Abstract
Intercellular adhesion molecule-5 (ICAM-5) is a dendritically polarized membrane glycoprotein in telencephalic neurons, which shows heterophilic binding to leukocyte β2-integrins. Here, we show that the human ICAM-5 protein interacts in a homophilic manner through the binding of the immunoglobulin domain 1 to domains 4–5. Surface coated ICAM-5-Fc promoted dendritic outgrowth and arborization of ICAM- 5–expressing hippocampal neurons. During dendritogenesis in developing rat brain, ICAM-5 was in monomer form, whereas in mature neurons it migrated as a high molecular weight complex. The findings indicate that its homophilic binding activity was regulated by nonmonomer/monomer transition. Thus, ICAM-5 displays two types of adhesion activity, homophilic binding between neurons and heterophilic binding between neurons and leukocytes.
Keywords: leukocyte, integrin, adhesion, dendrite, neuron
Introduction
Neuron–neuron contact mediated by adhesion molecules is an important way to influence brain architecture (Hynes and Lander 1992; Goodman and Shatz 1993; Walsh and Doherty 1997). Since neurons are polarized, extending an axon and dendrites from the soma (Craig and Banker 1994), neural cell adhesion molecules can be divided into axon-associated cell adhesion molecules (AxCAMs) and dendrite-associated cell adhesion molecules (DenCAMs; Yoshihara et al. 1994; Brümmendorf and Rathjen 1996). Whereas a great deal is known about the roles of AxCAMs in axonal elongation, fasciculation, and guidance by mechanisms involving transmembrane signaling pathways (Sonderegger and Rathjen 1992; Walsh and Doherty 1997), little is known about DenCAMs. However, DenCAMs are supposed to guide the formation of dendritic fasciculation and arborization, and to function as counter-receptors for AxCAMs in synaptogenesis.
Intercellular adhesion molecule-5 (ICAM-5; telencephalin; Yoshihara et al. 1994; Mizuno et al. 1997) is a cell surface glycoprotein that belongs to the immunoglobulin superfamily and shares 38–55% amino acid identity with the other ICAMs, which play important roles as adhesion molecules in the hematopoietic system (Springer 1994; Gahmberg 1997; Hayflick et al. 1998). Many cell surface proteins involved in cell adhesion belong to the immunoglobulin superfamily. Such proteins contain a variable number of immunoglobulin domains, which are ∼100 amino acids long and form two β-sheets. Human ICAM-5 consists of nine extracellular immunoglobulin domains with a total of 832 amino acids, a 28-amino acid transmembrane segment, and a 64-amino acid cytoplasmic domain. Like other ICAMs, it binds to the leukocyte integrin CD11a/CD18 (Mizuno et al. 1997; Tian et al. 1997) and it may be an important regulator of the immune response in the central nervous system.
ICAM-5 has several features that make it a candidate for a DenCAM. Firstly, it is localized to cell somata and dendrites of neurons, and neurons expressing ICAM-5 have more extensive dendritic branches than neurons without ICAM-5 (Benson et al. 1998). Secondly, it is expressed only in the telencephalon, the most rostral segment of the brain, and not in the caudal segments (Yoshihara and Mori 1994). Thirdly, the onset of its expression temporally parallels the onset of dendritic elongation and synaptogenesis during the postnatal period. These facts together support the hypothesis that ICAM-5 may provide a brain segment-specific cue for synaptogenesis or dendrite–dendrite interactions in the telencephalon. Indeed, earlier studies have shown that ICAM-5 promotes embryonic hippocampal neurite outgrowth (Tamada et al. 1998) and is involved in hippocampal long-term potentiation (Sakurai et al. 1998). However, the molecular basis has remained poorly understood.
Here, we attempted to address this issue by studying purified recombinant human ICAM-5 proteins, ICAM-5 transfected into a human neural crest derived cell line (Paju), and ICAM-5 in developing rat brain. We found that ICAM-5 induced neurite outgrowth of Paju cells and rat hippocampal neurons through homophilic interaction, and it also promoted the dendritic arborization of hippocampal neurons through homophilic binding. The adhesive domains in ICAM-5 were mapped, and we propose that the homophilic activity of ICAM-5 is regulated by a monomer/nonmonomer transition.
Materials and Methods
Cell Culture and Immunocytostaining
Wild-type Paju cells (Paju-WT) are a human neural crest-derived cell line, which was cultured in RPMI 1640 and 10% FCS (BioWhittaker) on glass coverslips. Paju-ICAM-5 was obtained by transfection of Paju-WT with the human ICAM-5 in the vector pEF-BOS (Tian et al. 1997; Tamada et al. 1998) and was cultured as above, but in the presence of 0.5 mg/ml G418 (Sigma-Aldrich). For mAb blocking assay, Paju-ICAM-5 cells were cultured in the presence of 100 μg/ml mAbs to ICAM-5 for 3 d. The antibodies were made by immunizing mice with purified ICAM-5-Fc and antibody-producing clones identified by flow cytometry analysis. Control cells were stained with 10 μg/ml mAb TL-3 after culture.
After methanol fixation, the cells were stained with FITC-conjugated rabbit anti–mouse antiserum (Jackson ImmunoResearch Laboratories). Cells were observed by immunofluorescence microscopy (Olympus Provis 70) at 400×.
Production of Recombinant Proteins
Truncated ICAM-5 extracellular domains were cloned by PCR from human ICAM-5 cDNA, sequenced, and inserted into the pEF-Fc (Tian et al. 1997; Tamada et al. 1998) expression vector. The recombinant proteins were purified from transiently transfected COS-1 (American Type Culture Collection) cells using protein A–Sepharose CL 4B (Amersham Pharmacia Biotech) and their purities checked by Western blotting using HRP-conjugated anti-human Ig (Amersham Pharmacia Biotech). Soluble ICAM-5 immunoglobulin domain 1–9 (D1–9) without Fc was purified from a baculovirus-expression system (Bac-to-bac system; GIBCO BRL).
Flow Cytometry and ELISA
106 Paju-WT and Paju-ICAM-5 cells were first incubated with 100 μg/ml recombinant ICAM-5-Fc, ICAM-1-Fc, and NCAM-Fc proteins, followed by FITC-conjugated anti-human IgG (Dako) at room temperature for 30 min. After washings, samples were analyzed with FACScan and CellQuest software (Becton Dickinson). The mean fluorescent value of Paju-ICAM-5 was subtracted with that of Paju-WT for each recombinant protein. For ELISA, 5 μg/ml recombinant proteins were coated on microtiter plates, blocked with BSA (Sigma-Aldrich), and then incubated first with biotinylated domains 1–2 Fc (D1–2-Fc; Pierce) of ICAM-5, and then with HRP-conjugated streptavidin (Pierce Chemical Co.) at 37°C for 1 h, and the color measured at 492 nm. For mAb blocking assay, 5 μg/ml ICAM-5 D1–9 was coated and preincubated with anti–ICAM-5 mAbs at room temperature for 30 min, followed by the steps mentioned above.
