Abstract
When centrosomes are destroyed during prophase by laser microsurgery, vertebrate somatic cells form bipolar acentrosomal mitotic spindles (Khodjakov, A., R.W. Cole, B.R. Oakley, and C.L. Rieder. 2000. Curr. Biol. 10:59–67), but the fate of these cells is unknown. Here, we show that, although these cells lack the radial arrays of astral microtubules normally associated with each spindle pole, they undergo a normal anaphase and usually produce two acentrosomal daughter cells. Relative to controls, however, these cells exhibit a significantly higher (30–50%) failure rate in cytokinesis. This failure correlates with the inability of the spindle to properly reposition itself as the cell changes shape. Also, we destroyed just one centrosome during metaphase and followed the fate of the resultant acentrosomal and centrosomal daughter cells. Within 72 h, 100% of the centrosome-containing cells had either entered DNA synthesis or divided. By contrast, during this period, none of the acentrosomal cells had entered S phase. These data reveal that the primary role of the centrosome in somatic cells is not to form the spindle but instead to ensure cytokinesis and subsequent cell cycle progression.
Keywords: centrosome, cell cycle progression, cytokinesis, mitosis, vertebrates
Introduction
Despite over a century of study, the function(s) of the centrosome remain vague, and recent papers on this topic have succeeded more in defining what this organelle does not do than revealing its true functions (for reviews see Stearns and Winey 1997; Hyman 2000). We now know, for example, that the organization of radial microtubule (Mt) arrays and the formation of mitotic spindles poles, which were traditionally attributed to the centrosome, can occur even in the absence of this organelle via the coordinated action of molecular motors and structural proteins (for reviews see Merdes and Cleveland 1997; Compton 1998; Hyman and Karsenti 1998; Hyman 2000; Merdes et al. 2000).
The fact that there are no viable vertebrate somatic cells lacking centrosomes reveals that this organelle is normally essential. As a result, the only way to determine how the absence of a centrosome affects cell behavior is to remove it at defined points in the cell cycle. In an early effort, Zorn et al. 1979 generated karyoplasts lacking centrosomes by centrifuging L929 cells in the presence of cytochalasin B. Based on an EM analysis, they concluded that centrosomes can regenerate and that this event is a prerequisite for the next mitosis. These conclusions were subsequently challenged, however, by Maniotis and Schliwa 1991, who found that the centrosome did not regenerate in BSC-1 cells after being surgically removed with a microneedle, and that in its absence cells became permanently blocked at G2. In spite of their discrepancies, both of these studies suggest that the centrosome is required for progress through the cell cycle. However, there are problems with both of these studies, not the least of which is that the methods used removed much of the cytoplasm.
We have developed a method to selectively destroy the centrosome. The basis for this approach was originally defined by Berns (for review see Berns et al. 1991), who showed that, when focused through a high NA microscope objective lens, pulses of green (532 nm) laser light can destroy any structure visible in a living cell (Strahs and Berns 1979). By combining this with green fluorescent protein (GFP) labeling, we have improved the laser microsurgery technique so that it can now be used to destroy otherwise invisible organelles including the centrosome (Khodjakov et al. 1997). In our initial study, we demonstrated that a bipolar mitotic spindle forms in vertebrates, even if both centrosomes are destroyed during prophase (Khodjakov et al. 2000). This study left several important questions unanswered, such that whether the acentrosomal spindles produced acentrosomal daughter cells and, if so, what their fate is. Here, we report our findings relevant to these questions.
Materials and Methods
Cell Culture
Culture conditions and isolation of CVG-2 and PtKG-23 have been reported previously (Khodjakov and Rieder 1999).
Laser Microsurgery
Centrosome ablation by laser microsurgery has been described previously (Cole et al. 1995; Khodjakov et al. 1997, Khodjakov et al. 2000). In brief, pulses of 532-nm Nd:YAG laser light are focused by a 60× 1.4 NA lens so the effective waist of the beam is ∼0.5 μm in the specimen plane (Cole et al. 1995). Centrosomes are then destroyed by exposing them to pulses of laser light until the γ-tubulin/GFP fluorescence is completely abolished. This typically takes ∼10 s and requires two to three series of 20–30 laser pulses.
