Abstract
Oncoprotein 18 (Op18) is a microtubule-destabilizing protein that is negatively regulated by phosphorylation. To evaluate the role of the three Op18 phosphorylation sites in Xenopus (Ser 16, 25, and 39), we added wild-type Op18, a nonphosphorylatable triple Ser to Ala mutant (Op18-AAA), and to mimic phosphorylation, a triple Ser to Glu mutant (Op18-EEE) to egg extracts and monitored spindle assembly. Op18-AAA dramatically decreased microtubule length and density, while Op18-EEE did not significantly affect spindle microtubules. Affinity chromatography with these proteins revealed that the microtubule-destabilizing activity correlated with the ability of Op18 to bind tubulin. Since hyperphosphorylation of Op18 is observed upon addition of mitotic chromatin to extracts, we reasoned that chromatin-associated proteins might play a role in Op18 regulation. We have performed a preliminary characterization of the chromatin proteins recruited to DNA beads, and identified the Xenopus polo-like kinase Plx1 as a chromatin-associated kinase that regulates Op18 phosphorylation. Depletion of Plx1 inhibits chromatin-induced Op18 hyperphosphorylation and spindle assembly in extracts. Therefore, Plx1 may promote microtubule stabilization and spindle assembly by inhibiting Op18.
Keywords: microtubule dynamics, spindle assembly, phosphorylation, Plx1, chromatin
Introduction
The accurate segregation of chromosomes during cell division is a crucial process that depends upon the formation of the mitotic spindle, which is composed primarily of microtubule polymers of α/β tubulin heterodimers. The radial interphase microtubule array disassembles at the onset of mitosis, and the highly dynamic microtubules become stabilized around chromosomes to form a bipolar structure (Desai and Mitchison 1997). Key organizing forces for spindle assembly include centrosomes that constitute focal microtubule nucleating centers and kinetochores and chromosome arms that capture and stabilize microtubules (Zhang and Nicklas 1995; Heald et al. 1997; Hyman 2000). The organizing force of chromosomes is especially apparent in female meiotic cells and other systems lacking centrosomes, in which microtubules polymerized around condensed chromosomes are sorted into a bipolar array by microtubule-based motor proteins (Gard 1993; McKim and Hawley 1995; Heald 2000). A useful tool for examining the role of chromatin in this process is plasmid DNA-coated beads, which induce bipolar spindle assembly in the absence of centrosomes and kinetochores when incubated in cytoplasmic extracts prepared from metaphase-arrested Xenopus laevis eggs (Heald et al. 1996; Walczak et al. 1998). However, the targets downstream of chromatin that directly modulate microtubule dynamics during spindle assembly have not yet been identified.
Potential microtubule effectors include a large family of microtubule-associated proteins (MAPs), which generally stabilize microtubules, and destabilizing proteins including Oncoprotein 18 (Op18), the KinI family of kinesin-related proteins, and the severing factor katanin (Cassimeris 1999; Andersen 2000). It is not clear to what extent these different molecules control microtubule dynamics during spindle assembly. We would like to identify proteins that control microtubule dynamics during mitosis and determine whether they are regulated by chromatin.
Op18, also termed Stathmin, is one factor that may be regulated by chromatin. A 17-kD soluble protein, Op18 exhibits a complex phosphorylation pattern and is overexpressed in some forms of cancer (Lawler 1998; Curmi et al. 2000). Phosphorylation negatively regulates the microtubule-destabilizing activity of Op18 (Marklund et al. 1996). In Xenopus egg extracts, Op18 is basally phosphorylated in interphase and hyperphosphorylated in mitosis in the presence of mitotic chromatin, indicating that a factor or factors on chromosomes may promote microtubule polymerization and spindle assembly by inactivating Op18 (Andersen et al. 1997). However, the role of Op18 during mitosis is not clear. While a mouse knock-out is viable (Schubart et al. 1996), inhibiting Op18 in cells using antisense RNA interferes with cell-cycle progression (Luo et al. 1994) and its depletion alters spindle assembly kinetics in Xenopus egg extracts (Andersen et al. 1997). Op18 constitutes one potential link between chromosomes and microtubule stability that we would like to elucidate.
Op18 is capable of destabilizing pure microtubules in vitro by two different mechanisms, catastrophe promotion and tubulin sequestering (Belmont and Mitchison 1996; Curmi et al. 1997; Howell et al. 1999b). However, it is controversial which mechanism predominates in vivo, and how Op18 activity is modulated by phosphorylation (Curmi et al. 1997; Di Paolo et al. 1997; Horwitz et al. 1997; Jourdain et al. 1997; Gavet et al. 1998). In this study, we analyzed the regulation of Op18 in Xenopus egg extracts by studying the effects of phosphorylation site mutants of Op18 on spindle assembly, microtubule destabilization, and tubulin binding. We used plasmid DNA-coated beads in an assay to identify Plx1 as a chromatin-associated kinase that influences Op18 phosphorylation. Our results indicate that Op18 functions at least in part by sequestering tubulin in egg extracts, and that Op18-dependent microtubule destabilization is inhibited during spindle assembly by kinases such as Plx1.
Materials and Methods
Expression and Purification of Recombinant Op18
Xenopus Op18 cDNAs encoding wild-type and Ser-to-Ala mutants were obtained from Dr. S. Andersen (University of California, San Diego, San Diego, CA). Glutamic acid substitutions were generated by PCR mutagenesis. For high level expression in Escherichia coli, we mutated the second residue, Cys, to Ala and added an NH2-terminal 6× His tag. For ZZ fusion constructs, the ZZ tag was inserted between the 6× His tag and Op18 coding sequence. All constructs were expressed in BL21/DE3, pLysS, grown at 37°C and induced for 3 h with 1 mM IPTG. All proteins were purified using Ni-NTA agarose chromatography (QIAGEN) and dialyzed into XB (10 mM Hepes, pH 7.7, 1 mM MgCl2, 0.1 mM CaCl2, 100 mM KCl, 50 mM sucrose) (Murray 1991).
Xenopus Egg Extracts and Spindle Assembly Reactions
Cytostatic factor-arrested (CSF) extracts were prepared from Xenopus eggs arrested in metaphase of meiosis II as described (Murray 1991). Demembranated sperm nuclei and rhodamine-labeled tubulin were added to extracts on ice, along with wild-type Op18 or phosphorylation site mutants. Half-spindle assembly reactions were incubated at 20°C for 30–45 min (Sawin and Mitchison 1991; Desai et al. 1999). Once spindle assembly was observed, the reactions were diluted and spun onto coverslips for analysis by fluorescence microscopy (Sawin and Mitchison 1991; Desai et al. 1999). All images were taken at the same exposure with a CCD camera and processed using Adobe Photoshop.
