Abstract
Background
NMDA receptor open channel blockers phencyclidine (PCP) and dizocilpine (MK-801) elicit schizophrenia-like symptoms in humans and in animal models. Group II metabotropic glutamate receptor agonists reverse the behavioral effects of PCP and MK-801 in animal models. N-Acetylaspartylglutamate (NAAG), third most prevalent neurotransmitter in the mammalian nervous system, is a selective group II metabotropic glutamate receptor agonist. We previously reported that ZJ43, a potent inhibitor of the enzymes that inactivate synaptically released NAAG, reduced motor and stereotypic effects of PCP in the rat.
Methods
To confirm the efficacy of NAAG peptidase inhibition in decreasing motor behaviors induced by PCP and MK-801, ZJ43 was tested in additional schizophrenia models.
Results
ZJ43 reduced MK-801-induced motor activation in a mouse model that has been used to characterize the efficacy of a wide range of pharmacotherapies for this human disorder. In a second mouse strain, the peptidase inhibitor reduced PCP-induced stereotypic movements. ZJ43 also reduced PCP-induced negative symptoms in a resident-intruder assay. The group II metabotropic glutamate receptor antagonist, LY341495, blocked the effect of NAAG peptidase inhibition in these mouse models of positive and negative PCP- and MK-801-induced behaviors. Additionally, LY341495 alone increased some PCP-induced behaviors suggesting that normal levels of NAAG act to moderate the effect of PCP via a group II mGluR.
Conclusion
These data support the proposal that NAAG peptidase inhibition and elevation of synaptic NAAG levels represent a new therapeutic approach to treating the positive and negative symptoms of schizophrenia that are modeled by open channel NMDA receptor antagonists.
Keywords: Phencyclidine, MK-801, N-acetylaspartylglutamate, NAAG, group II metabotropic glutamate receptor, mGluR3, schizophrenia, NMDA receptors, LY341495
Introduction
Nearly one percent of humans express symptoms of schizophrenia. The efficacy of dopamine D2 antagonists, haloperidol and chlorpromazine, in treating schizophrenia supports the view that dopaminergic neurons contribute to the expression of this disorder (1), while studies using drugs that affect NMDA receptors suggest that glutamatergic pathways also are involved in schizophrenia (2; 3; 4; 5; 6, 7; 8). For example, open channel NMDA receptor antagonists phencyclidine (PCP), ketamine and dizocilpine (MK-801) induce schizophrenia-like positive, negative and cognitive symptoms in humans and behaviors in animals. Drugs that are useful in treating schizophrenic patients moderate these PCP- and MK-801-induced behaviors. Group II metabotropic glutamate receptor (mGluR2 and mGluR3) agonists reduce these symptoms in humans and animal models (9; 10; 11; 12; 13). Further, polymorphisms in the human mGluR3 gene meet the criterion of association with risk of schizophrenia in three independent studies (reviewed in Harrison and Weinberger, 14). Consistent with a role for glutamate and NMDA receptors in expression of schizophrenia, D-serine, D-alanine and D-cycloserine, positive modulators of NMDA receptors show promise as adjuvant therapy for this disorder (15; 16). Additionally, NMDA receptor deficits have been identified in vivo in medication-free schizophrenic patients (17)
The peptide neurotransmitter N-acetylaspartylglutamate (NAAG) is widely distributed in the central and peripheral nervous systems at millimolar concentrations (18; 19; 20). NAAG is a mGluR3 selective group II mGluR agonist (21; 22) and is codistributed with different small amine transmitters including glutamate and GABA (reviewed in 23; 24). One function of NAAG is the activation of presynaptic mGluRs to inhibit transmitter release (reviewed in 25). Synaptically released NAAG is inactivated by two extracellular peptidases, glutamate carboxypeptidase II and III (26; 27; 28; 29). Inhibition of these peptidases reduces symptoms in animal models of glutamate-mediated clinical conditions including stroke, inflammatory and neuropathic pain, and traumatic brain injury (25; 30).
Consistent with the efficacy of group II mGluR agonists in moderating the schizophrenia-like behaviors elicited by PCP and MK-801, inhibition of NAAG peptidases by a novel NAAG analogue, ZJ43, also is effective in reducing PCP-evoked motor and stereotypic movements in rats (31). The present study tests the hypothesis that inhibition of NAAG peptidase and the consequent increase in NAAG activation of group II mGluRs, is effective across different models of positive and negative symptoms elicited by PCP and MK-801.
Materials and Methods
Animals
The experimental protocols used in this research were approved by the Georgetown University Animal Care and Use Committee consistent with guidelines of the US National Institutes of Health.
Adult C57BL/6J mice and NIH Swiss mice (National Cancer Institute, Frederick Research Center, USA) and DAB/2 mice (Taconic Farms, MD, USA) were maintained on a 12:12 h light-dark cycle. Food and water were available ad libitium. Mice were housed 5 to a cage, except as noted for the resident-intruder assay. Behavioral testing was performed between 10 am and 4 pm. Animals were weighed prior to drug administration and observation.
Drugs
ZJ43 was synthesized by Acenta Discovery, Inc., as described in Olszewski et al., (31), PCP and MK-801 ([+]-5-methyl-10, 11-dihydro-5H-dibenzo[a,d]cyclohepten-5, 10-imine maleate; dizocilpine) were from Sigma Aldrich (St. Louis, MO, USA). LY341495 was from Tocris Cookson Ltd. (Bristol, UK). LY341495 is a highly selective group II mGluR antagonist (32). All compounds were dissolved in saline for i.p. injection.