Images were captured by a MicroMax 5 Hz cooled charge-coupled device camera (Princeton Instruments) and saved as 8-bit TIFF files. The imaging system is driven by Image Pro software (Media Cybernetic).
Long-Term Imaging
After laser microsurgery, the position of the experimental cell was marked on the coverslip, and the culture was transferred to a phase–contrast microscope equipped with a Rose chamber heater (Rieder and Cole 1998). Time-lapse images were then captured every 15 min for ≤80 h using a video-rate charge-coupled device camera (model 100; Paultek Imaging), and the media was changed every 24 h by perfusion. Illumination was obtained from a 100W Tungsten filament, filtered to remove UV (GG400) and infrared (KG5) components, made monochromatic (GIF 546), and shuttered (UniBlitz Electronics) between exposures (1 s/image).
Immunofluorescence Microscopy
Immunofluorescence staining and imaging were conducted as previously described (Khodjakov and Rieder 1999). The following primary antibodies were used: rat monoclonal anti–α-tubulin (clone YL1/2; gift of Dr. J.V. Kilmartin, Medical Research Council, Cambridge, UK) at 1:100, and anti–γ-tubulin (number T6557; Sigma-Aldrich) at 1:300. BrdU labeling and visualization was conducted using a BrdU staining kit (number 93-3943; Zymed) according to manufacturer's instructions.
Some images (see Fig. 1 and Fig. 2) were collected as a Z-series (200-nm steps) and deconvolved using Delta Vision 2.1 deconvolution software (Applied Precision), and they are presented as maximal intensity projections.
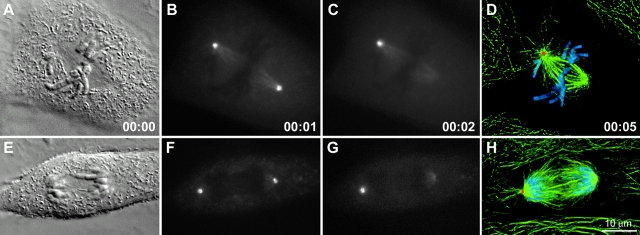
Figure 1.
Astral Mts rapidly disassemble when centrosomes are destroyed during spindle assembly. (A–D) After ablating one of two centrosomes during prometa- phase (compare B with C), this cell was fixed 5 min later and stained for α-tubulin (green), γ-tubulin (red), and DNA (blue). Notice that the acentrosomal pole lacks astral Mts. (E–H) Similar to A–D, but the centrosome was ablated during late anaphase. As in prometaphase and metaphase cells, the acentrosomal pole was depleted of astral Mts within 5 min (H), although some free Mts are present in the cytoplasm. PtKG cells. Time in h:min. Images in D and H are maximal intensity projections through the cell volume after deconvolution.
Figure 2.