Microtubule Pelleting in Extracts
25 μl of CSF extract was incubated with 500 sperm nuclei/μl to stimulate microtubule polymerization for 30 min at 20°C in the presence of 6 or 12 μM wild-type (WT) or mutant recombinant Op18. 0.5 ml of 30% glycerol/BRB80/1% Triton X-100 (BRB80: 80 mM K-Pipes, 1 mM MgCl2, 1 mM EGTA, pH 6.8) was added to the reaction, and then spun through 1 ml of 40% glycerol/BRB80 cushion in a microcentrifuge. The supernatant was aspirated, the interface between supernatant and cushion was washed, and the pellet was resuspended in SDS sample buffer. The samples were run on 10% SDS-PAGE and immunoblotted for α tubulin (mAb N-356; Amersham Pharmacia Biotech).
Affinity Chromatography with Op18
15 μM of ZZ-WT, ZZ-AAA, ZZ-EEE Op18 or control buffer was incubated with 100 μl of high-speed Xenopus extract (CSF extract spun for 50 min at 50,000 rpm in a TLS-55 rotor at 4°C) for 1 h on a rotator at room temperature. The reaction was then combined with IgG Sepharose (Amersham Pharmacia Biotech) that had been washed three times in XB + LPC. After incubation at room temperature for 1 h on a rotator, the Sepharose was pelleted, extract was removed, and the Sepharose washed six times with 500 μl of XB + LPC. Proteins were eluted by sequential incubations with increasing concentrations of MgCl2 in 50 mM Tris, pH 7.5 (0.25, 0.5, and 1 M). The eluted proteins were precipitated in methanol/chloroform and the pellet was resuspended in 50 μl of SDS-PAGE sample buffer. 10–20 μl were loaded on 12% SDS-PAGE and silver stained, or 10% gels were used for Western blot analysis of α tubulin.
Analysis of Chromatin Assembly on DNA Beads
DNA-coated beads were prepared as described (Heald et al. 1996, Heald et al. 1998). To analyze recruitment of chromatin proteins, DNA, or uncoupled control beads were incubated in CSF Xenopus egg extracts (CSF extracts) that were induced to enter interphase by addition of CaCl2 to 0.4 mM (Desai et al. 1999). Cycloheximide (0.2 mg/ml) was added to the reactions that were to remain in interphase. After a 2-h incubation at 20°C, an additional 0.5 vol of extract was added. Fresh mitotic extract was added to generate mitotic chromatin samples, or calcium-released interphase extract was added to generate interphase samples. After an additional 45 min, the cell cycle state of the extracts was monitored by adding 0.2 mg/ml rhodamine-labeled tubulin to a small aliquot of the extracts and visualizing the microtubule cytoskeleton in a squash sample (Sawin and Mitchison 1991). An extensive microtubule network was apparent in interphase extracts and absent from extracts in mitosis. Beads were retrieved on ice using magnets, washed once with 1 ml Buffer 1 (10 mM Hepes, pH 7.6, 2 mM MgCl2, 0.5 mM EGTA, 10 mM β-glycerophosphate, 10 mM sucrose, 50 mM KCl, 10 μg/ml LPC [leupepetin, Pepstatin A, and chymostatin], 1 mM DTT), and then three times with Buffer 1 plus 0.05% Triton X-100, before resuspending in SDS sample buffer. 1 μg DNA or equivalent uncoupled beads recovered from 200-μl extract was loaded in each lane. 13% SDS-PAGE was used to visualize histones with a silver stain protocol optimized for basic proteins (Wray et al. 1981). 6% gels were used for Western blot analysis. For analysis of nucleosomes, DNA beads were incubated for 2 h in interphase extract at a ratio of 1 μg DNA/100 μl extract. Micrococcal nuclease analysis was performed as described except that 2 μg of DNA was digested with 3.75 U of enzyme (Sandaltzopoulos et al. 1994). One third of the reaction was stopped after 1, 2, and 5 min for analysis by agarose gel electrophoresis.
In Vitro Op18 Kinase Assays
Interphase and mitotic chromatin beads assembled as described above using 3 μg plasmid DNA for each reaction. The beads were magnetically retrieved on ice and 1 μl of the extract was preserved for kinase analysis by adding 19 μl EB (80 mM β-glycerophosphate, pH 7.3, 20 mM EGTA, 15 mM MgCl2, 10 μg/ml LPC, 1 mM DTT) and freezing in liquid nitrogen. For both interphase and mitotic reactions, the beads were washed two times with 400 μl of buffer 1 while still on the magnet, resuspended well in 200 μl of buffer 2, and again retrieved on ice. After three additional washes with buffer 2, the beads were transferred to a fresh tube and the proteins were eluted using 0.5 M KCl in buffer 2. The extract, beads, or 0.5 M KCl eluates were incubated with recombinant Op18 and [γ-32P]ATP in a filter binding kinase assay as described (Felix et al. 1989) or analyzed by SDS-PAGE and autoradiography.
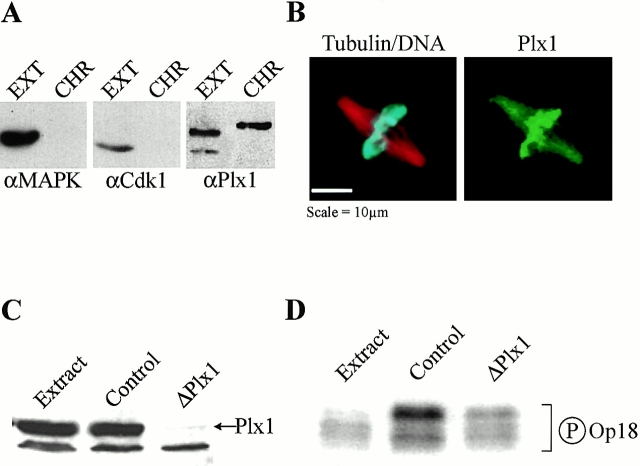
Immunodepletion of Plx1 and Immunoprecipitation of Op18 from Xenopus Egg Extracts
Polyclonal rabbit antibodies were produced against recombinant 6× His-Plx1-COOH-terminal protein (amino acids 225–598) by the Berkeley Antibody Co. and affinity purified as described (Kumagai et al. 1998). 15–20 μg of anti–Plx1 or rabbit IgG (control) was bound to 60–75 μl of Protein A–conjugated Dynabeads 280 (Dynal) in a total volume of 200–250 μl TBS-0.1% Triton X-100 and incubated 2 h or overnight at 4°C on a rotator. After coupling, the beads were washed with TBS-0.1% Triton X-100, 2× 200μl CSF-XB+ and divided in half for two successive rounds of depletion. Between washes, the beads were retrieved on a magnet. After removing the buffer, the beads were resuspended gently in 125 μl of extract and placed on ice for 90 min. The reaction was mixed periodically by flicking the tube gently. After the first round of depletion, the beads were retrieved on a magnet on ice for 10 min. The second set of beads was resuspended in this extract and placed on ice for an additional 90 min. After the second round of depletion, the beads were retrieved as above and the extract was used for chromatin assembly as described above. Chromatin beads were then retrieved and incubated with 20 μl fresh Plx1-depleted egg extract in the presence of [γ-32P]ATP for 30 min before Op18 was immunoprecipitated as described (Andersen et al. 1997).