Open Field Stereotypic Movement Assessment
Adult male DBA/2 male mice, 25–30 grams in weight, were placed individually in a MED ASSOCIATES (St. Albans, VT) ENV-515 open field chamber (43 × 43 cm) with evenly spaced infrared beams and detectors. After a 10 minute habituation interval, each animal was injected i.p. first time with saline or ZJ43 (10–200 mg/kg) or LY341495 (1 or 3 mg/kg) alone or ZJ43 plus LY341495, and placed back into the chamber for another 10 minutes. Mice then were given a second i.p. injection with PCP (6 mg/kg) or saline. The motor behavior was continuously scored for 15 minutes starting 10 minutes after the second injection. Stereotypic movements were assessed automatically by the infrared sensors. Stereotypic index or counts was defined as the total number of vertical or horizontal sensor breaks within a predefined “Box” within the open field. A “Box Size” of 4 beams (defined in Med Associates Activity Monitor 5 open field chamber and software) was selected to maximize the detection of stereotypic head weaving and discriminate these movements from ambulatory movements across boxes within the open field.
MK-801 Induced Jumping
Adult male NIH-Swiss mice were habituated for 5 minutes in a clear plastic open field chamber (18cm × 29cm × 12cm high) that was identical in size to their home cage.
Mice then were injected i.p. with either saline or LY341495 (1mg/kg) or ZJ43 (200 mg/kg) with or without LY341495 and placed back into the chamber. After another 5 minutes, mice were injected with saline or MK-801 (1 mg/kg). Mice were then continuously observed and jumping episodes were scored during the next 30 minutes by an observer who was naïve with respect to the drug treatments. A single jumping episode consisted of a series of continuous uncontrolled vertical movements lasting from 1–5 seconds. Data are reported as the total jumping episodes observed over the 30 minute interval.
Resident-Intruder Assay
Male DBA/2 mice, 30–45 days old, were housed as either “resident” or “intruder” mice in the same animal room. Resident mice were isolated in individual cages for 7–14 days and intruder mice were continuously housed in groups of five. On the test day, resident mice were injected with either saline or ZJ43 (150 mg/kg, i.p.) or LY341495 (1mg/kg, i.p.) or ZJ43 plus LY341495 and returned to the cage for 10 minutes prior to injection with saline or PCP (6 mg/kg, i.p.). These resident mice were then returned to their home cages and 5 minutes later an intruder mouse was introduced into the resident’s home cage. The resident mouse’s behavior was video recorded for ten minutes. The videos of resident behaviors were scored separately by two observers who were blinded to the treatments. A series of behaviors were scored including the following: crouch defense, when experimental mouse crouched when in contact with the intruder mouse; following, when experimental mouse walked after the intruder mouse; flight, when experimental mouse ran away from the intruder mouse; leap, when experimental mouse jumped off the ground in response to the presence of the intruder.
Statistical Analysis
Data were analyzed by one-way ANOVA using SPSS software 11.0 with significant differences noted with p<0.05. When ANOVA was significant, the Post Hoc comparisons using Student-Newman-Keul test identified pair-wise group differences.
Results
DBA/2 mice treated with PCP (6 mg/kg, i.p.) exhibited a 2-fold increase in stereotypic motor activity in the open field assay system (Figure 1) compared to mice injected with saline, saline with ZJ43 or saline with the group II mGluR antagonist LY341495 (1 or 3mg/kg, i.p.). ZJ43 (150 mg/kg, i.p.) given 10 minutes before PCP significantly reduced this behavior. LY341495 at 3 mg/kg, but not 1 mg/kg, blocked the effect of ZJ43. The group II antagonist at 3 mg/kg also significantly increased the effect PCP in the absence of ZJ43, an effect taken to suggest that endogenous NAAG or glutamate activation of group II mGluR reduces the effects of PCP even in the absence of peptidase inhibitor. ZJ43 reduced the effect of PCP in a dose- dependent manner when tested between 10 and 150 mg/kg (i.p.) (Figure 2).
Figure 1.