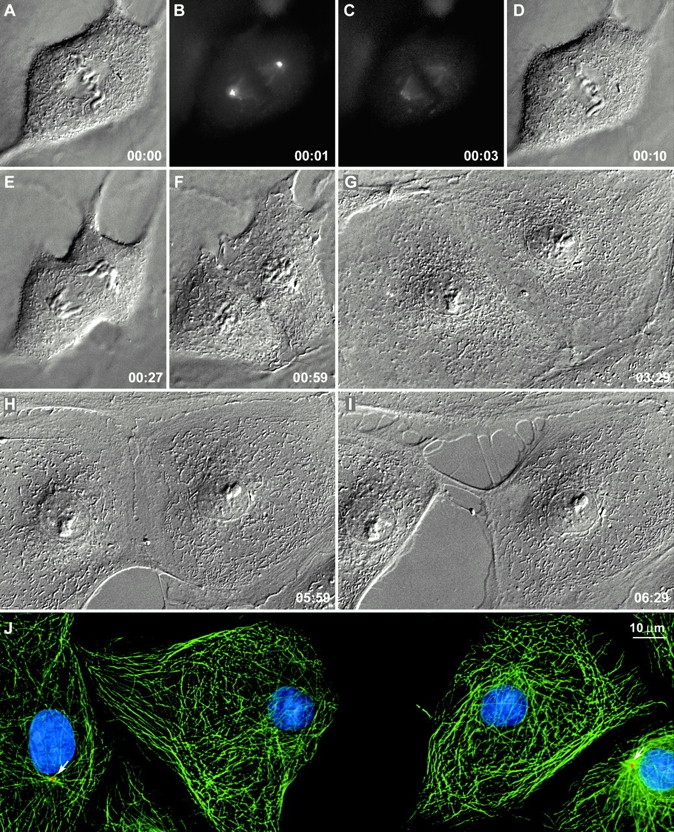
Cells can divide normally without centrosomes. After destroying both centrosomes during metaphase (B and C), this cell was followed by time-lapse differential interference contrast microscopy. It underwent a normal telophase (E and F) and cytokinesis (E–H), and ∼5 h later the midbody broke as the two daughter cells began to migrate from each other (H and I). At this time, the culture was fixed and stained as described in the legend to Fig. 1. Note that the two acentrosomal daughter cells contained normal numbers of Mts but that, when compared with neighboring centrosomal cells (J, arrows), the Mt lacked a sharp focus. PtKG cells. Time in h:min. The image in I is a maximal intensity projection through the cell volume after deconvolution.
Online Supplemental Material
Time-lapse movies (Supplemental Videos 1–4) of the all cells presented in the published figures are available at http://www.jcb.org/cgi/content/full/153/1/237/DC1. Additionally, an example of a CVG cell (not shown as a printed figure), which formed multiple asynchronous cleavage furrows, is available as a movie.
Results and Discussion
Astral Microtubules Rapidly Disappear upon Centrosome Destruction
The only noticeable difference between spindles formed in the presence or absence of centrosomes is that the latter lack the radial arrays of astral Mts normally associated with mitotic centrosomes (Khodjakov et al. 2000). To define the time course for astral Mts disassembly, we destroyed just one centrosome during mitosis and then fixed cells various times after the operation for an immunofluorescence analysis. We found that the irradiated pole always lost its associated astral Mts within 5 min, whereas the control pole retained a normal number of Mts (Fig. 1). Live-cell studies on cells permanently expressing α-tubulin/GFP confirmed this conclusion and revealed that the disappearance of astral Mts actually occurs within 2–3 min (not shown).
Destroying both Centrosomes during Metaphase Does Not Prevent Anaphase or Formation of the Cleavage Furrow
To determine if acentrosomal spindles produce acentrosomal daughter cells, we destroyed both centrosomes during prometaphase or metaphase in 10 PtKG-23 and 10 CVG-2 cells, and then followed them by time-lapse microscopy. We found that the outcome was always the same regardless of when the centrosomes were destroyed: after all chromosomes had achieved an equatorial alignment, the bipolar acentrosomal and anastral spindle subsequently entered anaphase, during which time, the chromatids moved towards the ends of the spindle.
In most cases (7/10 PtKGs and 5/10 CVGs), ablating both centrosomes during metaphase did not affect the later stages of mitosis. The cell exhibited normal chromatid separation and cytokinesis, and eventually severed the midbody to form two acentrosomal daughter cells (Fig. 2). At the light microscopy level, the morphology of these cells (Fig. 2 I) was the same as surrounding centrosome-containing cells, and they also appeared to contain normal numbers of Mts (Fig. 2 J). The only feature distinguishing acentrosomal cells from controls was that they lacked the sharp Mt focus normally associated with the centrosome (Fig. 2 J, arrows).