Results
Phosphorylation Site Mutants Prevent or Mimic Op18 Phosphorylation
Xenopus Op18 contains three phosphorylation sites, Ser16, 25, and 39 (Maucuer et al. 1993; Andersen et al. 1997). To study how phosphorylation regulates Op18 in extracts, we generated phosphorylation site mutants, changing the residues to either Ala (to block phosphorylation) or Glu (to mimic phosphorylation), and evaluated the effects of these different Op18 proteins on spindle assembly in egg extracts (Fig. 1 A). Recombinant proteins were added to 6 μM, approximately the concentration of endogenous Op18 (Belmont and Mitchison 1996). When added to Xenopus sperm spindle assembly reactions, the wild-type protein (Op18-WT) caused microtubule depolymerization, reducing spindle size and microtubule density. Individual and double Ser-to-Ala or single Ser-to-Glu mutations did not dramatically affect Op18 activity (data not shown), indicating that no single site modulates the activity of Op18. However, changing all three sites (Op18-AAA) caused complete microtubule depolymerization, demonstrating that all three sites are likely to be important in regulating microtubule destabilization. In contrast, changing all three sites to Glu (Op18-EEE) largely neutralized the effects of exogenous Op18 on microtubule stability (Fig. 1 A).
Figure 1.

Differential effects of phosphorylation site mutants of Op18 on spindle assembly reactions in Xenopus egg extracts. (A) Effects of 6 μM Op18-WT, AAA (all three Serine phosphorylation sites mutated to Alanine) or EEE (all three Serine phosphorylation sites mutated to Glutamic acid) on spindle assembly in Xenopus egg extracts. Microtubules appear red and sperm chromosomes blue. (B) Biochemical quantification of depolymerizing activities shown by antitubulin immunoblot of microtubules pelleted from extracts in the presence of 6 or 12 μM Op18-WT, -AAA, and -EEE. The control (CON) is buffer addition in both A and B.
To confirm our observations using a more quantitative assay, we pelleted the microtubules polymerized in the Op18 addition experiments and measured their abundance by immunoblotting for α tubulin (Fig. 1 B). Op18-AAA caused a drastic reduction in the microtubule pellet, while Op18-EEE had a more minor effect than Op18-WT even at 12 μM. These results highlight the importance of phosphorylation in regulating the activity of Op18 and indicate that the glutamic acid substitutions are able to at least partially mimic phosphorylation in cytoplasmic extracts.
Op18 Activity Correlates with Tubulin Binding in Extracts
To investigate the differential effects of the Op18-AAA and -EEE in extracts, we performed affinity experiments with the different recombinant proteins (Fig. 2). We fused tandem IgG-binding domains of protein A (termed ZZ), to Op18-WT, -AAA, and -EEE, which did not affect their relative microtubule depolymerizing activities (not shown), and permitted specific retrieval of the proteins from extracts using IgG-coated Sepharose beads for biochemical analysis. SDS-PAGE of salt elutions showed few obvious reproducible differences between proteins bound to Op18-WT, -AAA, and -EEE, except for a band at ∼50 kD (Fig. 2 A), which immunoblot analysis revealed as tubulin (Fig. 2 B). Interestingly, the Op18-ZZ-AAA bound tubulin very efficiently while Op18-ZZ-WT bound less and ZZ-Op18-EEE did not bind at all. This binding is independent of tubulin polymerization as it also occurred in the presence of nocodazole (data not shown). The differential binding of Op18 to tubulin indicates a role for phosphorylation-dependent tubulin affinity in determining Op18 activity. Analogous to the results of functional studies, single Ser to Ala or Glu substitutions behaved like Op18-WT with respect to tubulin binding in extracts, again indicating that phosphorylation at all three sites is important for Op18 regulation (data not shown).
Figure 2.
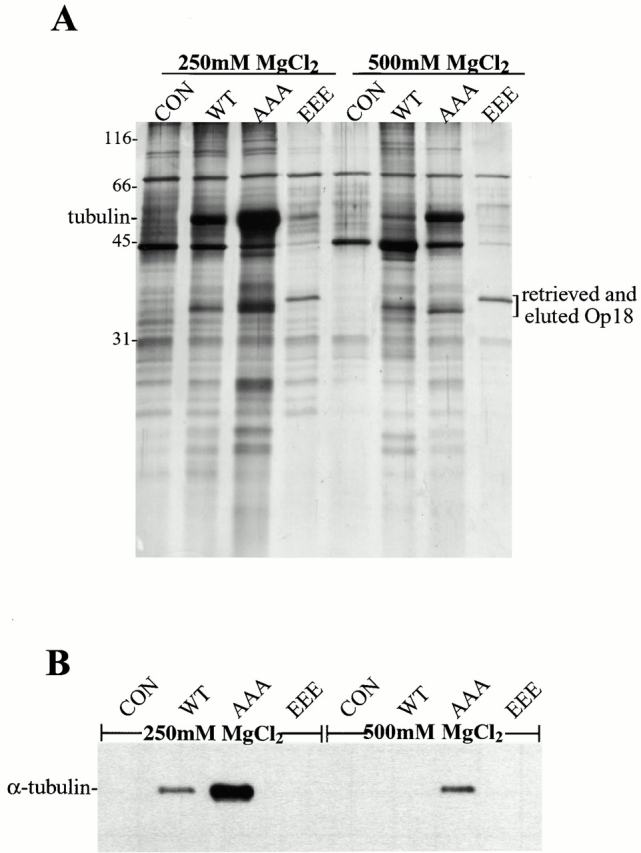
Nonphosphorylatable Op18 binds tightly to tubulin in extracts. (A) Silver-stained gel of MgCl2 elutions of control, ZZ-WT, -AAA, and -EEE Op18 bound to IgG Sepharose retrieved from extracts. (B) The elutions from A were blotted for α tubulin. The control (CON) is buffer addition in both A and B.