Native PAGE and Western Blotting
Brains of embryonic (embryonic day 19, E19), newborn (postnatal day 1–10, P1-10), and adult rats were homogenized in 10 mM Tris, 1 mM EDTA, 10 mM Chaps, 300 mM NaCl, and 1 mM PMSF. Paju-ICAM-5 cells were treated with 5 μM cytochalasin D (Calbiochem-Novabiochem) or 0.5 mg/ml ICAM-5 D1–2-Fc protein for 8 or 4 h, respectively, and were then lysed with the same buffer. Brain homogenates or cell lysates were run on native PAGE with PhastGel 4-15 (Amersham Pharmacia Biotech) and blotted onto nitrocellulose membranes. ICAM-5 protein was detected by a polyclonal antibody against the cytoplasmic part of mouse ICAM-5 (Tian et al. 1997).
Neurite Outgrowth Assay
0.5 μg of D1–2-Fc or D1–9-Fc of ICAM-5 and ICAM-1-Fc proteins were coated in drops on coverslips in 24-well plates. After blocking, the coverslips with BSA, 104/well Paju-WT and Paju-ICAM-5 cells were seeded and cultured for 30–36 h. For mAb inhibition assay, 50 μg/ml mAb 179B was incubated with Paju-WT and Paju–ICAM-5 cells. Cells were observed under phase-contrast microscope (Olympus Provis 70) at 400×. The length of the neurites was measured as the distance between the soma and the tip of the neurite counted in three random fields.
Hippocampus and cerebellum were dissected from 19-d-old rat embryos and treated with 0.5 mg/ml papain for 10 min (Worthington Biochemical Corp.) in HBSS (GIBCO BRL). After washing in HBSS, neurons suspended in Neurobasal medium (GIBCO BRL), 2% B27 supplement (GIBCO BRL), 25 μM l-glutamic acid (Sigma-Aldrich), and 1% l-glutamine (GIBCO BRL) were seeded at 104/well density in 8-chamber slides (Nunc) coated with laminin (50 μg/ml; Sigma-Aldrich), HB-GAM (Rauvala and Peng 1997; 50 μg/ml), poly-dl-ornithine (100 μg/ml; Sigma-Aldrich), or ICAM-5 recombinant proteins (100 μg/ml). For inhibition assay, 100 μg/ml of each antibody or recombinant protein was added to the culture medium. Neurons were cultured for 48 or 72 h, and were then fixed with 4% paraformaldehyde for 25 min. After permeabilization with 0.2% Triton X-100 for 10 min, the neurons were visualized with the dendritic marker, antimicrotubule associated protein-2 (anti–MAP-2) mAb (Boehringer), the axonal marker antitau mAb (Boehringer), or rabbit anticytoplasmic mouse ICAM-5 antiserum for 1 h at room temperature (Benson et al. 1998), followed by incubation with TRITC-conjugated goat anti–rabbit and FITC-conjugated goat anti–mouse antibodies (Jackson ImmunoResearch Laboratories, Inc.). The proportion of neurite-bearing cells (processes longer than 1 diam of the cell body) was counted in four random fields using a fluorescence microscope (Olympus Provis 70) at 400×.
Statistical Analysis
For all the quantitations, statistical analysis of variance (ANOVA) was used to compare the different groups in each experiment.
Results
Expression of ICAM-5 in Transfected Neural Crest Cells
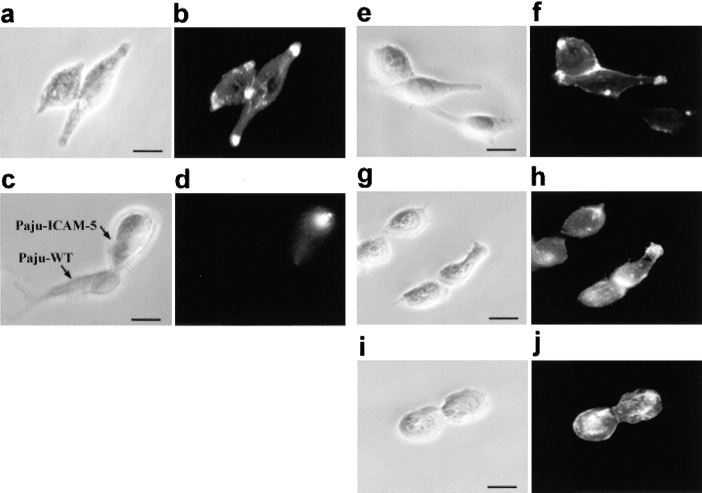
To study neuronal functions of ICAM-5, we used the human neural crest-derived cell line, Paju (Zhang et al. 1996). This cell line does not constitutively express ICAM-5, and therefore we transfected it with human ICAM-5. When we stained the Paju-ICAM-5 cells with mAbs against ICAM-5, we observed that ICAM-5 did not distribute evenly on the membrane. It showed a bimodal distribution to the uropods of Paju-ICAM-5 cells and to cell–cell contact sites (Fig. 1, a and b). When Paju-WT cells were cocultivated with Paju-ICAM-5 cells, localization of ICAM-5 to cell–cell contact sites was no longer observed, although the uropodal expression remained (Fig. 1c and Fig. d; Table ). When Paju-ICAM-5 cells were cultured in the presence of anti–ICAM-5 mAbs, we found that mAbs that do not inhibit leukocyte integrin-ICAM-5 adhesion, like TL-1 (Fig. 1g and Fig. h) and 179K, did not influence ICAM-5 colocalization much, whereas antileukocyte-adhesive mAbs, like TL-3 (Fig. 1i and Fig. j) and 179B, blocked the colocalization at cell–cell contact sites. With control antibody, ICAM-5 remained at sites of cell contact (Fig. 1 e and f; Table ).
Figure 1.
Colocalization of ICAM-5 at cell–cell contact sites in Paju neuronal cells. Human ICAM-5 transfected into Paju cells was expressed at cell–cell contact sites and the uropods of Paju-ICAM-5 cells (a and b), but not in Paju-WT/Paju-ICAM-5 contact regions (c and d). When Paju-ICAM-5 cells were cultured in the presence of the antiadhesive-ICAM-5 mAbs, like TL-3 (i and j), the localization of ICAM-5 at contact sites was diminished as compared with treatment with control mAb (antiglycophorin A; e and f) or the mAb TL-1 treatment (g and h), which does not affect adhesion. a, c, e, g, and i, Phase-contrast photographs; b, d, f, h, and j, immunofluorographs. Bars, 20 μm.
Table 1.
ICAM-5 Localization at Cell–Cell Contact Sites in the Paju Neuronal Crest Cell Line
| mAb treatment | Paju-WT/Paju-ICAM-5 | Paju-ICAM-5/Paju-ICAM-5 |
|---|---|---|
| % (n) | % (n) | |
| None | 5.7 (35) | 91.2 (35) |
| TL-1 (not antiadhesive) | 88.2 (34) | |
| TL-3 (antiadhesive) | 14.3 (35) | |
| 179B (antiadhesive) | 8.6 (35) | |
| 179K (not antiadhesive) | 90.9 (33) |
Data are presented as the percentage of cell–cell contact sites with ICAM-5 localized at the sites. The concentration of each mAb was 100 μg/ml.