In the remaining cases (3/10 PtKGs and 5/10 CVGs), cytokinesis failed, and a binucleated cell was produced lacking a centrosome. This failure rate was significantly higher than in nonirradiated control cells in which cytokinesis fails <5% of the time. In controls, the mitotic spindle is positioned near the geometric center of the cell with the spindle axis parallel to the long axis of the cell. As the shape of the cell changes during mitosis, the spindle rotates to maintain its proper orientation (Rieder and Hard 1990; O'Connell and Wang 2000). However, in cells with acentrosomal spindles, this compensatory change in spindle position did not occur. As result, anaphase was often initiated with the spindle roughly perpendicular to the cell's long axis (Fig. 3), or well off its geometric center (not shown). Under both conditions, when the chromatids reached the cell periphery, they stopped moving, even if the distance separating the chromatid groups was not sufficient to insure complete separation of longest chromosome arms (Fig. 3 F). As result, thin chromatin bridges connecting daughter nuclei often formed (Fig. 3 L) that are known to inhibit the terminal stages of cytokinesis (Mullins and Biesele 1977).
Figure 3.
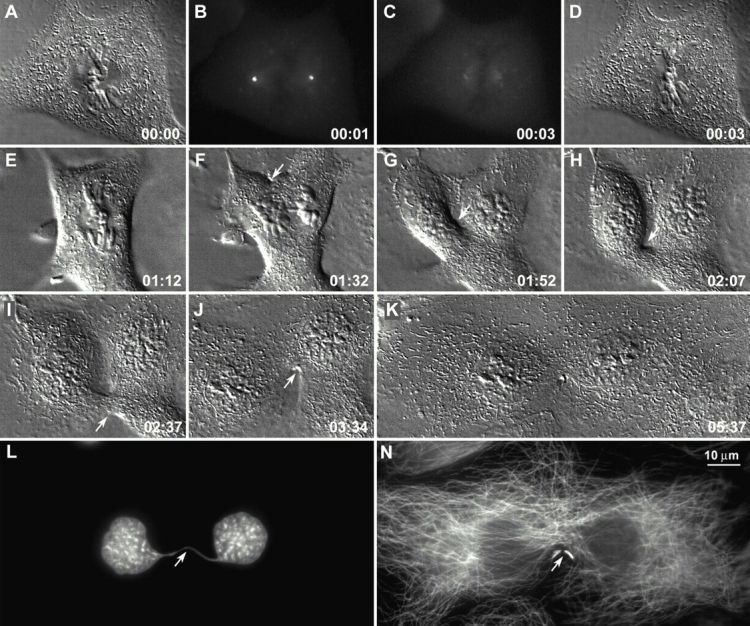
Cytokinesis frequently fails when both centrosomes are destroyed during metaphase. Centrosome ablation was performed described in the legend to Fig. 2. After the operation, the spindle remained stationary as the cell changed shape around it so that its long axis became oriented perpendicular to the long axis of the spindle (D and E). This, in turn, restricted chromatid separation during anaphase (F). During cytokinesis, a (unilateral) furrow formed, first on one side (arrows in F–H), and then on the other (I and J, arrows), but ultimately both regressed (K). The culture was fixed ∼6 h after operation and stained for DNA and Mts. An immunofluorescence analysis revealed that the separated nuclei were connected by a thin chromatin bridge (L, arrow), that the cell lacked sharp Mt foci, and that despite its failure to cleave it had developed a well-formed midbody (M, arrow). Time in h:min.
Cytokinesis in the acentrosomal cells could also fail due to abnormalities in the formation and/or propagation of the furrow. Unilateral furrows were commonly formed only on one side of the spindle (Fig. 3, F–H, arrowhead) that wandered through the cytoplasm, could impact chromosomes, and could turn, but that ultimately relaxed (Fig. 3). In some cells, such furrows formed well removed from the spindle equator, and in others multiple bilateral furrows were formed, all of which ultimately relaxed (time-lapse videos available at http://www.jcb.org/cgi/content/full/153/1/237/DC1).