Chromatin Assembles on DNA-Coated Beads in Xenopus Egg Extracts
Op18 hyperphosphorylation occurs in the presence of plasmid DNA-coated beads in mitotic Xenopus egg extracts (Andersen et al. 1997), indicating that chromatin-associated proteins may contribute to Op18 regulation. To determine whether magnetic plasmid DNA-coated beads are appropriate for biochemical analysis of chromatin activities, we incubated them in Xenopus egg extracts and evaluated chromatin assembly by two criteria: association of chromatin proteins and assembly of nucleosomes. To analyze bound proteins, DNA or uncoupled control beads retrieved from interphase or mitotic extracts were washed and analyzed by SDS-PAGE and immunoblot (Fig. 3A and Fig. B). Complex patterns of proteins were found associated with DNA beads, of which Xenopus histones were prominent. Many fewer proteins were associated with control beads, which did not bind histones. Although similar in their complexity, several differences were apparent among proteins bound to interphase and mitotic DNA beads (Fig. 3 A, arrowheads). We have examined the association of two proteins known to be recruited to sperm chromatin (Hirano and Mitchison 1994) (Fig. 3 B). Xklp1, a chromosomal kinesin (Vernos et al. 1995), was highly enriched on mitotic beads. Topoisomerase II was found on both interphase and mitotic beads, with bands of lower mobility recovered on beads from the mitotic extract, indicating hyperphosphorylation (Taagepera et al. 1993). Chromatin assembly on DNA beads was also evaluated by partial digestion with micrococcal nuclease (Fig. 3 C). A pattern of resistant fragments representing mono- and dinucleosomal DNA was apparent. Therefore, DNA attached to beads assembles chromatin and appears to be a legitimate source for an analysis of chromatin-associated Op18 kinase activity.
Figure 3.
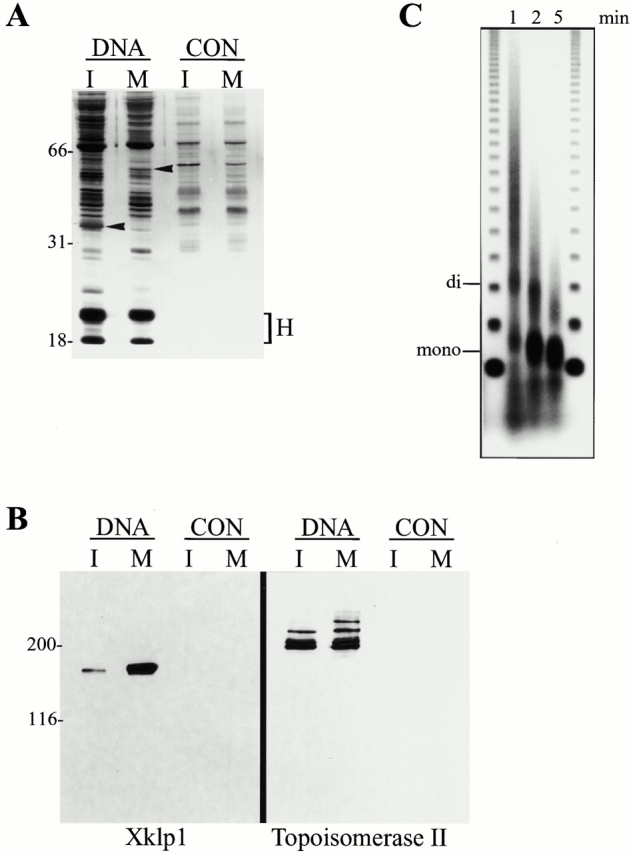
Chromatin assembles on DNA beads. (A) Silver stained gel of proteins associated with DNA beads and control beads in interphase (I) and mitotic (M) extracts. Arrowheads point to bands that are different between I and M. Histones (H) are indicated. (B) Western blot of the same samples from A probed with antibodies to Xklp1 and Topoisomerase II. (C) Micrococcal nuclease digestion of DNA beads for 1, 2, or 5 min; mononucleosomes (mono) and dinucleosomes (di) are indicated. Outer lanes are 123-bp markers.
A Mitotic Chromatin-associated Kinase Activity Phosphorylates Op18 In Vitro
An appealing model for regulation of microtubule dynamics during spindle assembly contends that Op18 is locally inactivated by mitotic chromatin, thereby leading to polarized growth of microtubules around chromosomes (Andersen et al. 1997). The simplest explanation is that a chromatin-associated kinase phosphorylates Op18, but since inhibition of protein phosphatase 2A (PP2A) also promotes Op18 phosphorylation (Andersen et al. 1997; Tournebize et al. 1997), the chromatin-associated activity could also be a phosphatase inhibitor.
To examine whether Op18 could be directly phosphorylated by chromatin, we devised an in vitro kinase assay using recombinant Op18 and proteins eluted from chromatin beads. Both total mitotic extract and mitotic chromatin beads could phosphorylate Op18, while interphase extract or chromatin had much less activity (Fig. 4 A). The kinase activity could be eluted from mitotic chromatin beads with 0.5 M KCl, yielding between 2× and 10× more activity toward Op18 than total mitotic extract, indicating the presence of a specific kinase activity enriched on mitotic chromatin (Fig. 4 B). We performed the in vitro kinase assay using single site Ser-to-Ala mutants of Op18 and confirmed that Ser39, a cdc2 consensus site, was the major site phosphorylated by total mitotic extract (Brattsand et al. 1994; Andersen et al. 1997, and data not shown). In contrast, all three sites were phosphorylated to some extent by mitotic chromatin proteins (data not shown), indicating either the presence of a relatively nonspecific chromatin-associated kinase or that multiple different kinases capable of phosphorylating Op18 are bound to the mitotic chromatin beads. No phosphorylation by extract or chromatin proteins was observed of the Op18-AAA mutant, indicating that Ser16, 25, and 39 are the only sites regulated by kinase activity (data not shown).
Figure 4.
Chromatin-associated proteins phosphorylate recombinant Op18 in vitro. (A) Autoradiogram of recombinant Op18 showing [γ32P]phosphate incorporation after incubation with interphase or mitotic extract, or chromatin beads isolated from interphase or mitotic extract. (B) Quantification of in vitro Op18 kinase data comparing total extract (I, M extract) and 0.5 M KCl-eluted chromatin proteins (I, M eluate).