Homophilic Binding of Recombinant ICAM-5 Fragments
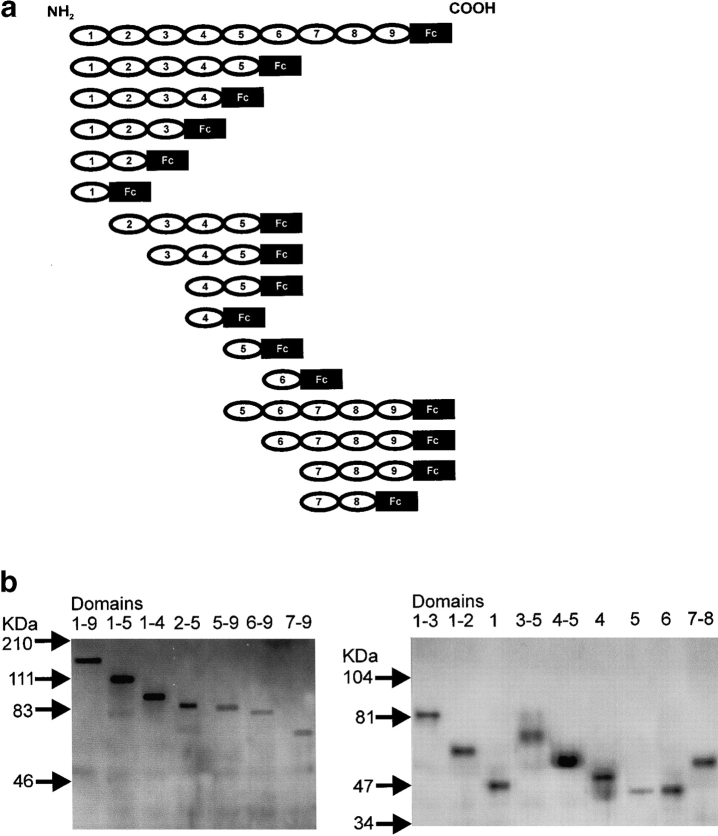
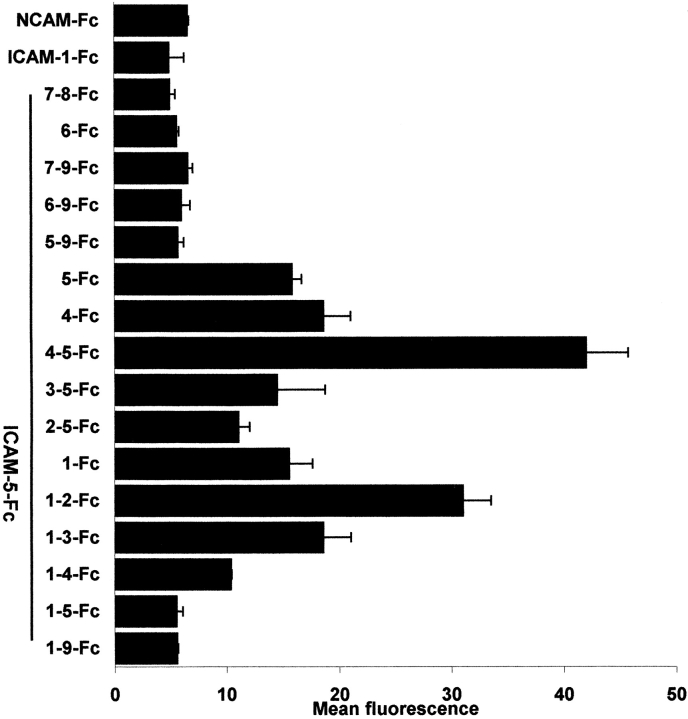
Since these findings suggested that ICAM-5 can bind in a homophilic manner, we studied this possibility in more detail by constructing a series of extracellular domain deletion constructs of ICAM-5 (Fig. 2). These truncated ICAM-5 proteins were linked at their COOH terminals to the Fc portion of human IgG1. The purity of the recombinant proteins was at least 90% (Fig. 2 b). After isolation, these constructs were incubated with Paju-WT and Paju-ICAM-5 cells, and the binding was analyzed by flow cytometry. Whereas recombinant proteins containing domains 1–9, 1–5, 5–9, 6–9, 7–9, 6, and 7–8 ICAM-5-Fc proteins, as well as ICAM-1-Fc and NCAM-Fc proteins showed little binding to Paju-ICAM-5, truncated proteins containing domains 1–4, 1–3, 1–2, 1, 2–5, 3–5, 4–5, 4, and 5 showed significant binding, especially the domains 1–2 and 4–5 constructs (Fig. 3). The results indicated that the homophilic interactions mainly involve the first five domains of ICAM-5, and the reason for the low binding of domains 1–9-Fc and domains 1–5-Fc proteins might be that these proteins were inhibited by intramolecular association.
Figure 2.
ICAM-5 recombinant molecules. a, A schematic representation of the ICAM-5 constructs used. The domains are numbered from the NH2-terminal. Fc, Immunoglobulin Fc domain. b, Western blot of purified ICAM-5 proteins. The positions of the molecular weight markers are shown to the left. The left-hand gel was made using 8% acrylamide and the right-hand gel was made using 10% acrylamide.
Figure 3.
ICAM-5 binds in a homophilic manner. Paju-WT and Paju-ICAM-5 cells were stained with truncated ICAM-5-Fc proteins and analyzed by flow cytometry. The values obtained with Paju-WT were subtracted from the Paju-ICAM-5 values. Note that D1–2-Fc and D4–5-Fc of ICAM-5 gave especially strong binding to Paju-ICAM-5 cells, as compared with the other proteins.
Further support for homophilic interaction was obtained when we coated truncated, isolated ICAM-5-Fc proteins on plastic, and detected that biotinylated D1–2-Fc bound especially well to various constructs containing domains 4–5 (Fig. 4 a). We further studied the effects of anti–ICAM-5 mAbs on the interaction of D1–2 with immobilized D1–9 (without Fc), and observed that mAbs TL-3, 179B, and 179I (recognizing D1) almost completely blocked the binding, whereas mAbs 179K, 179D, and 246E (recognizing D2) were inactive or even increased binding (Fig. 4 b). These data indicate that the homophilic interaction takes place mainly through the binding of the NH2-terminal domain 1 to domains 4–5. The homophilic binding of ICAM-5 was Ca2+-independent, and EDTA treatment had no effect (not shown).
Figure 4.