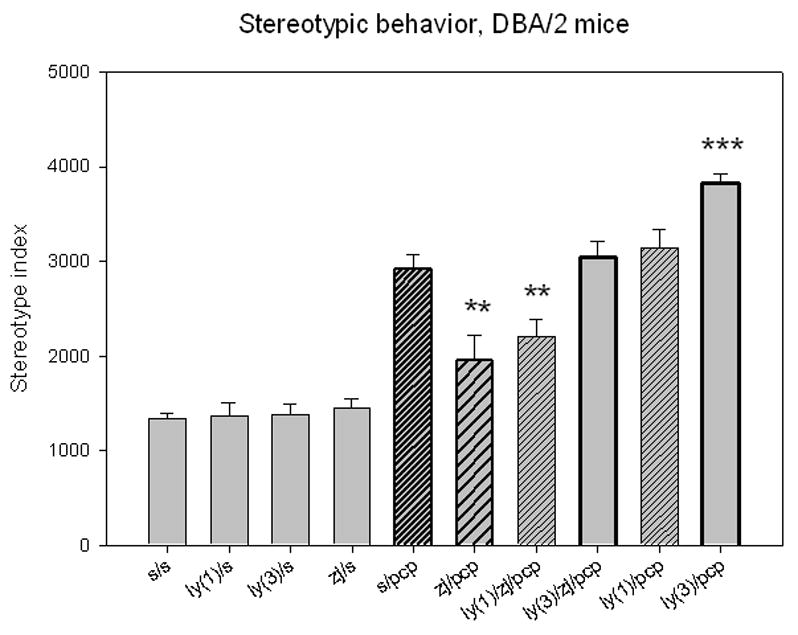
N-Acetylaspartylglutamate (NAAG) peptidase inhibition reduces phencyclidine (PCP)-induced stereotypic movements in DBA/2 mice. Mice (10 to 15 mice per group) were given two i.p. injections 10 minute apart and their activity monitored in an open field chamber 10 minutes after the last injection. Injection with the group II antagonist LY341495 (1 or 3 mg/kg) or the NAAG peptidase inhibitor, ZJ43 (150mg/kg), followed by saline had the same effect as two injections of saline. Saline followed by 6 mg/kg of PCP induced a significant increase in stereotypic behavior versus each of the other saline groups (p < 0.001). ZJ43 (150 mg/kg) significantly decreased the effects of PCP on stereotypic behavior and this action of ZJ43 was reversed by coinjection of 3mg/kg LY341495 with ZJ43 10 minutes prior to injection of PCP. Preinjection of mice with 3 mg/kg LY341495 alone prior to PCP injection significantly increased the stereotypy above that obtained with saline PCP. (* denotes p<0.05; ** denotes p< 0.01; *** denotes p< 0.001 in this and following figures)
Figure 2.
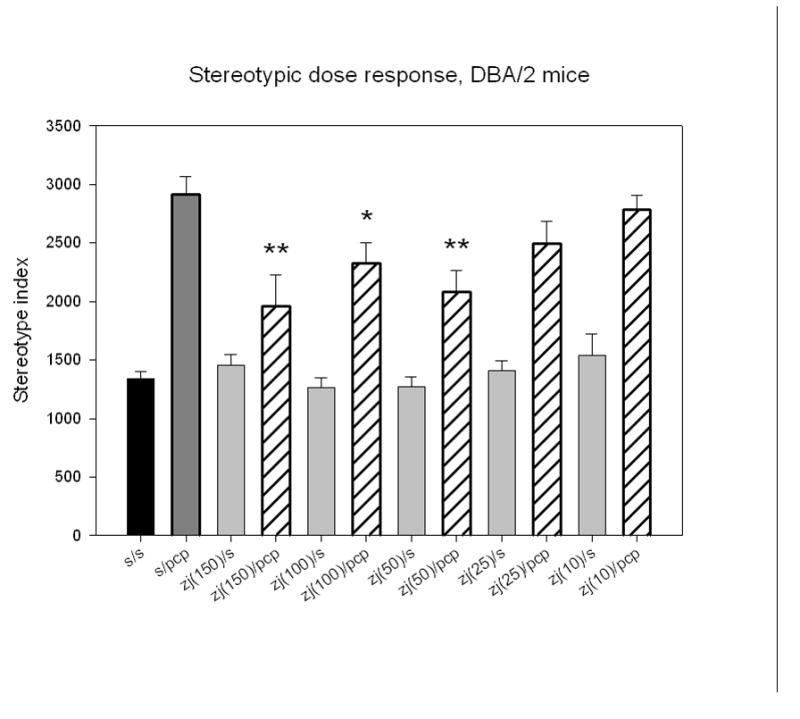
ZJ43 dose response in DBA/2 mice treated with 6 mg/kg PCP. Groups of 10–15 mice were given i.p. injections of 25, 50, 100 and 150 mg/kg ZJ43 followed 10 minutes later by PCP and their stereotypic movements recorded in the open field chamber for 10 minutes. 50 mg/kg ZJ43 was the lowest dose to produce a significant reduction in the motor behavior induced by treatment with saline-PCP. All ZJ43 effects were completely reversed by the group II mGluR antagonist LY341495.
In order to determine if the effects of NAAG peptidase inhibition were specific for PCP or generalized to other NMDA open channel antagonists that are known to induce schizophrenia-like behaviors, ZJ43 was tested in the NIH Swiss mouse model that has been used to study a series of therapeutic approaches to schizophrenia (33; 34; 35). These mice respond to MK-801 with a characteristic jumping behavior (36). At a dose of ZJ43 (200 mg/kg, i.p.) alone that had no effect on open field locomotor activity in the NIH Swiss mice (saline-saline, 3460 ± 360 cm traveled versus 3600 ± 267 cm for ZJ43-saline mice), ZJ43 significantly decreased the jumping response induced by MK-801 (1 mg/kg, i.p.) (Figure 3). While LY341495 induced no jumping behavior when paired with saline, it dramatically synergized with MK-801 in this model to produce a nearly 3-fold increase in jumping over PCP alone, and blocked the effect of ZJ43 on MK-801.
Figure 3.