Plx1 Depletion Inhibits both Op18 Phosphorylation and Spindle Assembly
To identify potential candidates for a mitotic chromatin-associated Op18 kinase, we evaluated the presence of several known kinases in chromatin bead eluates by immunoblot. In contrast to cdc2 and p44 MAPK, which were not enriched in the bead eluate, Xenopus polo-like kinase (Plx1) was present at high levels on chromatin isolated from mitotic extracts (Fig. 5 A). By immunofluorescence, Plx1 was enriched on sperm chromatin but also found throughout spindle microtubules in metaphase (Fig. 5 B), as described previously in Xenopus embryos (Qian et al. 1999). To determine whether Plx1 plays a role in Op18 phosphorylation and spindle assembly around chromatin beads, we assembled chromatin in Plx1-depleted egg extracts and tested its ability to induce Op18 hyperphosphorylation and spindle assembly. Two rounds of immunodepletion removed >99% of Plx1 (Fig. 5 C). In control-depleted mitotic extract, addition of chromatin beads caused a dramatic increase in the phosphate incorporation of endogenous Op18, and the predominance of a lower mobility form of the protein, indicating hyperphosphorylation (Andersen et al. 1997). Plx1 depletion reduced the chromatin-induced hyperphosphorylation of endogenous Op18 by >75% (Fig. 5 D). These results indicate that Plx1 is a chromatin-associated Op18 kinase or that it is required to activate an Op18 kinase.
Figure 5.
Plx1 is a chromatin-associated kinase and its depletion inhibits chromatin-induced Op18 hyperphosphorylation. (A) Immunoblot analysis of total extract (EXT) and proteins eluted from mitotic chromatin beads using 0.5 M KCl (CHR) probed with antibodies against MAP kinase, cdk1, and Plx1. (B) Immunofluorescence micrograph showing Plx1 localization to chromatin and microtubules in sperm spindles assembled in Xenopus egg extracts. Microtubules appear red, sperm chromosomes blue, and Plx1 green. (C) Immunoblot of total extract (Extract), IgG-depleted (Control), and Plx1-depleted (ΔPlx1) extracts shows that >99% of Plx1 has been removed. (D) Autoradiogram showing [γ32P]phosphate incorporation in Op18 immunoprecipitated from mitotic extract (Extract) or in IgG-depleted (Control) or Plx1-depleted (ΔPlx1) mitotic extract + chromatin beads.
To test the physiological relevance Plx1-induced Op18 phosphorylation, spindle assembly was evaluated in the control- and Plx1-depleted reactions (Fig. 6 A). Plx1 depletion resulted in a dramatic decrease in the formation of bipolar spindles around plasmid DNA-coated beads. In the absence of Plx1, many fewer bead clusters induced microtubule polymerization, yielding on average four times fewer mitotic microtubule arrays compared with control reactions (Fig. 6 B). In addition, the microtubule structures that formed were often aberrant. In three separate experiments, the percentage of structures that could be classified as normal bipolar spindles averaged 7% in Plx1-depleted reactions, compared with 56% in the control reactions (Fig. 6 C). Instead, mitotic structures formed in the absence of Plx1 often lacked sufficient microtubules to form a bipolar array, or microtubule-chromatin interactions were impaired, yielding monopolar and bent spindle structures (Fig. 6 A). These defects may be due in part to decreased Op18 phosphorylation, which would prevent its local inactivation around chromatin and microtubule stabilization. Therefore, Plx1 is an important chromatin-associated kinase that functions during spindle assembly in Xenopus egg extracts and may act in part by regulating Op18.
Figure 6.
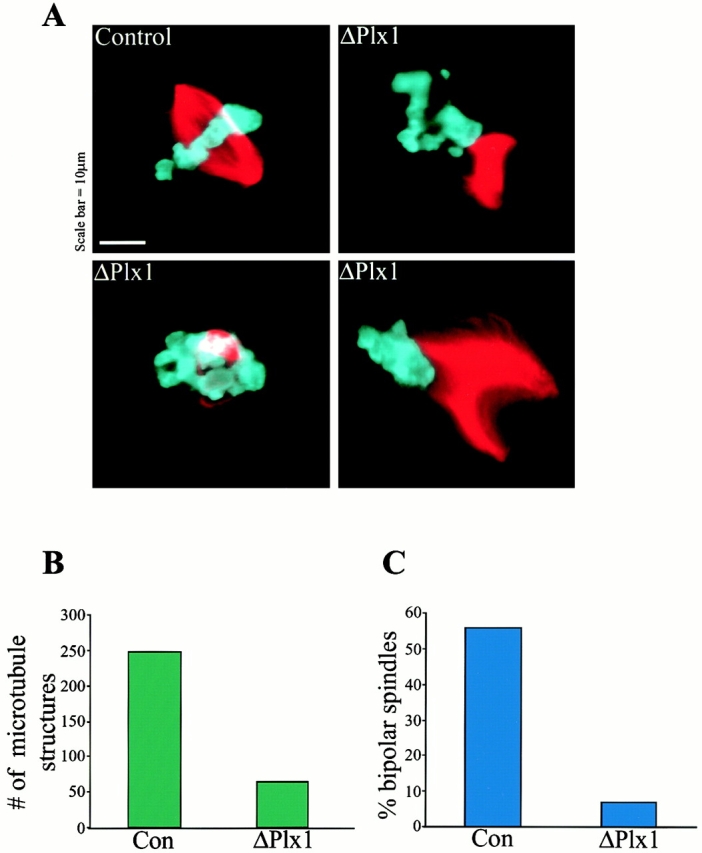
Plx1 depletion causes defects in microtubule polymerization and spindle assembly. (A) Representative microtubule structures found in control- and Plx1-depleted extracts. (B) Quantification of spindle defects showing the decrease in total microtubule structures formed in Plx1-depleted (ΔPlx1, 64) compared with IgG-depleted (Con, 247), and (C) the small percentage of normal bipolar spindles formed in Plx1-depleted extract. A total of 933 structures were counted in three separate experiments.
Discussion
A Chromatin-associated Kinase Contributes to Op18 Regulation and Spindle Assembly
The initial observation that plasmid DNA-coated beads are sufficient to induce assembly of bipolar mitotic spindles in Xenopus egg extracts indicated that the immobilized DNA recruits chromatin proteins sufficient to stabilize and organize microtubules in the absence of centrosomes and kinetochores (Heald et al. 1996). We show here that known chromosomal proteins are recruited to the DNA beads and nucleosomes assemble, indicating that functional chromatin indeed assembles on the immobilized plasmid DNA. To dissect how chromatin directs spindle assembly, we would like to identify both the microtubule effectors and the chromatin-associated proteins that regulate this process.