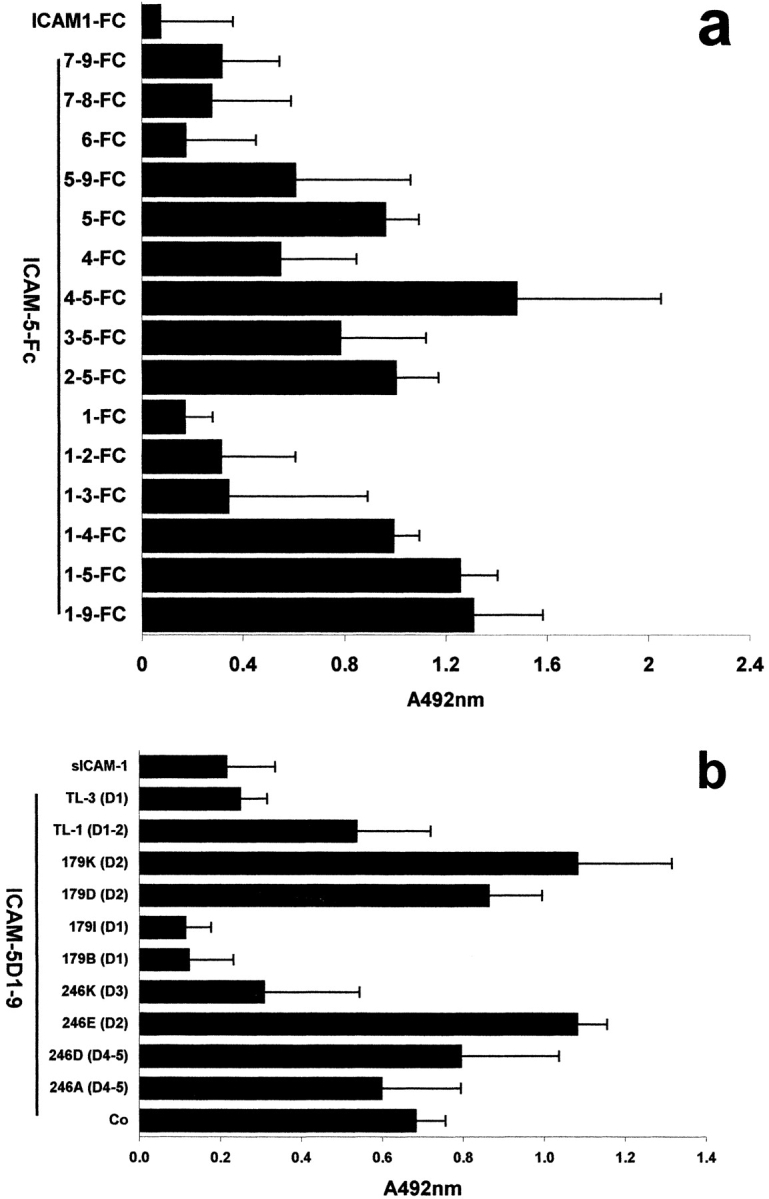
Homophilic binding of ICAM-5 D1–2-Fc to different ICAM-5-Fc constructs. When the binding of biotinylated D1–2-Fc of ICAM-5 to immobilized truncated ICAM-5-Fc proteins was studied, direct binding was observed between D1–2 and D4–5 containing proteins (a). The ICAM-5 mAbs 179B, 179I, and TL-3 (all recognizing domain 1) blocked the binding of D1–2-Fc to immobilized D1–9 of ICAM-5 (b). The Ig-domains of ICAM-5, which are recognized by the respective antibodies are shown in parentheses. Co, Control mouse IgG. SDs are shown.
High Molecular Weight Form of ICAM-5
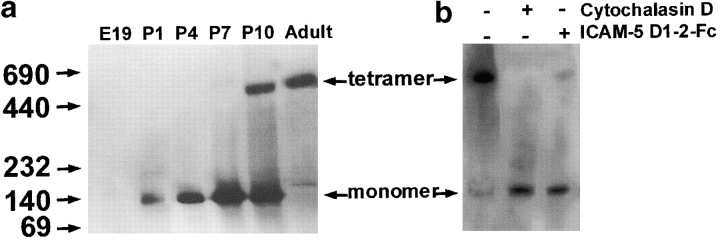
Earlier studies have failed to detect any aggregation of ICAM-5–transfected mouse fibroblasts (Yoshihara et al. 1994). This is now understandable in light of our findings that the complete external part of ICAM-5 does not bind to transfected cells. The rabbit ICAM-5 molecule has been proposed to exist as a tetramer (Oka et al. 1990), and therefore we thought that it would be important to study ICAM-5 during development. Fig. 5 a shows that during the first postnatal week of development of the central nervous system in the rat, the molecule existed as a monomer (Fig. 5 a) and at the tenth day of postnatal development a high molecular weight form corresponding in molecular weight to a tetramer began to appear (Fig. 5 a). In the brain of adult rats, almost all ICAM-5 molecules migrated as the high molecular weight form (Fig. 5 a). Also, in transfected Paju-ICAM-5 cells, ICAM-5 mainly existed as nonmonomers (Fig. 5 b). Disruption of the actin cytoskeleton in Paju-ICAM-5 cells by cytochalasin D (Fig. 5 b) brought most of ICAM-5 into the monomer form. Incubation of Paju-ICAM-5 cells with the ICAM-5 D1–2-Fc protein (Fig. 5 b) also resulted in monomers.
Figure 5.
Membrane-bound ICAM-5 undergoes monomer/nonmonomer transition in rat brain (a) and Paju-ICAM-5 cells (b). Brain homogenates or Paju-ICAM-5 cell lysates with or without indicated pretreatments were run on PhastGel 4-15 under native conditions, and were then blotted with a polyclonal antibody against the cytoplasmic part of ICAM-5. In rat brain, ICAM-5 was not expressed at E19, but present after birth. At postnatal 1 (P1), 4 (P4), and 7 (P7) days, ICAM-5 mainly existed as monomers of 140 kD, while it partially changed to nonmonomers (which could be tetramers) of 550 kD at P10. Brains from adult rats contained only nonmonomers. In Paju-ICAM-5 cells (b), ICAM-5 existed mainly as nonmonomers, with a minor monomer component. If the cells were pretreated with 5 μM cytochalasin D or 0.5 mg/ml ICAM-5 D1–2-Fc protein, most of membrane-bound ICAM-5 was brought into monomers.
ICAM-5 Homophilic Interaction Promotes Neurite Outgrowth from Paju Cells and Is Involved in Dendritogenesis and Arborization of Rat Hippocampal Neurons
When we coated ICAM-5 D1–9-Fc on plastic, and then seeded Paju-WT or Paju-ICAM-5 cells, we observed neurites extending from Paju-ICAM-5 cells (Fig. 6 b), but not from Paju-WT cells (Fig. 6 a). When coated on ICAM-5 D1–2, the effect was more pronounced (Fig. 6 d). In the presence of mAb 179B, the neurite extension from Paju-ICAM-5 cells was inhibited (Fig. 6 f). We found significantly less neurites from either type of cells on ICAM-1-Fc coated surfaces (Fig. 6g and Fig. h; Table ).
Figure 6.
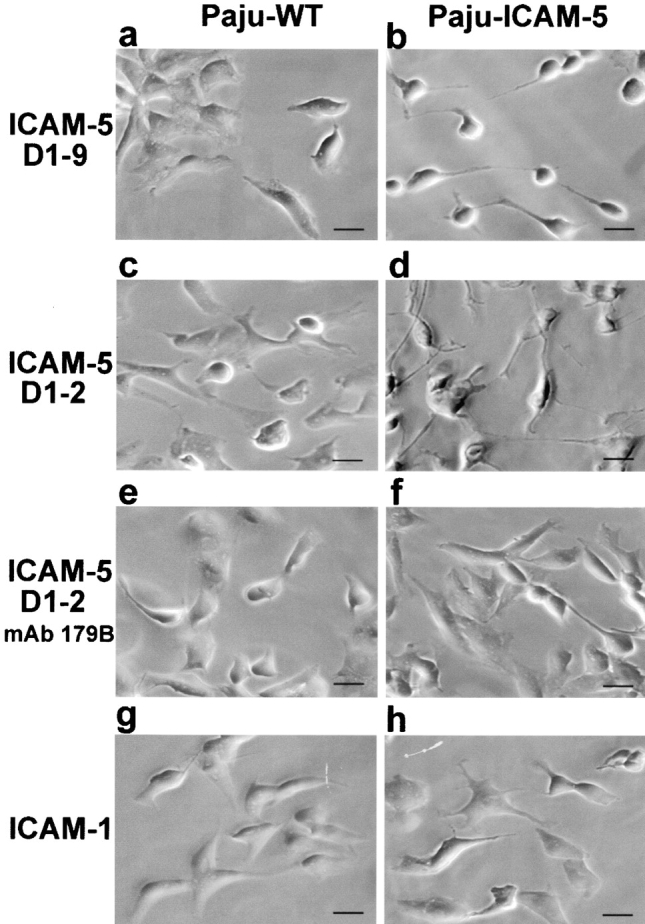
Paju-ICAM-5 cells induced for neurite outgrowth by ICAM-5. When Paju-WT and Paju-ICAM-5 cells were seeded on immobilized ICAM-5 D1–9-Fc or D1–2-Fc proteins, neurite outgrowth was clearly seen with Paju-ICAM-5 cells, but not with Paju-WT cells. mAb 179B inhibited neurite outgrowth of Paju-ICAM-5 cells, but had no effect on Paju-WT cells. Neither Paju-ICAM-5 nor Paju-WT cells developed neurites on ICAM-1-Fc protein. Bars, 20 μm.