The fact that most of our experimental cells exited mitosis and formed two daughters reveals that centrosomes, per se, are not required for progression through mitosis, as might be expected, for example, from the observation that cyclin B degradation starts in the spindle poles (Clute and Pines 1999; Wakefield et al. 2000). In most cells, the duration of metaphase was within the range established for control PtK1 cells (23 min with a range of 9–48 min; Rieder et al. 1994), but in those cells where the spindle was improperly oriented, metaphase sometimes lasted 60–70 min (Fig. 3). This is consistent with O'Connell and Wang 2000 (see also Muhua et al. 1998) report that preventing proper spindle orientation induces a slight prolongation of metaphase in normal rat kidney cells.
Until recently, the putative primary function of centrosomes was to define the poles of the mitotic spindle and to orchestrate its formation. However, it is now clear that bipolar spindles can be formed via acentrosomal pathway(s), even in cell types that normally possess centrosomes (for reviews see Merdes and Cleveland 1997; Compton 1998; Hyman 2000). The work presented here reveals that in vertebrates such acentrosomal spindles are fully functional, i.e., they support normal chromosome separation during anaphase and, at least under favorable conditions, cytokinesis and the formation of independent daughter cells lacking centrosomes.
Our data also reveal that acentrosomal spindles are no longer able to reposition themselves in response to ensuing changes in cell shape, and this is correlated with defects in the formation and/or propagation of cleavage furrows. Overall, these types of abnormalities support the hypothesis that astral Mts orient the spindle (Shaw et al. 1997; O'Connell and Wang 2000; Faulkner et al. 2000) and help define the cleavage plane by providing spatial queues (Rappaport and Ebstein 1965). Thus, the role of the centrosome during mitosis in animals is not to form the spindle, but rather to organize and maintain the astral Mt arrays to ensure proper cytokinesis (de Saint Phalle and Sullivan 1998; Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999).
Acentrosomal Cells Arrest during G1
The fact that our laser microsurgery approach can be used to reproducibly generate cells that begin a new cycle in the absence of a centrosome allowed us to address the question of whether centrosomes are required for cell cycle progression. For these experiments, we ablated only one of the two centrosomes during metaphase when the centrosomes were maximally separated from the chromosomes. This approach minimized possible collateral damage to the DNA, which is known to impede cell cycle progression (Elledge 1996; Kaufmann and Paules 1996). Also, since the two resultant progeny cells were born from the same mother, they were identical except for the presence or absence of a centrosomes, and any nonspecific effects of the irradiation would be expected to equally affect both.
We used CVG cells for these experiments because, as typical fibroblasts, they grow individually. PtKG, on the other hand, are typical epithelia in which progression of an individual cell through the cycle depends on many variables including, for example, whether it is located within or at the periphery of a cell sheet—a fact that complicates individual cell analyses. To determine if the progeny of our operation underwent DNA synthesis during the observation period, we perfused them with media containing BrdU immediately after the operation, and the media was replenished with fresh medium containing BrdU every 24 h. All cultures were then fixed ∼72 h after operation.
To determine if something produced within the cell by the laser operation inhibits progression through the cell cycle, we irradiated four CVG metaphase spindles next to one of the centrosomes. In all cases, all of the resultant eight daughter cells replicated their DNA during our 72-h observation period, and two divided.
We completely destroyed one of the two centrosomes in 25 metaphase CVG cells, 16 of which subsequently underwent cytokinesis to produce two daughter cells. Of the 16 centrosome-containing control cells generated during this study, 3 underwent the next mitosis during the 72-h observation period (not shown). The other 13 centrosome-containing controls remained in interphase during this time, but all had incorporated BrdU and duplicated their centrosomes by the time of fixation (Fig. 4). By contrast, during this same period, 3 of the acentrosomal cells rounded and died, after extensive blebbing (not shown) characteristic of apoptosis (Wyllie et al. 1980), and none of the other 13 acentrosomal cells had incorporated BrdU at the time of fixation 72 h later (Fig. 4 M).
Figure 4.