The microtubule-destabilizing protein Op18 represents one potential target regulated by chromatin during spindle assembly. Op18 is active during mitosis in Xenopus egg extracts, as its immunodepletion causes a significant decrease in the catastrophe frequency and results in accelerated microtubule polymerization around chromatin (Andersen et al. 1997; Tournebize et al. 1997). In several systems, nonphosphorylatable Op18 mutants dramatically inhibit microtubule polymerization, indicating that phosphorylation during mitosis is crucial for Op18 regulation (Fig. 1) (Marklund et al. 1996; Horwitz et al. 1997; Larsson et al. 1997). The observation that DNA-coated beads induced Op18 hyperphosphorylation in egg extracts led us to investigate chromatin-associated kinase activities that could regulate Op18 phosphorylation and spindle assembly.
To investigate Op18 phosphorylation by chromatin, we developed an in vitro kinase assay and showed that a mitotic chromatin-specific activity phosphorylates Op18. By examining chromatin eluates for known kinases, we identified Xenopus polo-like kinase Plx1 as a chromatin-associated kinase. We propose a role for Plx1 in regulating Op18 based on the effects of its depletion, which reduced mitotic chromatin-induced Op18 hyperphosphorylation in extracts. Plx1 depletion also caused defects in spindle assembly, including reduced microtubule polymerization around DNA beads, which could result from a failure to inactivate Op18. Similar microtubule polymerization defects were also observed in Xenopus sperm nuclei reactions in Plx1-depleted extracts (Budde, P.P., and R. Heald, unpublished results), indicating that this mechanism is not specific to DNA bead spindle assembly. Therefore, we propose that Plx1 contributes to Op18 inactivation during mitosis, thereby promoting microtubule growth around chromosomes.
Plx1 Plays Multiple Roles in Mitosis
Plx1 is a member of the Polo kinase family of Serine/Threonine kinases, which are involved in mitotic progression in many organisms. Plx1 was originally identified in Xenopus egg extracts as a Cdc25-regulatory kinase (Kumagai and Dunphy 1996), thereby playing a role in entry into mitosis. Plx1 in frog embryos is localized to centrosomes early in mitosis, on chromosomes and the spindle at metaphase, and at the midbody late in mitosis (Qian et al. 1999). We have found a similar localization of Plx1 to chromatin and spindle microtubules in Xenopus egg extracts (Fig. 5 B). Injection of Plx1 antibodies arrests cleavage in Xenopus embryos and results in monopolar spindles in Xenopus embryonic cells (Qian et al. 1998). In other species, Plks (Polo-like kinases) have been localized throughout the spindle (Golsteyn et al. 1995; Logarinho and Sunkel 1998; Wianny et al. 1998; Moutinho-Santos et al. 1999), to chromosomes (Llamazares et al. 1991; Arnaud et al. 1998), and evidence indicates functional roles in centrosome assembly and separation (Sunkel and Glover 1988; Lane and Nigg 1996), exit from mitosis (Charles et al. 1998; Descombes and Nigg 1998; Kotani et al. 1998; Shirayama et al. 1998), and cytokinesis (Ohkura et al. 1995; Carmena et al. 1998; Bahler et al. 1998; reviewed in Glover et al. 1998; Nigg 1998). Despite the multiple roles that Plks play in mitosis, only a handful of Plk substrates have been identified. Although Op18 appears to be regulated by Plx1, we do not yet know whether Op18 is a direct substrate of the chromatin-associated pool. While a direct interaction is supported by the localization of Plx1 to chromatin, Plx1 is found throughout the spindle and is also likely present in a soluble pool. Plx1 inhibition causes defects in spindle assembly and function, including a decrease in microtubule polymerization that could result from problems in Op18 regulation. However, it is likely that Plx1 regulates multiple substrates within the spindle.
Global and Local Op18 Regulation
Protein phosphatase 2A has also been implicated in Op18 regulation, as its inhibition by okadaic acid causes an increase in Op18 phosphorylation and microtubule length in mitotic Xenopus egg extracts (Andersen et al. 1997; Tournebize et al. 1997). It is not known whether PP2A directly dephosphorylates Op18 or functions in a more complex pathway. Unlike okadaic acid, mitotic chromatin does not appear to cause Op18 hyperphosphorylation by inhibiting its dephosphorylation (Andersen et al. 1997). Therefore, we favor a model in which PP2A regulates the global level of Op18 phosphorylation, while kinase activity on chromatin suppresses Op18 activity locally, promoting microtubule growth and spindle assembly. Interestingly, Plk activity is itself activated by phosphorylation and can be dephosphorylated by PP2A (Hamanaka et al. 1995). PP2A has also been shown to be antagonistic to Plx1, at least in Cdc25 activation (Karaiskou et al. 1999). Therefore, PP2A may regulate Op18 phosphorylation directly and/or through Plx1. Phosphorylation of Plx1 is likely to play a role in this process and may occur on chromatin, as indicated by a shift in mobility of the chromatin-associated form (Fig. 5 A).
Recent studies in Xenopus indicate that at the top of one cascade regulating microtubule stability during spindle assembly is RCC1, a chromatin-bound nucleotide exchange factor that generates RanGTP, which causes release of microtubule effectors from transport factors in the vicinity of chromatin (Gruss et al. 2001; Nachury et al. 2001; Wiese et al. 2001). It will be interesting to determine whether Plx1-dependent microtubule stabilization through Op18 regulation has any relationship to the Ran pathway or represents a redundant mechanism to stabilize microtubules during spindle assembly.
Phosphorylation Site Mutants Reveal that Op18 Activity Correlates with Tubulin Binding
How does Op18 phosphorylation regulate its microtubule destabilizing activity? We have determined that all three phosphorylation sites of Xenopus Op18, Ser 16, 25, and 39, are important for its regulation, since single and double serine-to-alanine mutations at these sites did not significantly alter the microtubule-destabilizing activity of recombinant proteins added to extracts. In contrast, serine-to-alanine mutations that prevented phosphorylation at all three sites hyperactivated the protein, while triple glutamic acid substitutions mimicking phosphorylation largely attenuated Op18 function. Activity of the recombinant proteins correlated strongly with their ability to bind tubulin dimers in Xenopus egg extracts. Interaction between tubulin and Op18 has been difficult to study in vivo, as coimmunoprecipitation techniques disrupt their interaction (Howell et al. 1999a). Using ZZ-tagged Op18 constructs that can easily be retrieved from extracts has enabled us to probe the interaction of recombinant Op18 with endogenous tubulin. We have shown that AAA binds strongly to tubulin while EEE does not bind at all, indicating that tubulin sequestering could play a role in Op18 function in vivo, but that this activity is highly sensitive to phosphorylation of Op18. In contrast to the well-established interaction between Op18 and tubulin dimers (Gigant et al. 2000; Steinmetz et al. 2000; Wallon et al. 2000), there have been no reports of Op18 association with microtubule ends, which would be expected if it functioned as a catastrophe promoter.