Table 2.
Neurite Outgrowth of Paju Cells
| Length of neurites | ||
|---|---|---|
| Substrate | Paju-WT | Paju-ICAM-5 |
| μm (n) | μm (n) | |
| ICAM-1-Fc | 4 ± 2 (70) | 3 ± 2 (68) |
| ICAM-5 D1–2-Fc | 4 ± 1 (75) | 43 ± 10 (72) |
| ICAM-5 D1–2-Fc + mAb 179B | 6 ± 3 (66) | 12 ± 3 (65) |
Each substrate was coated at a concentration of 10 μg/ml. mAb 179B was used at 50 μg/ml. Length of neurites is shown in micrometers, mean ± SD.
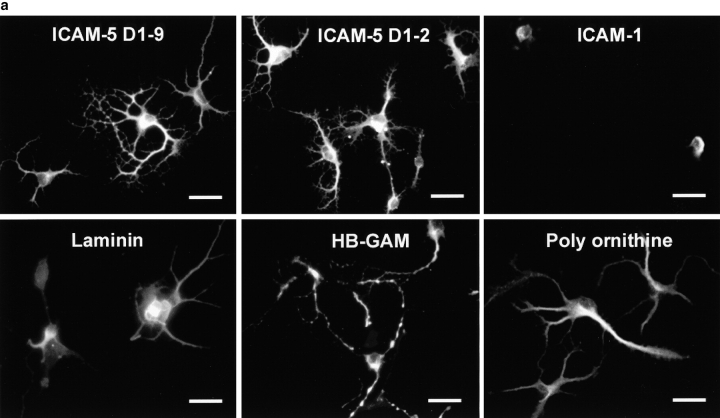
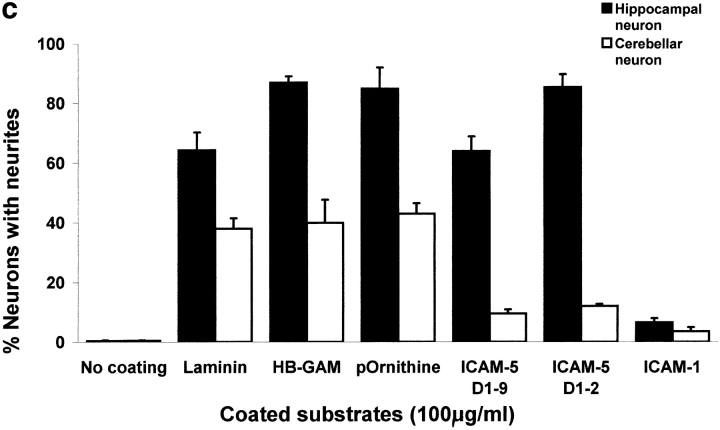
We then tested if ICAM-5 is involved in the dendritic outgrowth using ICAM-5–expressing rat hippocampal neurons and rat cerebellar neurons, which do not express ICAM-5. By staining the neurons with the dendritic marker, MAP-2, and the axonal marker, tau (Fig. 7), we found that rat hippocampal neurons were induced for dendritic outgrowth on surfaces coated with ICAM-5 D1–9-Fc, D1–2-Fc, laminin, heparin-binding growth-associated molecule (HB-GAM), or poly-dl-ornithine (Fig. 7 a), but not on ICAM-1-Fc. Interestingly, the morphology of dendrites induced by the different molecules was different from one another; the most dramatic difference was seen on ICAM-5 coated surfaces (Fig. 7, a and b), which induced a network of dendritic arbors. When we calculated the number of major dendritic branches extending from the cell soma, there was no significant difference in neurite induction by these molecules (with exception of ICAM-1; P > 0.01). Fig. 7 b, left, shows double staining of MAP-2 (green) and ICAM-5 (red). Coexpression of MAP-2 and ICAM-5 was seen (yellow), indicating that ICAM-5 is largely dendrite-associated. Fig. 7 b, right, shows that ICAM-5 (red) is strongly stained on neurons grown on ICAM-5 and little tau staining (green) was seen. In contrast, when cells were coated on laminin, tau staining was evident. Furthermore, the axonal arbors were more complex on laminin than on ICAM-5 (Fig. 7 b, right), while the dendritic arbors were more extensive on ICAM-5 than on laminin (Fig. 7 b, left). In comparison to hippocampal neurons, the cerebellar neurons, which do not express ICAM-5, showed significantly less capability of dendritic outgrowth on ICAM-5 coated surfaces than on the surfaces coated with the other molecules (Fig. 7 c). Table shows a quantitation of the ICAM-5–induced dendrite outgrowth.
Figure 7.
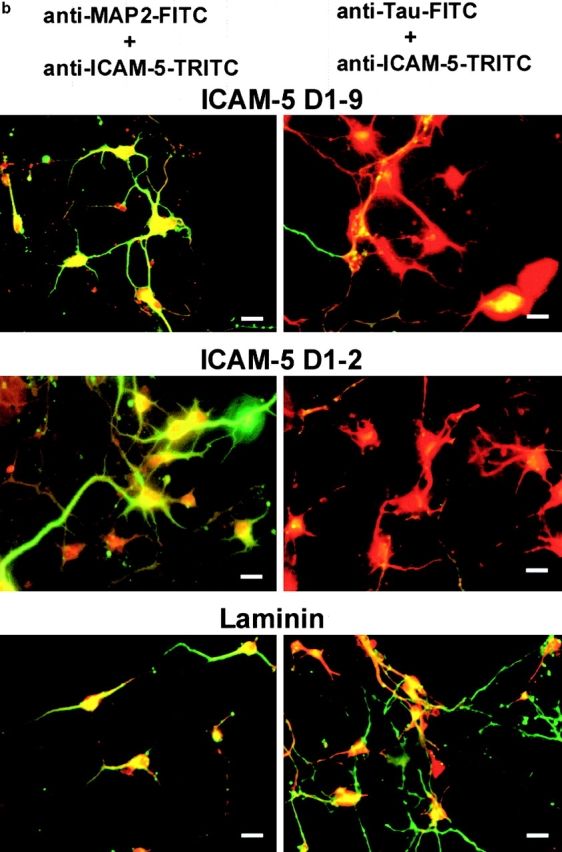
ICAM-5 promotes dendritic outgrowth from rat hippocampal neurons. Rat E19 hippocampal or cerebellar neurons were cultured on different substrate coated surfaces for 48 (a) and 72 h (b), and were then visualized by staining with the dendritic marker, MAP-2 (a and b), and axonal marker, tau (b) together with anti–ICAM-5 polyclonal antibody. The proportion of neurons with neurites was quantitated (c). All photographs were taken at 400×. Bars, 20 μm. In a, neurites from hippocampal neurons were induced on ICAM-5 D1–9, ICAM-5 D1–2, laminin, HB-GAM, or poly-dl-ornithine coated surfaces, but not on ICAM-1 coated plates. Note the more elaborate network of neurites induced by ICAM-5 than on laminin, HB-GAM, or poly-dl-ornithine. b, Shows double-staining of the dendritic marker MAP-2 with FITC-labeled antibody (green, left) and ICAM-5 with TRITC-labeled antibody (red). On the right, the expression of the axonal marker tau has been studied using FITC-labeled antibody and ICAM-5 has been stained with TRITC. The yellow staining in the left shows that MAP-2 and ICAM-5 are largely coexpressed, whereas it is evident from the right that ICAM-5 and axons have a different distribution. The results show that laminin promoted the development of an axonal network much better than ICAM-5, whereas ICAM-5 promoted dendritic outgrowth. c, Shows that ICAM-5 D1–2 and D1–9 induced similar amounts of neurite-containing hippocampal neurons (black bars) as the other substrates, except for ICAM-1, which had almost no neurite-promoting effect. Cerebellar neurons (white bars) showed less neurite outgrowth than hippocampal neurons, especially on ICAM-5 coated surfaces. In all cases, 100 neurons were examined. SDs are shown.
