor in memory consolidation has been proposed based on previous research in young rodents tested for passive avoidance (35) and aged rats tested on the Morris water maze (6). In these studies, D1/D5 agonists improved passive avoidance and water maze performance, whereas D1/D5 antagonists impaired passive avoidance performance. In the hippocampus, the majority of dopamine receptors are of the D1/D5 subtype (36). No change in D1/D5 receptor density has been observed with aging (6, 37), but there are reports of a decrease in dopamine levels (31, 32). Moreover, there is also evidence that phosphodiesterase type IV is increased in the hippocampus relative to other brain areas with aging (38). One mechanism by which the D1/D5 agonists could facilitate memory and L-LTP in aged mice is through activation of the cAMP signaling pathway. Rolipram presumably also produces its effect by increasing cAMP as a result of inhibiting phosphodiesterase. This would increase PKA activity and CREB phosphorylation, potentially reversing the hippocampus-dependent memory and L-LTP defects. Consistent with this idea, administration of rolipram in aged rats was found to reverse an age-related decrease in CRE-binding activity (33). In addition, D1/D5 agonists and rolipram might also improve age-related defects in spatial memory by modulating the cholinergic system in the hippocampus (6, 39–41). In either case, the demonstration of a defect in L-LTP in vitro correlated with age-related memory loss puts that loss in the context of a known molecular pathway and thus provides a rational framework for understanding the amelioration by D1/D5 agonists and phosphodiesterase inhibitors as well as for designing additional new therapies.
Acknowledgments
We thank V. Winder, R. Gordon, S. Lee, and H. Choi for help with behavioral experiments, C. Lam for help with figures, H. Ayers and M. Pellan for typing the manuscript, and M. Osman for animal care. This work was supported by the Dana Foundation and by the Alzheimer’s Disease Research Center Grant AG08702.
ABBREVIATIONS
- E-LTP
early phase long-term potentiation
- L-LTP
late phase LTP, PKA, protein kinase A
References
- 1.Barnes C A. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 2.Bartus R T, Dean R L, Beer B. Neurobiol Aging. 1980;1:145–152. doi: 10.1016/0197-4580(80)90008-1. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher M, Pelleymounter M A. Neurobiol Aging. 1988;9:363–369. doi: 10.1016/s0197-4580(88)80082-4. [DOI] [PubMed] [Google Scholar]
- 4.Uttl B, Graf P. Psychol Aging. 1993;8:257–273. doi: 10.1037//0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Perlmutter M, Metzger R, Nezworski T, Miller K. J Gerontol. 1981;36:59–65. doi: 10.1093/geronj/36.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Hersi A I, Rowe W, Gaudreau P, Quirion R. Neuroscience. 1995;69:1067–1074. doi: 10.1016/0306-4522(95)00319-e. [DOI] [PubMed] [Google Scholar]
- 7.Rapp P R, Amaral D G. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- 8.Baxter M G, Gallagher M. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 9.Deupree D L, Bradley J, Turner D A. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- 10.Geinisman Y, DeToledo-Morrell L, Morrell F, Heller R E. Progress Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- 11.Rapp P R, Gallagher M. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squire L R, Barondes S H. Brain Res. 1973;56:215–225. doi: 10.1016/0006-8993(73)90336-3. [DOI] [PubMed] [Google Scholar]
- 13.Kandel E R, Spencer W A. Physiol Rev. 1968;48:65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Frey U, Krug M, Reymann K G, Matthies H. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen P V, Abel T, Kandel E R. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y-Y, Kandel E R. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 17.Huang Y-Y, Nguyen P V, Abel T, Kandel E R. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y-Y, Kandel E R. Proc Natl Acad Sci USA. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barad M, Bourtchouladze R, Winder D G, Golan H, Kandel E R. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey U, Huang Y-Y, Kandel E R. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 21.Abel T, Nguyen P V, Barad M, Deuel T A S, Kandel E R, Bourtchouladze R. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 22.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 23.Bach M E, Hawkins R D, Osman M, Kandel E R, Mayford M. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 24.Son H, Hawkins R D, Martin K, Kiebler M, Huang P L, Fishman M C, Kandel E R. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 25.Beatty W W. Neurobiol Aging. 1988;9:557–561. doi: 10.1016/s0197-4580(88)80113-1. [DOI] [PubMed] [Google Scholar]
- 26.Tanila H, Sipila P, Shapiro M, Eichenbaum H. J Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes C A, Susler M S, Shen J, McNaughton B L. Nature (London) 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- 28.Norris C M, Korol D L, Foster T C. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenzweig E S, Rao G, McNaughton B, Barnes C A. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Shankar S, Teyler T J, Robbins N. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- 31.Godefroy F, Bassant M H, Weil-Fugazza J, Lamour Y. Neurobiol Aging. 1989;10:187–190. doi: 10.1016/0197-4580(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 32.Luine V, Bowling D, Hearns M. Brain Res. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- 33.Asanuma M, Nishibayashi S, Iwata E, Kondo Y, Nakanishi T, Vargas M G, Owaga N. Mol Brain Res. 1996;41:210–215. doi: 10.1016/0169-328x(96)00098-8. [DOI] [PubMed] [Google Scholar]
- 34.West M J. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 35.Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina J. Proc Natl Acad Sci USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokoloff P, Schwartz J C. Trends Pharmacol Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 37.Levin E D, Torry D, Christopher N C, Yu X, Einstein G, Schwartz-Bloom R D. Brain Res Bull. 1997;43:295–304. doi: 10.1016/s0361-9230(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 38.Tohda M, Murayama T, Nogiri S, Nomura Y. Biol Pharm Bull. 1996;19:300–302. doi: 10.1248/bpb.19.300. [DOI] [PubMed] [Google Scholar]
- 39.Hersi A I, Richard J W, Gaudreau P, Quirion R. J Neurosci. 1995;15:7150–7157. doi: 10.1523/JNEUROSCI.15-11-07150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fibiger H C. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- 41.Vogelsberg V, Neff N H, Hadjiconstantinou M. J Neurochem. 1997;68:1062–1070. doi: 10.1046/j.1471-4159.1997.68031062.x. [DOI] [PubMed] [Google Scholar]