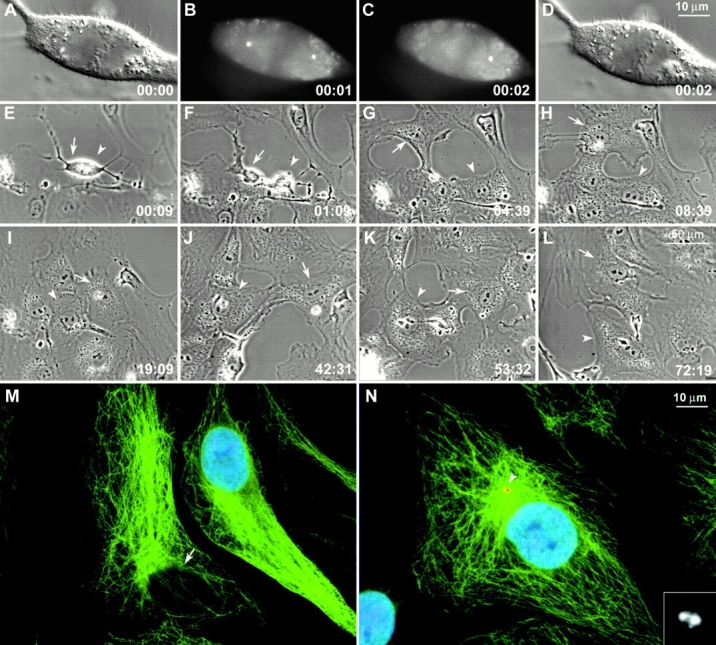
Cells lacking centrosomes do not enter DNA synthesis. One of two centrosomes was ablated during metaphase in a CVG cell that then divided to form a cell lacking a centrosome (E–L, arrows), and a cell containing a centrosome (E–L, arrowheads). Both progeny underwent postmitotic flattening and exhibited similar behaviors over the next three days. The culture was then fixed ∼72 h after operation and stained for α-tubulin (green), γ-tubulin (red), and BrdU (blue). The cell lacking a centrosome had not incorporated BrdU (M, arrow) and lacked the sharp Mt focus normally associated with a centrosome (the centrosome in the neighboring cell seen in M was in a different focal plane). By contrast the centrosome-containing daughter cell (N) had incorporated BrdU and contained a typical array of cytoplasmic Mts that focused onto a duplicated centrosome (inset in N). CVG cells. Time in h:min. Bars, 10 μm.
Except for the 3 cells that died, the remaining 13 acentrosomal cells could not be distinguished morphologically from surrounding centrosome-containing controls: each exhibited a similar behavior (polarization, locomotion, etc.) and distribution of mitochondria and Golgi apparatus (not shown). However, at the end of 72 h, acentrosomal cells contained no discrete γ-tubulin–containing structures, and their Mt arrays lacked a sharp focus, i.e., there was no evidence of centrosome reformation (Fig. 4 M). In a separate set of experiments, we analyzed 10 PtKG and 10 CVG cells by serial section electron microscopy, 24–48 h after ablating the centrosome, and never found any evidence of centriole/centrosome regeneration (not shown).
These data reveal that acentrosomal daughter cells, produced during mitosis from cells containing an acentrosomal spindle pole, invariably arrest in interphase. This general conclusion is consistent with that of Maniotis and Schliwa 1991 who found that acentrosomal BSC-1 cells, generated by removing the centrosome (and a large part of cytoplasm) with a microneedle, never entered mitosis. However, these authors found that acentrosomal cells could initiate and presumably complete DNA synthesis, which, on the surface, appears in conflict with our finding that acentrosomal CVG cells produced by laser ablation arrest before S period (i.e., during G1). This discrepancy could simply be due, however, to the different protocols used to produce acentrosomal cells. Maniotis and Schliwa 1991 conducted their experiments on nonsynchronized interphase cells, immediately added BrdU, and then followed the cells for ≤26 h. Under these conditions, those acentrosomal cells that incorporated BrdU (11/14) could have been near the G1/S transition, or even in S, when the centrosome was removed. By contrast, in our experiments, the centrosomes were destroyed during mitosis, before initiating the next cell cycle—in essence, before the cell was born. The fact that none of our acentrosomal cells incorporated BrdU clearly reveals that the centrosome is required during early G1 for cell cycle progression, such as, passage through START. However, at some point after this time, it may be dispensable for cell cycle progression.