Table 3.
Quantitation of Rat Hippocampal Neurons Expressing Axons or Dendrites
| Percentage of neurons with | ||
|---|---|---|
| Coated protein | Axons | Dendrites |
| % | % | |
| Laminin | 72 ± 8.0 | 20 ± 9.2 |
| ICAM-5 D1–2 | 7.5 ± 2.5 | 63 ± 6.5 |
| ICAM-5 D1–9 | 6.5 ± 3.8 | 52 ± 8.2 |
80 neurons were studied for each protein. 50 μg/ml of laminin and 100 μg/ml of ICAM-5 was used for coating. The cells were grown for 72 h. Axons were identified by antitau staining and dendrites were identified by double-staining with anti–ICAM-5 and MAP-2.
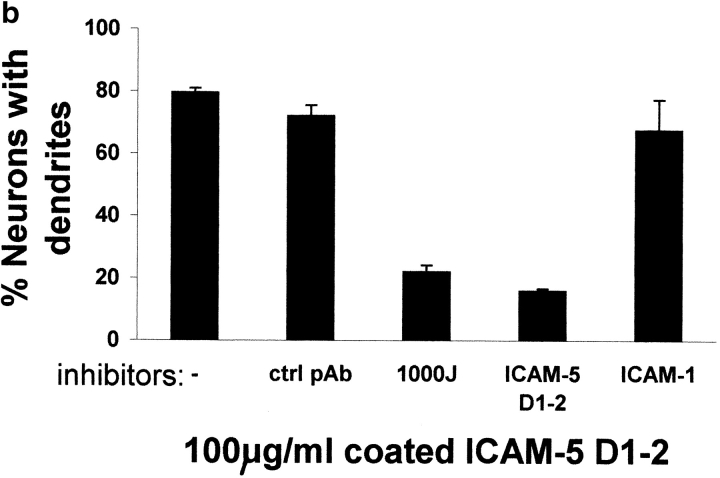
To block the function of endogenous ICAM-5 expressed on hippocampal neurons, we coated them with ICAM-5 D1–2-Fc and then incubated the hippocampal neurons with antibodies and ICAMs in the medium. We found that an antiserum against rat ICAM-5 (pAb 1000J) significantly inhibited dendritic outgrowth, as compared with a control serum (Fig. 8 a). Soluble ICAM-5 D1–2-Fc, but not ICAM-1-Fc, also showed a significant blocking effect. The effect of these proteins was quantitated and shown in Fig. 8 b.
Figure 8.
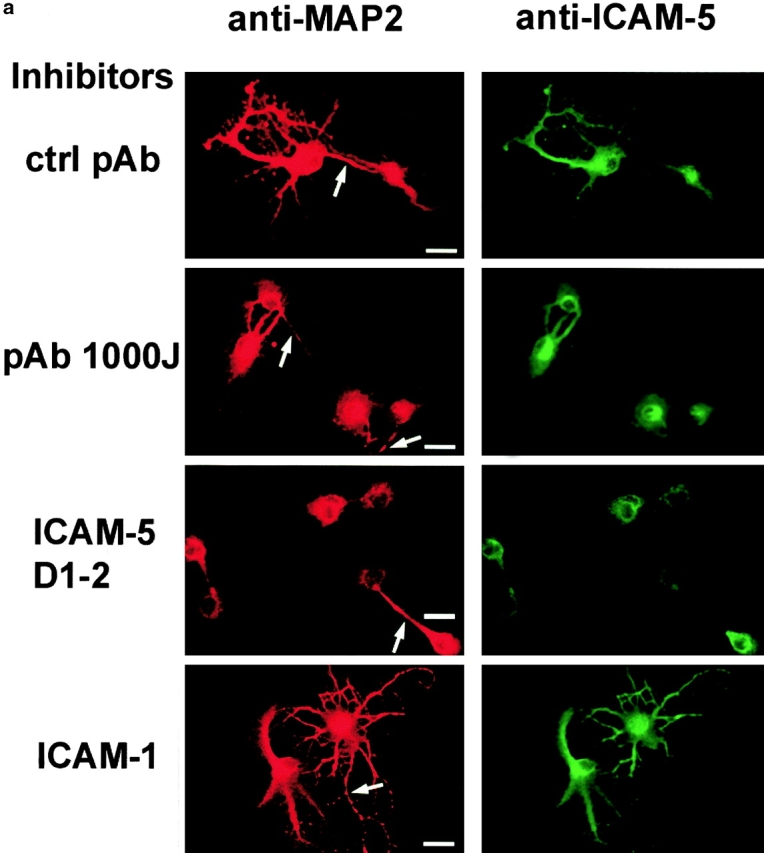
Inhibition of dendritic outgrowth from hippocampal neurons by anti–ICAM-5 antiserum or soluble ICAM-5 D1–2 or ICAM-1. a, Rat E19 hippocampal neurons were cultured on ICAM-5 D1–2 coated surfaces for 48 h. The neurons were stained by anti–MAP-2 (red) and anti–ICAM-5 (green). The polyclonal antibody against rat ICAM-5, 1000J (100 μg/ml), significantly inhibited dendrite outgrowth, compared with the control polyclonal serum (ctrl pAb). A similar inhibitory effect was seen with soluble ICAM-5 D1–2 (100 μg/ml), but not with ICAM-1. Arrows indicate neurites that may be axons stained by anti–MAP-2, but not by anti–ICAM-5. Bars, 20 μm. In b, the number of dendrite-bearing hippocampal neurons subjected to different treatments was counted. 100 neurons in each case were examined.
Discussion
Neurons are known for their elaborate subcellular structures that enable the transmission of signals from one cell to another. To be meaningful, they must make precise connections with their respective target cells (Goodman and Shatz 1993). This developmental process includes axonal and dendritic elongation, axonal guidance, and adhesive interactions between axonal growth cones and soma-dendritic membranes of target neurons that give rise to the formation of synaptic connections. Although much is known about axonal elongation and guidance (Tessier-Lavigne and Goodman 1996; Cook et al. 1998), little is known about dendritic development and how dendrites participate in synaptic interaction.