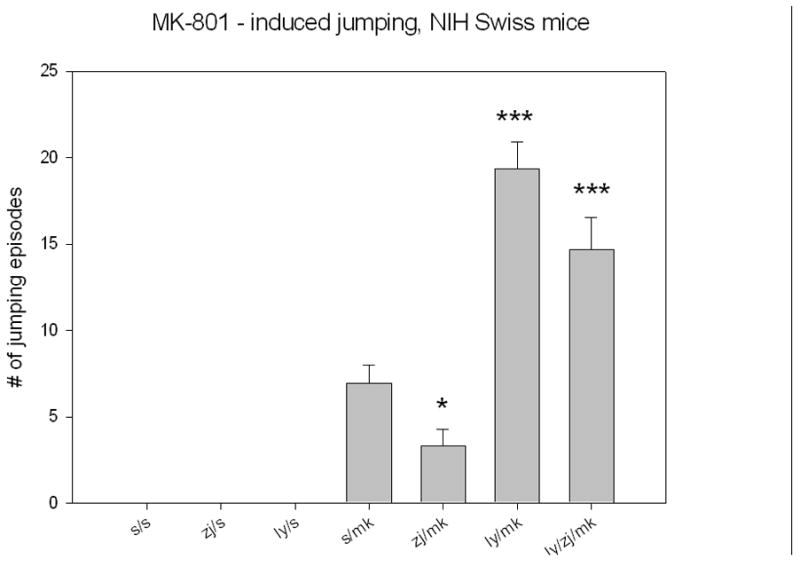
Adult male NIH Swiss mice respond to 1 mg/kg dizocilpine (MK-801) with a pronounced jumping behavior that is reduced by treatment 5 minutes earlier with 200 mg/kg (i.p.) ZJ43 (s-mk, n = 27; ZJ-mk, n =16). Mice injected twice with saline (n-5), ZJ43 + saline (n = 5) or LY341495 + saline (n = 5) exhibited no jumping behavior during the 30 minutes observation interval. Strikingly, pretreatment with the group II mGluR antagonist increases the effect of MK-801 nearly 3-fold (n = 25) as did pretreatment with the antagonist and the peptidase inhibitor (n = 19).
Schizophrenics and control human subjects treated with PCP exhibit negative symptoms, including social withdrawal, that are modeled in animals using the resident-intruder assay. Resident DBA/2 mice injected with PCP (6 mg/kg, i.p.) had a significantly reduced tendency to follow the uninjected intruder mice (Figure 4). As in the other experiments, ZJ43 (150 mg/kg, i.p.) substantially reduced this effect of PCP. LY341495 did not affect this PCP-induced behavior and completely reversed the effects of the NAAG peptidase inhibitor. Indeed, the mice treated with the antagonist together with ZJ43 and PCP had a trend to fewer (p = 0.085) following episodes than the saline + PCP mice, again consistent with a role for the group II receptor in reducing the effects of PCP. PCP treated resident mice also exhibit a “crouch defense posture” in response to the introduction of the intruder mouse (2.2 ± 0.4 episodes for saline-saline mice, n = 18 versus 3.1 ± 0.5 episodes for saline-PCP mice, n = 42). ZJ43 reduced the induction of the crouch defense behavior elicited by PCP (2.0 ± 0.2 episodes for ZJ43-PCP mice, n = 43; p = 0.06). While reducing the degree to which resident mice followed the intruder mouse, PCP also induced the resident mouse to flee and climb or jump up the side of the cage following introduction of the intruder (Figure 5). ZJ43 reduced this behavior and the effects of the peptidase inhibitor were blocked by LY341495.
Figure 4.
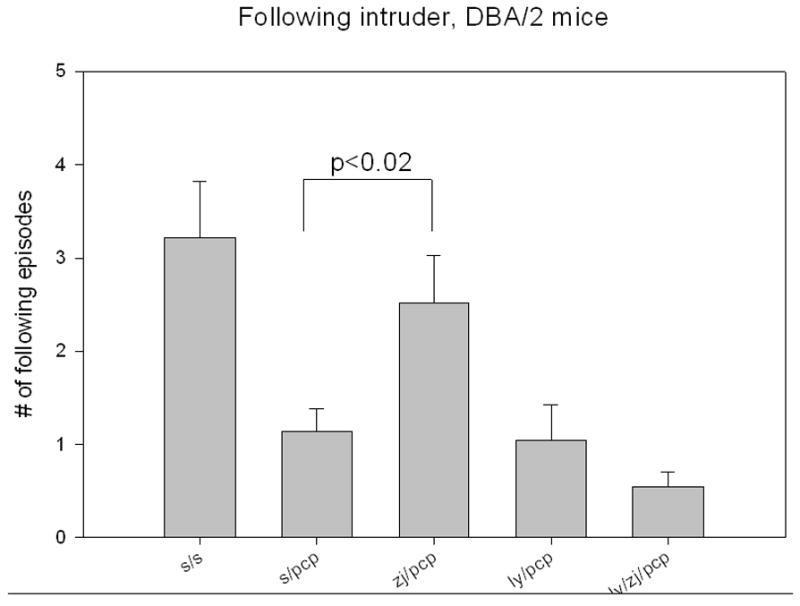
Following behavior of resident mice after the introduction of the intruder into the home cage. The resident-intruder assay is taken as an index of negative phencyclidine (PCP)-induced behaviors. Adult male DBA/2 mice were housed in groups of five (intruder mice) or alone (resident mice) for 7–14 days. After an intruder was introduced into the home cage of a resident mouse, the saline treated resident mouse (n = 18) followed the intruder 3 times on average over the next 10 minutes. In contrast, the mice treated with 6 mg/kg (i.p.) followed the intruder significantly less (p. < 0.01, n = 42). ZJ43 (150mg/kg, i.p., n = 43) reversed the effects of PCP in this assay while the effects of ZJ43 were blocked by the group II mGluR antagonist LY341495 (1 mg/kg, i.p., n = 24).
Figure 5.