All of our centrosome-containing control cells either incorporated BrdU or divided during our 72-h observational period. The reason why more of these cells did not enter another mitosis is unknown, but it likely means that our filming conditions are not optimal. Cell cycle progression in most mammalian cells, and in particular the G2/M transition, is light sensitive and easily inhibited or delayed by the illumination used during microscopy (Rieder and Cole 2000). Regardless, however, the fact that all of our control cells replicated their DNA (14/16) and/or underwent mitosis (3/16), under conditions in which none of their acentrosomal sisters even incorporated BrdU, reveals that the presence of the centrosome is required for the G1/S transition.
Morphologically our acentrosomal cells can only be distinguished from their centrosomal sisters by their lack of a sharp cytoplasmic Mt focal point. This raises the issue of whether the cell cycle arrest we see in acentrosomal cells is due to this abnormal organization of Mts or to the lack of the centrosome as an organelle. Current evidence favors the later idea: when CHO cells synchronized in mitosis are replated in the presence of nocodazole, they appear to progress through the cell cycle with normal kinetics (Young et al. 2000). If this is true, it means that a pathway necessary for progression requires the centrosome independent of the Mt array it organizes. Whether the presence of a centrosome is monitored by a bona fide cell cycle checkpoint, or if it is required simply to provide a product necessary for cell cycle progression, remains to be determined.
Finally, we never observed centrosome regeneration. In contrast, when antibodies to polyglutamylated tubulin are loaded into cells, the centrosome as a structural and functional entity disappears, but as the antibody concentration drops, the centrosome reassembles (Bobinnec et al. 1998). This implies that centrosome re-formation requires a template that is destroyed by our laser ablation approach but that remains intact and functional after antibody-induced dispersion of the centrosome. The nature of this template is unknown, but our data suggest that it is located within the centrosome and that it cannot be re-formed in the absence of another centrosome (or template).
Supplemental Material
Acknowledgments
We thank Mr. R. Cole for assistance with laser microsurgery and Drs. M. Koonce and G. Sluder for stimulating discussions and critical comments on the manuscript. We also acknowledge use of the Wadsworth Center's Video Light Microscopy core facilities.
This work was supported by National Institutes of Health grants 59363 (A. Khodjakov) and 40198 (C.L. Rieder).
Note Added in Proof. The observation that acentrosomal cells become arrested during G1 also has been reported recently by Hinchcliffe et al. (Hinchcliffe, E.D., F.J. Miller, M. Cham, A. Khodjakov, and G. Sluder. 2001. Science. 291:1547–1550).
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: GFP, green fluorescent protein; Mt, microtubule.
References
- Berns M.W., Wright W.H., Wiegand S.R. Laser microbeam as a tool in cell biology. Int. Rev. Cytol. 1991;129:1–44. doi: 10.1016/s0074-7696(08)60507-0. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Khodjakov A., Mir L.M., Rieder C.L., Edde B., Bornens M. Centriole disassembly and in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P., Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- Cole R.W., Khodjakov A., Wright W.H., Rieder C.L. A differential interference contrast-based light microscopic system for laser microsurgery and optical trapping of selected chromosomes during mitosis in vivo. J. Microsc. Soc. Amer. 1995;1:203–215. [Google Scholar]
- Compton D.A. Focusing on spindle poles. J. Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- de Saint Phalle B., Sullivan W. Spindle assembly and mitosis without centrosomes in parthenogenetic Sciara embryos. J. Cell Biol. 1998;141:1383–1391. doi: 10.1083/jcb.141.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J. Cell cycle checkpointspreventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Faulkner N.E., Dujardin D.L., Tai C.Y., Vaughan K.T., O'Connell C.B., Wang Y.-L., Vallee R.B. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Hyman A. CentrosomesSic transit gloria centri. Curr. Biol. 2000;10:R276–R278. doi: 10.1016/s0960-9822(00)00406-1. [DOI] [PubMed] [Google Scholar]