A number of factors are known to be involved in axonal guidance, including diffusible or surface-bound growth-promoting molecules and various adhesion molecules (Jessell 1988), such as cadherins (Takeichi 1991; Tamura et al. 1998), integrins (Hynes 1992), and immunoglobulin superfamily molecules (Sonderegger and Rathjen 1992; Goodman and Shatz 1993). In contrast, only ICAM-5 has been shown to have a restricted expression to soma-dendritic membranes of telencephalic neurons (Yoshihara et al. 1994; Benson et al. 1998). The immunofluorescence studies show that ICAM-5 had a similar distribution as the dendritic marker MAP-2. MAP-2 evidently also stained some axons (Fig. 7 b), probably due to the fact that the neurons at this stage are not absolutely polarized. In contrast, axon staining with antitau showed a different distribution and little overlap was seen in cells grown on ICAM-5. When coated on laminin, a number of tau+ neurites were seen. The stainings indicate that ICAM-5 promotes the expression of dendritic proteins, such as MAP-2, much better than laminin, whereas laminin promotes the expression of axonal proteins such as tau.
Although the function of ICAM-5 as a brain-specific leukocyte-binding protein is well established (Mizuno et al. 1997; Tian et al. 1997), its strong and specific expression in the telencephalon, especially in the cerebrum and hippocampus, indicated that it may possess other functions. Here, we describe that ICAM-5 induces dendritic extension and branching through homophilic adhesion, the effect of which is dramatically different from that of laminin (Höpker et al. 1999) or HG-GAM (Rauvala and Peng 1997), which are known to mainly promote axonal outgrowth, and that this activity can be blocked by mAbs against ICAM-5.
The first indication of homophilic binding was obtained when we observed that ICAM-5 transfected in Paju cells was enriched at the cell–cell contact sites. However, when we made the entire external part of ICAM-5 by recombinant methods, it did not show homophilic binding. This issue was then studied by making truncated shorter constructs of the molecule. We then found that the binding activity was mediated by homophilic binding of the NH2-terminal domain 1 to domains 4–5. Constructs containing domains 1–2 were more efficient in binding than the single first domain. The finding that mAbs reacting with domain 2 did not inhibit binding further indicates that domain 2 is not directly involved in binding. In fact, some antibodies to D2 enhanced binding, which could be due to conformational changes in D1. The reason for the inability of the isolated D1–9 construct to mediate binding could be its strong tendency to form higher aggregates, possibly tetramers in vitro.
When we performed an analysis of the calculated pI values of the ICAM-5 extracellular domains, we found that in all species of cloned ICAM-5, the pI values of domains 1–2 were ∼11.3, whereas those of domains 4–5 were ∼4.3, suggesting that the homophilic interaction of D1 with D4–5 could be at least partially mediated by electrostatic interactions between the respective domains. This characteristic of highly oppositely charged domains in ICAM-5 is unique among the ICAMs. This may be the reason that ICAM-1 did not show any homophilic binding. Nor did ICAM-1 bind ICAM-5, which shows that the binding is highly specific.
Earlier studies on neural adhesion molecules have shown that the homophilic binding of the neuronal cell adhesion molecule (NCAM) involves all of the five Ig domains (Ranheim et al. 1996), whereas in the case of L1 and cadherin, only Ig domain 2 in L1 (Zhao and Siu 1995) and domain 1 in cadherin (Tamura et al. 1998) are involved. The complex phenomena of homophilic binding of different cell adhesion molecules must underlie their different functions, not only in adhesion, but also in possible signaling events (Brümmendorf and Rathjen 1996).
Multimerization of cell surface molecules is commonly believed to enhance the binding to their ligands/receptors through an increase in avidity. Such examples are the homodimerization of cadherin (Tamura et al. 1998) and ICAM-1 (Miller et al. 1998), and the heterodimerization of NgCAM and axonin-1 (Kunz et al. 1998). The behavior of ICAM-5 is different from that of the other studied Ig domain-containing adhesion proteins.
We think that the inability of the ICAM-5 high molecular weight form to adhere is due to the fact that the homophilic binding of ICAM-5 requires a deeper accessibility of two opposite polypeptides. Furthermore, the studies with the truncated ICAM-5 molecules show that intramolecular associations may exist. The short constructs, like D1–2, can bind because they have their binding domains accessible, whereas longer constructs, like D1–5, are inactive because of interpolypeptide association between D1 and D4–5.
High local concentrations of ICAM-5 may promote high molecular weight complex formation as seen in Paju cells where the uropods are especially rich in the protein. The nonmonomer state must not, however, be permanent in vivo because when Paju-ICAM-5 cells were incubated with truncated ICAM-5 fusion proteins containing the two NH2-terminal domains, the ICAM-5 molecules cells were brought into monomers. How this phenomenon is regulated in vivo remains unknown. When Paju-ICAM-5 cells were treated with cytochalasin D, ICAM-5 completely switched into monomers. These facts indicate a linkage of ICAM-5 with cytoskeletal proteins, as with other ICAM molecules (Carpén et al. 1992; Heiska et al. 1996). ICAM-5 was previously observed to concentrate in dendritic growth cones and filopodia in hippocampal neurons (Benson et al. 1998). Experiments with cytochalasins to disrupt actin filaments within axonal growth cones have shown that the actin cytoskeleton is necessary for correct pathfinding in vivo (Forscher and Smith 1988; Sheetz et al. 1992; Chien et al. 1993), and for directed neurite outgrowth in vitro (Smith 1988; Sheetz et al. 1992). The binding of adhesion molecules on the surface of cells may mediate changes in the cytoskeletal scaffold, thereby resulting in stabilized adhesion or forward growth (Lin et al. 1994). The dynamic change of adhesiveness between monomers and nonmonomers of ICAM-5, which may be regulated by the cytoskeleton, offers an interesting possibility of regulation of dendritic outgrowth.
Regulation of ICAM-5 binding by nonmonomer/monomer transition could also be a means of influencing leukocyte integrin–ICAM-5 interaction. We have found that CD11a/CD18 binds to the first Ig domain of ICAM-5 (Tian et al. 2000), and some mAbs that blocked the ICAM-5-integrin interaction also blocked the ICAM-5 homophilic binding, so the binding sites for these two activities are partially overlapping.
It has been known that dendritogenesis and synaptogenesis take place during the early postnatal period (Goodman and Shatz 1993; Craig and Banker 1994). Therefore, we studied ICAM-5 during this time in rats where brain development has been extensively studied (Craig and Banker 1994). Interestingly, during the first postnatal week, ICAM-5 mainly existed as monomers, whereas at postnatal days 7–10, it gradually changed into nonmonomers. Whether the nonmonomer form is a tetramer or a complex of ICAM-5 with other proteins is not known. This transition coincides with dendritogenesis in the early postnatal period of the rodent central nervous system. Obviously, it is important that when neurons extend branches of dendrites they also need signals for distinguishing axonal growth cones from dendritic ones so that the correct synapses are formed. At an early stage of dendritogenesis, ICAM-5 monomers could promote dendritic elongation and arborization through homophilic interaction. When neurons become developmentally more mature, ICAM-5 mainly exists as nonmonomers, whereby it does not promote dendrite–dendrite/soma interactions. It is possible that promotion of dendrite–dendrite adhesion by monomeric ICAM-5 does not promote branching, and conversely, that the nonmonomeric form could induce more branching.