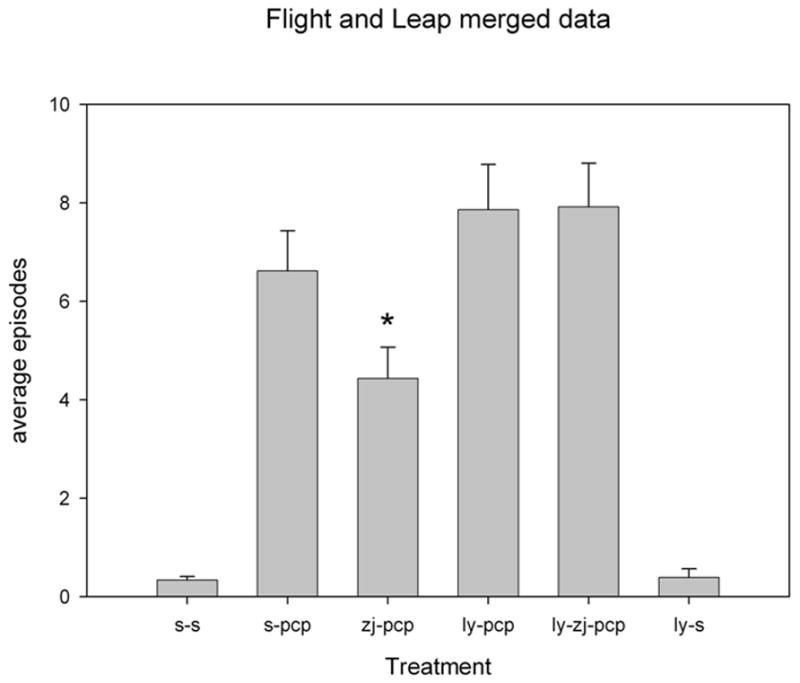
Escape behavior of resident mice following introduction of the intruder into the home cage. Escape behavior, including flight from the intruder and jumping up the side of the cage to escape from the intruder, was increased in mice treated with phencyclidine (PCP) as described in the legend to Figure 4. NAAG peptidase inhibition significantly reduced this PCP-induced escape behavior and LY341495 reversed the effect of ZJ43 (p < 0.01). The zj-pcp treated group was significantly different from the ly-pcp or the ly-zj-pcp groups (p< 0.005). The data are from the same groups as are described in Figure 4.
Discussion
Efficacy of NAAG Peptidase Inhibition
In the only previous study of the efficacy of NAAG peptidase inhibitors in an animal model of schizophrenia, we reported that ZJ43 significantly reduced the overall motor behavior induced by PCP as well as a series of PCP-induced stereotypic behaviors in rats (31). The data presented here confirm the efficacy of NAAG peptidase inhibition in PCP-induced stereotypic motor behaviors and extend these observations to MK 801-induced positive behaviors and PCP-induced negative symptoms.
These results on the efficacy of NAAG peptidase inhibition on PCP-induced motor activity in DBA mice provide the first dose-response data on the efficacy of a NAAG peptidase inhibitor in the PCP model. We conclude that the inhibitor has significant effects at i.p. doses between 50 and 150 mg/kg in the mouse (Figure 2). We previously found that rats injected i.p. with 50 and 150 mg/kg of ZJ43 had 1–3 μM brain concentrations of the drug thirty minutes later (Olszewski and Neale, unpublished). Given the IC50 and Ki of ZJ43 of 2.4 nM and 0.9 nM respectively when tested against human GCPII (37), these behaviorally effective doses of ZJ43 are clearly within the range required to substantially inhibit the activity of NAAG peptidases. Consistent with this conclusion, we have demonstrated that this dose range of ZJ43 significantly elevates extracellular NAAG levels and decreases glutamate release in the rat hippocampus following traumatic brain injury (38). While we do not yet know the full time course of inhibition of NAAG peptidases that is achieved by a single i.p. dose of ZJ43, we found that 150 mg/kg of ZJ43 retained near maximal effectiveness for at least 2 hours in vivo (unpublished) and that this effect was fully blocked by a single dose of LY431495 (38).
MK-801 is a high affinity analogue of PCP that interacts with the same open channel site on the NMDA ion channel as ketamine and PCP. MK-801-induced jumping behavior in NIH Swiss mice is a model of schizophrenia that we have long used for assessment of the efficacy of a broad spectrum of potential therapeutic treatments for schizophrenia, including D-cycloserine, topiramate, anabasine and galantamine (33; 34; 39; 35; 40). The efficacy of ZJ43 in reducing the MK-801- induced behavior in this model confirms that the effects of NAAG peptidase inhibition are not PCP specific but related to this class of open channel NMDA receptor antagonists that also includes ketamine. While there is no equivalent motor behavior schizophrenics or humans treated with PCP or MK-801, this jumping behavior model has been shown to have value in assessing drug treatments for this disorder (34). A striking result in these studies was the three-fold increase in jumping behavior induced by pretreatment of MK-801-treated mice with the group II mGluR antagonist LY341495. This is clearly synergy inasmuch as this group II antagonist had no effects when administered alone (Figure 3). While LY341495 was observed to increase the effects of PCP in rats (31) and in DBA/2 mice (Figure 1), these effects were less pronounced. These data are consistent with the conclusion that even in the absence of NAAG peptidase inhibition, there is sufficient activation of group II mGluRs to moderate the influence of the open channel NMDA receptor antagonists like MK-801 and PCP.