- Hyman A., Karsenti E. The role of nucleation in pattering microtubule networks. J. Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.K., Paules R.S. DNA damage and cell cycle checkpoints. FASEB J. 1996;10:238–247. doi: 10.1096/fasebj.10.2.8641557. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C.L. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 1999;146:585–596. doi: 10.1083/jcb.146.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., Rieder C.L. A synergy of technologiescombining laser microsurgery with green fluorescent protein tagging. Cell Motil. Cytoskelet. 1997;38:311–317. doi: 10.1002/(SICI)1097-0169(1997)38:4<311::AID-CM1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., Oakley B.R., Rieder C.L. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Maniotis A., Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991;67:495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- Megraw T.L., Li K., Kao L.R., Kaufman T.C. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila . Development. 1999;126:2829–2839. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- Merdes A., Cleveland D.W. Pathways of spindle pole formationdifferent mechanisms; conserved components. J. Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Heald R., Samejima K., Earnshaw W.C., Cleveland D.W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 2000;149:851–862. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhua L., Adames N.R., Murphy M.D., Shields C.R., Cooper J.A. A cytokinesis checkpoint requiring the yeast homologue of an APC-binding protein. Nature. 1998;393:487–491. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J.M., Biesele J.J. Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 1977;73:672–684. doi: 10.1083/jcb.73.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C.B., Wang Y.-L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell. 2000;11:1765–1774. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R., Ebstein R.P. Duration of stimulus and latent periods preceding furrow formation in sand dollar eggs. J. Exp. Zool. 1965;158:373–382. doi: 10.1002/jez.1401580311. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Cole R.W. Perfusion chambers for high-resolution video-light microscopic studies of vertebrate cell monolayerssome considerations and a design. In: Sluder G., Wolf D.E., editors. Video Microscopy. Academic Press; San Diego: 1998. pp. 253–275. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Cole R.W. Microscopy-induced radiation damage, microtubules, and progression through the terminal stage of G2 (prophase) in vertebrate somatic cells. Cold Springs Harb. Symp. Quant. Biol. 2000;65:1–8. doi: 10.1101/sqb.2000.65.369. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Hard R. Newt lung epithelial cellscultivation, use, and advantages for biomedical research. Int. Rev. Cytol. 1990;122:153–220. doi: 10.1016/s0074-7696(08)61208-5. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Schultz A., Cole R., Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.L., Yeh E., Maddox P., Salmon E.D., Bloom K. Astral microtubule dynamic in yeasta microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J. Cell Biol. 1997;139:985–994. doi: 10.1083/jcb.139.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Winey M. The cell center at 100. Cell. 1997;91:303–309. doi: 10.1016/s0092-8674(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Strahs K.R., Berns M.W. Laser microirradiation of stress fibers and intermediate filaments in non-muscle cells from cultured rat heart. Exp. Cell Res. 1979;119:31–45. doi: 10.1016/0014-4827(79)90332-x. [DOI] [PubMed] [Google Scholar]
- Vaizel-Ohayon D., Schejter E.D. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 1999;9:889–898. doi: 10.1016/s0960-9822(99)80393-5. [DOI] [PubMed] [Google Scholar]
- Wakefield J.G., Huang J.-Y., Raff J.W. Centrosomes have a role in regulating the destruction of cyclin B in early Drosophila embryos. Curr. Biol. 2000;10:1367–1370. doi: 10.1016/s0960-9822(00)00776-4. [DOI] [PubMed] [Google Scholar]
- Wyllie A.H., Kerr J.F.R., Currie A.R. Cell deaththe significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Young A., Dictenberg J.B., Purohit A., Tuft R., Doxsey S.J. Cytoplasmic synein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol. Biol. Cell. 2000;11:2047–2056. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn G.A., Lucas J.J., Kates J.R. Purification and characterization of regenerating karioplasts. Cell. 1979;18:659–672. doi: 10.1016/0092-8674(79)90121-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.