Based on our experiments, we propose a model for chromatin-regulated Op18 function (Fig. 7). While basal phosphorylation levels would prevent Op18 from sequestering a large fraction of tubulin pool, dephosphorylation by PP2A may generate a steady state level of active Op18. During mitosis, active Plx1 on chromosomes leads to Op18 hyperphosphorylation, preventing its interaction with tubulin dimers and causing local stabilization of microtubules that promotes spindle assembly. Future studies will elucidate the likely involvement of other kinases in Op18 regulation, as well as the relative roles of tubulin sequestering and direct catastrophe-promoting mechanisms in Op18 function.
Figure 7.
Model for Op18 regulation of microtubule dynamics in Xenopus. The curved line represents Op18 and P is a phosphate group. Unphosphorylated Op18 promotes microtubule depolymerization by sequestering tubulin. Op18 that encounters mitotic chromatin is phosphorylated by Plx1, preventing the Op18-tubulin interaction and promoting microtubule stabilization.
Acknowledgments
We are grateful to S. Andersen for Op18 constructs, I. Vernos, T. Hirano, and J. Ferrell for antibodies to Xklp1, Topoisomerase II, and MAP kinase, respectively. We thank T. Blank for help with micrococcal nuclease digests, J. Swedlow for advice regarding kinase assays, N. Rao and R. Yue for help generating Op18 constructs, A. Desai for a frog tubulin purification protocol, and G.O. Nads for sperm nuclei. We also thank members of the Heald, Welch, and Weis labs for helpful discussion, and Matthew Welch, Sarah Wignall, and Jennifer Banks for critical reading of the manuscript.
R. Heald is supported by the National Institutes of Health, The Pew Charitable Trust, and the Cancer Research Coordinating Committee.
Footnotes
Abbreviations used in this paper: CSF, cytostatic factor-arrested; MAP, mitogen-activating protein; PP2A, protein phosphatase 2A; WT, wild type.
References
- Andersen S. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000;10:261–267. doi: 10.1016/s0962-8924(00)01786-4. [DOI] [PubMed] [Google Scholar]
- Andersen S.S.L., Ashford A.J., Tournebize R., Gavet O., Sobel A., Hyman A.A., Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. doi: 10.1038/39382. [DOI] [PubMed] [Google Scholar]
- Arnaud L., Pines J., Nigg E.A. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107:424–429. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- Bahler J., Steever A.B., Wheatley S., Wang Y.-L., Pringle J.R., Gould K.L., McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L.D., Mitchison T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Brattsand G., Marklund U., Nylander K., Roos G., Gullberg M. Cell-cycle–regulated phosphorylation of oncoprotein 18 on Ser16, Ser25 and Ser38. Eur. J. Biochem. 1994;220:359–368. doi: 10.1111/j.1432-1033.1994.tb18632.x. [DOI] [PubMed] [Google Scholar]
- Carmena M., Riparbelli M.G., Minestrini G., Tavares A.M., Adams R., Callaini G., Glover D.M. Drosophila polo kinase is required for cytokinesis. J. Cell Biol. 1998;143:659–671. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 1999;11:134–141. doi: 10.1016/s0955-0674(99)80017-9. [DOI] [PubMed] [Google Scholar]
- Charles J.F., Jaspersen S.L., Tinker-Kulberg R.L., Hwang L., Szidon A., Morgan D.O. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae . Curr. Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Curmi P.A., Andersen S.S.L., Lachkar S., Gavet O., Karsenti E., Knossow M., Sobel A. The stathmin/tubulin interaction in vitro. J. Biol. Chem. 1997;272:25029–25036. doi: 10.1074/jbc.272.40.25029. [DOI] [PubMed] [Google Scholar]
- Curmi P.A., Nogues C., Lachkar S., Carelle N., Gonthier M.P., Sobel A., Lidereau R., Bieche I. Overexpression of stathmin in breast carcinomas points out to highly proliferative tumours. Brit. J. Cancer. 2000;82:142–150. doi: 10.1054/bjoc.1999.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Desai A., Murray A., Mitchison T.J., Walczak C.E. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Descombes P., Nigg E.A. The Polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., Antonsson B., Kassel D., Riederer B.M., Grenningloh G. Phosphorylation regulates the microtubule-destabilizing activity of stathmin and its interaction with tubulin. FEBS Lett. 1997;416:149–152. doi: 10.1016/s0014-5793(97)01188-5. [DOI] [PubMed] [Google Scholar]
- Felix M.A., Pines J., Hunt T., Karsenti E. A post-ribosomal supernatant from activated Xenopus eggs that displays post-translationally regulated oscillation of its cdc2+ mitotic kinase activity. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:3059–3069. doi: 10.1002/j.1460-2075.1989.tb08457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.L. Ectopic spindle assembly during maturation of Xenopus oocytesevidence for functional polarization of the oocyte cortex. Dev. Biol. 1993;159:298–310. doi: 10.1006/dbio.1993.1242. [DOI] [PubMed] [Google Scholar]
- Gavet O., Ozon S., Manceau V., Lawler S., Curmi P., Sobel A. The stathmin phosphoprotein familyintracellular localization and effects on the microtubule network. J. Cell Sci. 1998;111:3333–3346. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- Gigant B., Curmi P.A., Martin-Barbey C., Charbaut E., Lachkar S., Lebeau L., Siavoshian S., Sobel A., Knossow M. The 4 ANG x-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102:809–816. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Hagan I.M., Tavares A.A.M. Polo-like kinasesa team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- Golsteyn R.M., Mundt K.E., Fry A.M., Nigg E.A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss O.J., Carazo-Salas R.E., Schatz C.A., Guarguaglini G., Kast J., Wilm M., Le Bot N., Vernos I., Karsenti E., Mattaj I.W. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Hamanaka R., Smith M.R., O'Connor P.M., Maloid S., Mihalic K., Spivak J.L., Longo D.L., Ferris D.K. Polo-like kinase is a cell cycle–regulated kinase activated during mitosis. J. Biol. Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- Heald R. Motor function in the mitotic spindle. Cell. 2000;102:399–402. doi: 10.1016/s0092-8674(00)00044-1. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Vernos I., Murray A., Hyman T., Karsenti E. In vitro assays for mitotic spindle assembly and function. In: Celis J., editor. Cell BiologyA Laboratory Handbook. 2nd Ed. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1998. pp. 326–335. [Google Scholar]
- Hirano T., Mitchison T.J. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Horwitz S.B., Shen H.-J., He L., Dittmar P., Neef R., Chen J., U.K. Schubart The microtubule-destabilizing activity of metablastin (p19) is controlled by phosphorylation. J. Biol. Chem. 1997;272:8129–8132. doi: 10.1074/jbc.272.13.8129. [DOI] [PubMed] [Google Scholar]