The negative symptoms of schizophrenia, including social withdrawal, are in many ways the most threatening to patients’ general health because they support dissociation from social and medical networks that are important in tracking and responding to their basic and clinical needs (41). These symptoms are less than fully amenable to treatment with standard antipsychotics in many patients (42). The resident-intruder assay is used to assess PCP-induced social withdrawal in mice (43). The data presented in Figures 4 and 5 provide the first support for the proposal that NAAG peptidase inhibition may be effective as adjuvant therapy in treating those negative symptoms of schizophrenia that are emulated by PCP in the resident-intruder assay. A more comprehensive analysis of the negative effects of PCP and NAAG peptidase inhibition in which intruder mice are treated with the drugs and the behaviors of both resident and intruder mice are recorded (44) would be useful in confirming the efficacy of NAAG peptidase inhibition.
Models of NAAG and Schizophrenia
There are numerous animal models of the positive, negative, cognitive behaviors observed in schizophrenia. The motor activation models have no direct correlate in schizophrenics but derived initially from the dopamine model of this disorder in which d-amphetamine-induced motor activation was somewhat useful in predicting drug efficacy. Based on this and the numerous studies of the efficacy of group II mGluR agonists on PCP-induced motor activation (9, 10, 11, 12), we elected to initially focus studies of the efficacy of NAAG peptidase inhibition in these PCP and MK-801 models. In contrast, social withdrawal is a consistent feature of schizophrenia and variations on the resident-intruder social interaction test are widely used to assess drug efficacy.
Blockade of the effect of ZJ43 by the group II mGluR antagonist LY341495 supports the hypothesis that the efficacy of NAAG peptidase inhibition in these schizophrenia models is mediated by elevating synaptic levels of NAAG and its subsequent activation of a group II mGluR. We directly tested this hypothesis in a brain injury paradigm and found that systemic injection of ZJ43 results in increased levels of extracellular NAAG and that this effect was completely blocked by the group II antagonist (38). One model of the role of glutamate in schizophrenia and PCP-induced behaviors proposes that hypoactivity of NMDA receptors, as is modeled by PCP, leads to reduced GABA-ergic inhibition, and excess release of glutamate and dopamine, particularly in the prefrontal cortex (45; 46). Consistent with our behavioral data with ZJ43 and LY341495 and in the context of this model, a group II mGluR agonist reduces PCP-induced glutamate efflux as well as motor and cognitive behaviors (9). Similarly, we also found that ZJ43 induced elevation of synaptic NAAG levels reduces glutamate efflux (38).
Interpretation of data obtained from assays of post mortem schizophrenic brains has led to a different model of the role of NAAG and NAAG peptidase in this disorder. In this model, it is suggested that NAAG levels are elevated in selected brain regions of schizophrenics and that the peptide, acting as an NMDA antagonist, contributes to schizophrenic symptoms in a manner analogous to PCP (47; 48; 49). While there are reports that NAAG has antagonist-like effects on NMDA receptors (50; 51), questions remain as to the physiological relevance of these effects since they can be reversed by glycine or D-serine, two amino acids that are normally present in the extracellular space. In contrast, two reports found no significant antagonist action of NAAG on this receptor on hippocampal or cerebellar neurons (52; 53), while a third study found that in the prefrontal cortex, activation of group II mGluRs increases NMDA currents via protein kinase C (54). Further, while open channel NMDA antagonists like PCP, ketamine and MK801 elicit schizophrenia-like behaviors, there is no evidence that any other types of NMDA antagonists do so. This model stems from a report that NAAG levels were elevated and NAAG peptidase activity decreased in the prefrontal cortex and hippocampus of drug treated schizophrenics relative to tissue from non-drug treated controls. Importantly, however, this study also found no significant differences when these same human brain samples were compared between schizophrenic and neuroleptic treated non-schizophrenic controls (47). In contrast, more recent assays revealed elevated, rather than decreased, levels of the NAAG peptidase GCPII mRNA in the hippocampus of schizophrenic brain tissue relative to normal controls (55). Also troublesome for the hyperactivity theory of NAAG in schizophrenia, Nudmamud et al. (56) reported a significant and selective decrease in NAAG levels in the superior temporal cortex of schizophrenics relative to brain tissue from a matched group of normal controls and individuals with affective disorders. Finally, this model predicts that NAAG peptidase inhibition would itself induce PCP- or schizophrenia-like behaviors in animals or would be additive with the effects of PCP due to elevated levels of the peptide. The data here and in Olszewski et al. (31) represent the only experimental tests of this model and are clearly contrary to this prediction.
NAAG Peptidase Inhibition as Potential Pharmacotherapy
The ability of ZJ43 to increase NAAG activation of mGluR3 with the resulting attenuation of locomotor activity induced by PCP is not likely to be the consequence of non-specific moderation of drug induced increases in locomotor activity inasmuch as group II agonists attenuate PCP- but not d-amphetamine-induced locomotor activity (10). Consistent with a previous study (57), our data provide no evidence that these doses of either ZJ43 or LY341495 induce changes in basal locomotor activity. Also important in the assessment of the effects of ZJ43 is the finding that this compound has no detectable activity at a series of receptors and transporters including the NMDA and mGluR receptors (31; 58; 59; 51)
These data are consistent with the concept that NAAG peptidase inhibition represents a completely novel therapeutic approach to the design of adjuvant therapy for schizophrenia. Its foundation rests on NAAG’s selective activation of a group II mGluR and the substantial literature demonstrating the efficacy of synthetic group II mGluR agonists in the PCP model. This work clearly parallels more current studies on the efficacy of positive allosteric modulators of these receptors as potential new pharmacotherapies for this disorder (reviewed in 60). Yet to be determined are the effects of NAAG peptidase inhibition on the cognitive models of schizophrenia in which it has been shown, for example that a group II mGluR agonist moderates the effects of PCP on working memory (9) and in behaviors induced by chronic PCP treatments. Similarly, NAAG peptidase inhibition needs to be explored in acute PCP-induced decrement in prepulse inhibition of acoustic startle, where group II agonist are not effective but where a positive allosteric modulators of mGluR2 is effective against PCP (61).