Acknowledgments
We are grateful to Dr. Urmas Arumäe for help with hippocampal neuronal cultures. We thank Outi Nikkilä and Leena Kuoppasalmi for technical assistance and Yvonne Heinilä for secretarial help.
This study was supported by the Academy of Finland, the Sigrid Jusélius Foundation, and the Finnish Cancer Society.
Footnotes
Abbreviations used in this paper: AxCAM, axon-associated cell adhesion molecule; D, immunoglobulin domain; DenCAM, dendrite-associated cell adhesion molecule; E19, embryonic day 19; ICAM, intercellular adhesion molecule; MAP-2, microtubule-associated protein-2; P, postnatal day; WT, wild-type.
References
- Benson D.L., Yoshihara Y., Mori K. Polarized distribution and cell type-specific localization of telencephalin, an intercellular adhesion molecule. J. Neurosci. Res. 1998;52:43–53. doi: 10.1002/(SICI)1097-4547(19980401)52:1<43::AID-JNR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Rathjen F.G. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr. Opin. Neurobiol. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Carpén O., Pallai P., Staunton D.E., Springer T.A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and α-actinin. J. Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C.B., Rosenthal D.E., Harris W.A., Holt C.E. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11:237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- Cook G., Tannahill D., Keynes R. Axon guidance to and from choice points. Curr. Opin. Neurobiol. 1998;8:64–72. doi: 10.1016/s0959-4388(98)80009-3. [DOI] [PubMed] [Google Scholar]
- Craig A.M., Banker G. Neuronal polarity. Annu. Rev. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C.G. Leukocyte adhesionCD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- Goodman C.S., Shatz C.J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Hayflick J.S., Kilgannon P., Gallatin W.M. The intercellular adhesion molecule (ICAM) family of proteins. New members and novel functions. Immunol. Res. 1998;17:313–327. doi: 10.1007/BF02786454. [DOI] [PubMed] [Google Scholar]
- Heiska L., Kantor C., Parr T., Critchley D.R., Vilja P., Gahmberg C.G., Carpén O. Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to α-actinin. J. Biol. Chem. 1996;271:26214–26219. doi: 10.1074/jbc.271.42.26214. [DOI] [PubMed] [Google Scholar]
- Höpker V.H., Shewan D., Tessier-Lavigne M., Poo M.M., Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrinsversatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes R.O., Lander A.D. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Jessell T.M. Adhesion molecules and the hierarchy of neural development. Neuron. 1988;1:3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Kunz S., Spirig M., Ginsburg C., Buchstaller A., Berger P., Lanz R., Rader C., Vogt L., Kunz B., Sondereger P. Neurite fasciculation mediated by complexes of axonin-1 and Ng cell adhesion molecule. J. Cell Biol. 1998;143:1673–1690. doi: 10.1083/jcb.143.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Lamoureux P., Buxbaum R.E., Forscher P. Cytoskeletal reorganization underlying growth cone motility. Curr. Opin. Neurobiol. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Miller J., Knorr R., Ferrone M., Houdei R., Carron C.P., Dustin M.L. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J. Exp. Med. 1998;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yoshihara Y., Inazawa J., Kagamiyama H., Mori K. cDNA cloning and chromosomal localization of the human telencephalin and its distinctive interaction with lymphocyte function-associated antigen-1. J. Biol. Chem. 1997;272:1156–1163. doi: 10.1074/jbc.272.2.1156. [DOI] [PubMed] [Google Scholar]
- Oka S., Mori K., Watanabe Y. Mammalian telencephalic neurons express a segment-specific membrane glyprotein, telencephalin. Neurosci. 1990;35:93–103. doi: 10.1016/0306-4522(90)90124-m. [DOI] [PubMed] [Google Scholar]
- Ranheim T.S., Edelman G.M., Cunningham B.A. Homophilic adhesion mediated by the neural cell adhesion molecule involves multiple immunoglobulin domains. Proc. Natl. Acad. Sci. 1996;93:4071–4075. doi: 10.1073/pnas.93.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H., Peng H.B. HB-GAM (heparin-binding growth-associated molecule) and heparin type glycans in the development and plasticity of neuron-target contacts. Progress Neurobiol. 1997;52:127–144. doi: 10.1016/s0301-0082(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Sakurai E., Hashikawa T., Yoshihara Y., Kaneko S., Satoh M., Mori K. Involvement of dendritic adhesion molecule telencephalin in hippocampal long-term potentiation. Neuroreport. 1998;9:881–886. doi: 10.1097/00001756-199803300-00022. [DOI] [PubMed] [Google Scholar]
- Sheetz M.P., Wayne D.B., Pearlman A.L. Extension of filopodia by motor-dependent actin assembly. Cell. Motil. Cytoskel. 1992;22:160–169. doi: 10.1002/cm.970220303. [DOI] [PubMed] [Google Scholar]
- Smith S.J. Neuronal cytomechanicsthe actin-based motility of growth cones. Science. 1988;242:708–715. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Rathjen F.G. Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J. Cell Biol. 1992;119:1387–1394. doi: 10.1083/jcb.119.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tamada A., Yoshihara Y., Mori K. Dendrite-associated cell adhesion molecule, telencephalin, promotes neurite outgrowth in mouse embryo. Neurosci. Lett. 1998;240:163–166. doi: 10.1016/s0304-3940(97)00951-8. [DOI] [PubMed] [Google Scholar]
- Tamura K., Shan W.S., Hendrickson W.A., Colman D.R., Shapiro L. Structure–function analysis of cell adhesion by neural (N−) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Goodman C.S. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tian L., Yoshihara Y., Mizuno T., Mori K., Gahmberg C.G. The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J. Immunol. 1997;158:928–936. [PubMed] [Google Scholar]
- Tian L., Kilgannon P., Yoshihara Y., Mori K., Gallatin W.M., Carpén O., Gahmberg C.G. Binding of T lymphocytes to hippocampal neurons through ICAM-5 (telencephalin) and characterization of its interaction with the leukocyte integrin CD11a/CD18. Eur. J. Immunol. 2000;30:810–818. doi: 10.1002/1521-4141(200003)30:3<810::AID-IMMU810>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Walsh F.S., Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamilyrole in axon growth and guidance. Annu. Rev. Cell Dev. Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Mori K. Telencephalina neuronal area code molecule? Neurosci. Res. 1994;21:119–124. doi: 10.1016/0168-0102(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Oka S., Nemoto Y., Watanabe Y., Nagata S., Kagamiyama H., Mori K. An ICAM-related neuronal glycoprotein, telencephalin, with brain segment-specific expression. Neuron. 1994;12:541–553. doi: 10.1016/0896-6273(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Zhang K.Z., Westberg J.A., Hölttä E., Andersson L.C. BCL2 regulates neural differentiation. Proc. Natl. Acad. Sci. 1996;93:4504–4508. doi: 10.1073/pnas.93.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Siu C.H. Colocalization of the homophilic binding site and the neuritogenic activity of the cell adhesion molecule L1 to its second Ig-like domain. J. Biol. Chem. 1995;270:29413–29421. doi: 10.1074/jbc.270.49.29413. [DOI] [PubMed] [Google Scholar]