- Howell B., Deacon H., Cassimeris L. Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo J. Cell Sci 112 1999. 3713 3722a [DOI] [PubMed] [Google Scholar]
- Howell B., Larsson N., Gullberg M., Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin Mol. Biol. Cell 10 1999. 105 118b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A.A. Centrosomessic transit gloria centri. Curr. Biol. 2000;10:R276–R278. doi: 10.1016/s0960-9822(00)00406-1. [DOI] [PubMed] [Google Scholar]
- Jourdain L., Curmi P., Sobel A., Pantaloni D., Carlier M.-F. Stathmina tubulin-sequestering protein which forms a ternary T-2S complex with two tubulin molecules. Biochemistry. 1997;36:10817–10821. doi: 10.1021/bi971491b. [DOI] [PubMed] [Google Scholar]
- Karaiskou A., Jessus C., Brassac T., Ozon R. Phosphatase 2A and Polo kinase, two antagonistic regulators of Cdc25 activation and MPF auto-amplification. J. Cell Sci. 1999;112:3747–3756. doi: 10.1242/jcs.112.21.3747. [DOI] [PubMed] [Google Scholar]
- Kotani S., Tugendreich S., Fujii M., Jorgensen P.-M., Watanabe N., Hogg C., Hieter P., Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity. Mol. Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W.G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Guo Z., Emami K.H., Wang S.X., Dunphy W.G. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H.A., Nigg E.A. Antibody microinjection reveals as essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N., Marklund U., Gradin H.M., Brattsand G., Gullberg M. Control of microtubule dynamics by oncoprotein 18dissection of the regulatory role of multisite phosphorylation during mitosis. Mol. Cell. Biol. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S. Microtubule dynamicsif you need a shrink try stathmin/Op18. Curr. Biol. 1998;8:R212–R214. doi: 10.1016/s0960-9822(98)70128-9. [DOI] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B.A., Gonzalez C., Karess R.E., Glover D.M., Sunkel C.E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Logarinho E., Sunkel C.E. The Drosophila POLO kinase localises to multiple compartments of the mitotic apparatus and is required for the phosphorylation of MPM2 reactive epitopes. J. Cell Sci. 1998;111:2897–2909. doi: 10.1242/jcs.111.19.2897. [DOI] [PubMed] [Google Scholar]
- Luo X.-N., Mookerjee B., Ferrari A., Mistry S., Atweh G.F. Regulation of phosphoprotein p18 in leukemic cellscell cycle regulated phosphorylation by p34-cdc2 kinase. J. Biol. Chem. 1994;269:10312–10318. [PubMed] [Google Scholar]
- Marklund U., Larsson N., Gradin H.M., Brattsand G., Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:5290–5298. [PMC free article] [PubMed] [Google Scholar]
- Maucuer A., Moreau J., Mechali M., Sobel A. Stathmin gene familyphylogenetic conservation and developmental regulation in Xenopus . J. Biol. Chem. 1993;268:16420–16429. [PubMed] [Google Scholar]
- McKim K.S., Hawley R.S. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Moutinho-Santos T., Sampaio P., Amorim I., Costa M., Sunkel C.E. In vivo localisation of the mitotic POLO kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis in Drosophila . Biol. Cell. 1999;91:585–596. [PubMed] [Google Scholar]
- Murray A.W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nachury M.V., Maresca T.J., Salmon W.C., Waterman-Storer C.M., Heald R., Weis K. Importin β is a mitotic target of the small GTPase ran in spindle assembly. Cell. 2001;104:95–106. doi: 10.1016/s0092-8674(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. Polo-like kinasespositive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Hagan I.M., Glover D.M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- Qian Y.-W., Erikson E., Li C., Maller J.L. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis . Mol. Cell. Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.-W., Erikson E., Maller J.L. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol. Cell. Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandaltzopoulos R., Blank T., Becker P.B. Transcriptional repression by nucleosomes but not H1 in reconstituted preblastoderm Drosophila chromatin. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:373–379. doi: 10.1002/j.1460-2075.1994.tb06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E., Mitchison T.J. Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol. 1991;112:925–940. doi: 10.1083/jcb.112.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart U.K., Yu J.H., Amat J.A., Wang Z.Q., Hoffmann M.K., Edelmann W. Normal development of mice lacking metablastin (P19), a phosphoprotein implicated in cell cycle regulation. J. Biol. Chem. 1996;271:14062–14066. doi: 10.1074/jbc.271.24.14062. [DOI] [PubMed] [Google Scholar]
- Shirayama M., Zachariae W., Ciosk R., Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M.O., Kammerer R.A., Jahnke W., Goldie K.N., Lustig A., van Oostrum J. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:572–580. doi: 10.1093/emboj/19.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel C.E., Glover D.M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Taagepera S., Rao P.N., Drake F.H., Gorbsky G.J. DNA topoisomerase II-alpha is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM-2. Proc. Natl. Acad. Sci. USA. 1993;90:8407–8411. doi: 10.1073/pnas.90.18.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize R., Andersen S.S.L., Verde F., Doree M., Karsenti E., Hyman A.A. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I., Raats J., Hirano T., Heasman J., Karsenti E., Wylie C. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Vernos I., Mitchison T.J., Karsenti E., Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Wallon G., Rappsilber J., Mann M., Serrano L. Model for stathmin/OP18 binding to tubulin. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:213–222. doi: 10.1093/emboj/19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wianny F., Tavares A., Evans M.J., Glover D.M., Zernicka-Goetz M. Mouse polo-like kinase 1 associates with the acentriolar spindle poles, meiotic chromosomes and spindle midzone during oocyte maturation. Chromosoma. 1998;107:430–439. doi: 10.1007/s004120050327. [DOI] [PubMed] [Google Scholar]
- Wiese C., Wilde A., Moore M.S., Adam S.A., Merdes A., Zheng Y. Role of importin-β in coupling ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. doi: 10.1126/science.1057661. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V.P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zhang D.H., Nicklas R.B. Chromosomes initiate spindle assembly upon experimental dissolution of the nuclear envelope in grasshopper spermatocytes. J. Cell Biol. 1995;131:1125–1131. doi: 10.1083/jcb.131.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]