Acknowledgments
Acenta Discovery supported this research by providing ZJ43. Acenta Discovery has licensed to the patent for ZJ43 that is held by Georgetown University. This research was supported by grants from the Undergraduate Program at the Howard Hughes Medical Institute (JHN) and from the NIH (JHN) and gifts to Georgetown University by Nancy and Daniel Paduano (JHN).
Footnotes
Financial Disclosures. Dr. Kozikowski is the major owner of Acenta Discovery located in Tucson, Arizona. Acenta has licensed the patent to ZJ43 from Georgetown University. As such, Dr. Kozikowski could profit from this class of compounds should they ever become drugs. As Dr. Kozikowski’s major intellectual contribution to the present work is the invention of ZJ43 and related molecules, he had no direct involvement in collecting the data presented herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–86. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 3.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 4.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Current Opinion in Pharm. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Heresco-Levy U. Glutamatergic neurotransmission modulators as emerging new drugs for schizophrenia. Expert Opin Emerg Drugs. 2005;10:827–44. doi: 10.1517/14728214.10.4.827. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AW, 3rd, Chafee MV. Translational and developmental perspective on N-methyl-D-aspartate synaptic deficits in schizophrenia. Dev Psychopathol. 2006;18:853–76. [PubMed] [Google Scholar]
- 7.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:363–82. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 8.Morrison PD, Pilowsky LS. Schizophrenia: more evidence for less glutamate. Expert Rev Neurother. 2007;7:29–31. doi: 10.1586/14737175.7.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 10.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–70. [PubMed] [Google Scholar]
- 11.Cartmell J, Monn JA, Schoepp DD. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]
- 12.Swanson CJ, Schoepp DD. The group II metabotropic glutamate receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate ( LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J Pharmacol Exp Ther. 2002;303:919–27. doi: 10.1124/jpet.102.038422. [DOI] [PubMed] [Google Scholar]
- 13.Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179:303–9. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 14.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- 15.Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–85. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19:151–7. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- 17.Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–9. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 18.Curatolo AD, Arcangelo P, Lino A, Brancati A. Distribution of N-acetyl-aspartic and N-acetyl-aspartyl-glutamic acids in nervous tissue. J Neurochem. 1965;12:339–42. doi: 10.1111/j.1471-4159.1965.tb06771.x. [DOI] [PubMed] [Google Scholar]
- 19.Cangro CB, Namboodiri MA, Sklar LA, Corigliano-Murphy A, Neale JH. Immunohistochemistry and biosynthesis of N-acetylaspartylglutamate in spinal sensory ganglia. J Neurochem. 1987;49:1579–88. doi: 10.1111/j.1471-4159.1987.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrman S, Palkovits M, Cassidy M, Neale JH. The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J Neurochem. 1994;62:275–81. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- 21.Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer C, Kratzeisen C, Adam G, Lundstrom K, Malherbe P, Ohresser S, et al. Characterization of - LY354740 binding to rat mGlu2 and mGlu3 receptors expressed in CHO cells using Semliki Forest virus vectors. Neuropharmacology. 2000;39:1700–1706. doi: 10.1016/s0028-3908(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 23.Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–8. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- 24.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–52. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 25.Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477–84. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93:749–53. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, et al. Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem. 1997;69:2270–7. doi: 10.1046/j.1471-4159.1997.69062270.x. [DOI] [PubMed] [Google Scholar]
- 28.Luthi-Carter R, Barczak AK, Speno H, Coyle JT. Hydrolysis of the neuropeptide N-acetylaspartylglutamate (NAAG) by cloned human glutamate carboxypeptidase II. Brain Res. 1998;795:341–8. doi: 10.1016/s0006-8993(98)00244-3. [DOI] [PubMed] [Google Scholar]
- 29.Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, et al. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J Neurochem. 2004;89:627–35. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–26. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 31.Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S, et al. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–85. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- 32.Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, et al. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 33.Deutsch SI, Rosse RB, Schwartz BL, Powell DG, Mastropaolo J. Stress and a glycinergic intervention interact in the modulation of MK-801-elicited mouse popping behavior. Pharmacol Biochem Behav. 1999;62:395–8. doi: 10.1016/s0091-3057(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 34.Deutsch SI, Rosse RB, Billingslea EN, Bellack AS, Mastropaolo J. Topiramate antagonizes MK-801 in an animal model of schizophrenia. Eur J Pharmacol. 2002;449:121–5. doi: 10.1016/s0014-2999(02)02041-1. [DOI] [PubMed] [Google Scholar]
- 35.Mastropaolo J, Rosse RB, Deutsch SI. Anabasine, a selective nicotinic acetylcholine receptor agonist, antagonizes MK-801-elicited mouse popping behavior, an animal model of schizophrenia. Behav Brain Res. 2004;153:419–22. doi: 10.1016/j.bbr.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Rosse RB, Mastropaolo J, Sussman DM, Koetzner L, Morn CB, Deutsch SI. Computerized measurement of MK-801-elicited popping and hyperactivity in mice. Clin Neuropharmacol. 1995;18:448–57. doi: 10.1097/00002826-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Kozikowski AP, Zhang J, Nan F, Petukhov PA, Grajkowska E, Wroblewski JT, et al. Synthesis of Urea-Based Inhibitors as Active Site Probes of Glutamate Carboxypeptidase II: Efficacy as Analgesic Agents. J Med Chem. 2004;47:1729–1738. doi: 10.1021/jm0306226. [DOI] [PubMed] [Google Scholar]
- 38.Zhong C, Zhao X, Van KC, Bzdega T, Smyth A, Zhou J, et al. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J Neurochem. 2006;97:1015–25. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]
- 39.Deutsch SI, Rosse RB, Billingslea EN, Bellack AS, Mastropaolo J. Modulation of MK-801-elicited mouse popping behavior by galantamine is complex and dose-dependent. Life Sci. 2003;73:2355–61. doi: 10.1016/s0024-3205(03)00642-8. [DOI] [PubMed] [Google Scholar]
- 40.Chilton M, Mastropaolo J, Rosse RB, Bellack AS, Deutsch SI. Behavioral consequences of methyllycaconitine in mice: a model of alpha7 nicotinic acetylcholine receptor deficiency. Life Sci. 2004;74:3133–9. doi: 10.1016/j.lfs.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Javitt DC, Coyle JT. Decoding schizophrenia. Sci Am. 2004;290:48–55. doi: 10.1038/scientificamerican0104-48. [DOI] [PubMed] [Google Scholar]
- 42.Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or Cul-de-sac? Acta Psychiatr Scand. 2007;115:93–100. doi: 10.1111/j.1600-0447.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 43.Sams-Dodd F. Effects of diazepam, citalopram, methadone and naloxone on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Neurosci Biobehav Rev. 1998;23:287–93. doi: 10.1016/s0149-7634(98)00030-x. [DOI] [PubMed] [Google Scholar]
- 44.Tyler CB, Miczek KA. Effects of phencyclidine on aggressive behavior in mice. Pharmacol Biochem Behav. 1982;17:503–10. doi: 10.1016/0091-3057(82)90311-2. [DOI] [PubMed] [Google Scholar]
- 45.Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):S10–4. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- 46.Deutsch SI, Rosse RB, Schwartz BL, Mastropaolo J. A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol. 2001;24:43–9. doi: 10.1097/00002826-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–36. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- 48.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann NY Acad Sci. 2003;1003:318–27. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 49.Siekmeier PJ, Hasselmo ME, Howard MW, Coyle J. Modeling of context-dependent retrieval in hippocampal region CA1: implications for cognitive function in schizophrenia. Schizophr Res. 2007;89:177–90. doi: 10.1016/j.schres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- 51.Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–57. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- 52.Lea PM, 4th, Wroblewska B, Sarvey JM, Neale JH. beta-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the type 3 metabotropic glutamate receptor. J Neurophysiol. 2001;85:1097–106. doi: 10.1152/jn.2001.85.3.1097. [DOI] [PubMed] [Google Scholar]
- 53.Losi G, Vicini S, Neale J. NAAG fails to antagonize synaptic and extrasynaptic NMDA receptors in cerebellar granule neurons. Neuropharmacology. 2004;46:490–6. doi: 10.1016/j.neuropharm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J Physiol. 2004;554:765–77. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghose S, Weickert CS, Colvin SM, Coyle JT, Herman MM, Hyde TM, et al. Glutamate carboxypeptidase II gene expression in the human frontal and temporal lobe in schizophrenia. Neuropsychopharmacology. 2004;29:117–25. doi: 10.1038/sj.npp.1300304. [DOI] [PubMed] [Google Scholar]
- 56.Nudmamud S, Reynolds LM, Reynolds GP. N-acetylaspartate and N-Acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: a postmortem study. Biol Psychiatry. 2003;53:1138–41. doi: 10.1016/s0006-3223(02)01742-0. [DOI] [PubMed] [Google Scholar]
- 57.O’Neill MF, Heron-Maxwell C, Conway MW, Monn JA, Ornstein P. Group II metabotropic glutamate receptor antagonists LY341495 and LY366457 increase locomotor activity in mice. Neuropharmacology. 2003;45:565–74. doi: 10.1016/s0028-3908(03)00232-6. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Hirasawa S, Wroblewska B, Grajkowska E, Zhou J, Kozikowski A, et al. Antinociceptive effects of N-acetylaspartylglutamate (NAAG) peptidase inhibitors ZJ-11, ZJ-17 and ZJ-43 in the rat formalin test and in the rat neuropathic pain model. Eur J Neurosci. 2004;20:483–94. doi: 10.1111/j.1460-9568.2004.03504.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto T, Saito O, Aoe T, Bartolozzi A, Sarva J, Zhou J, et al. Local administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in peripheral pain in rats. Eur J Neurosci. 2007;25:147–58. doi: 10.1111/j.1460-9568.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- 60.Linden A-M, Schoepp DD. Metabotropic glutamate receptor targets for neuropsychiatric disorders. Drug Discovery Today: Therapeutic Strategies. 2006;3(4):507–17. [Google Scholar]
- 61.Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–7. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]


