Abstract
Intraflagellar transport (IFT) is a rapid movement of multi-subunit protein particles along flagellar microtubules and is required for assembly and maintenance of eukaryotic flagella. We cloned and sequenced a Chlamydomonas cDNA encoding the IFT88 subunit of the IFT particle and identified a Chlamydomonas insertional mutant that is missing this gene. The phenotype of this mutant is normal except for the complete absence of flagella. IFT88 is homologous to mouse and human genes called Tg737. Mice with defects in Tg737 die shortly after birth from polycystic kidney disease. We show that the primary cilia in the kidney of Tg737 mutant mice are shorter than normal. This indicates that IFT is important for primary cilia assembly in mammals. It is likely that primary cilia have an important function in the kidney and that defects in their assembly can lead to polycystic kidney disease.
Keywords: orpk, intraflagellar transport, primary cilia, kinesin-II, cytoplasmic dynein
Introduction
Defects in the Tg737 gene cause kidney and liver defects in mice that are very similar to those seen in humans with autosomal recessive polycystic kidney disease (Moyer et al. 1994). This disease affects ∼1 in 10,000 children born each year and may be responsible for a much higher proportion of stillbirths and prenatal deaths (Blyth and Ockenden 1971; Cole et al. 1987). The function of the Tg737 protein is unknown. Here we identify a protein in Chlamydomonas that is homologous to Tg737 and show that it is required for assembly of flagella.
The epithelial cells lining the collecting tubules of the kidney have very well developed primary cilia (Andrews and Porter 1974). The role of these cilia is unknown; however, they extend into the lumen of the tubule and may serve as sensory appendages. Precedence for primary cilia serving a sensory role is well established in vision and olfaction, as the outer segments of the rod and cone cells of the eye and the olfactory cilia of the nose have evolved from cilia and have retained primary cilia characteristics; e.g., the 9+0 microtubule arrangement. Primary cilia in other organisms such as Caenorhabditis elegans also serve a sensory role (White et al. 1976; Perkins et al. 1986).
Eukaryotic cilia and flagella are built and maintained by a process called intraflagellar transport (IFT) (Rosenbaum et al. 1999). Most well characterized in Chlamydomonas, IFT is a rapid movement of particles along the axonemal microtubules of cilia and flagella. The outward movement of these particles from the cell body to the tip of the flagellum is driven by FLA10 kinesin-II (Kozminski et al. 1993, Kozminski et al. 1995), whereas the transport of the particles from the tip back to the cell body is driven by DHC1b/DHC2 cytoplasmic dynein (Pazour et al. 1998, Pazour et al. 1999; Porter et al. 1999). The particles that are transported by IFT are composed of at least 17 protein subunits (Piperno and Mead 1997; Cole et al. 1998). The functions of the individual subunits are not known, but the proteins are conserved between green algae, nematodes, and vertebrates (Cole et al. 1998; Rosenbaum et al. 1999). In this manuscript, we describe the cloning of the IFT88 subunit of the Chlamydomonas IFT particle and show that cells missing this gene do not assemble flagella. We further show that IFT88 is homologous to the polycystic kidney disease gene Tg737 and that mice with mutations in this gene have shorter than normal primary cilia in their kidney.
Materials and Methods
Purification and Microsequencing of Chlamydomonas IFT88
16S IFT particles were purified from Chlamydomonas flagella as described in Cole et al. 1998. The IFT88 subunit was further purified by two-dimensional gel electrophoresis and transferred to ImmobilonPSQ (Millipore) as described previously (Cole et al. 1998). The spot corresponding to IFT88 was excised and digested with trypsin. Tryptic peptides were eluted from the membrane and fractionated by high performance liquid chromatography. Pure peptides, identified by mass spectrometry, were subjected to microsequence analysis in the UMMS Protein Sequencing Facility.
Cloning IFT88
Portions of the IFT88 peptide sequence (LEGETDQA and GIDPYCVE) were used to design two degenerate oligonucleotide PCR primers (GA[A/G] AC[C/G/T] GA[C/T] CA[A/G] GC[C/G/T] GA[C/T] AA[A/G] TA and GC [C/T]TC [A/C/G]AC [A/G]CA [A/G]TA [A/C/G]GG [A/G]TC [A/G]AT). These primers amplified a 365-bp fragment of genomic DNA that contained parts of two exons and a 132-bp intron. This fragment of genomic DNA was used to screen a Chlamydomonas cDNA library made from cells undergoing division (contact Drs. Pazour and Witman for cDNA libraries). Two positive clones were identified and sequenced by primer walking. These two clones were similar except for the sequences at their 5′ ends. IFT88cDNA-1 was longer than IFT88cDNA-2 and appeared to have a short region of polyA inappropriately fused to the 5′ end, probably the result of a cloning artifact. One Chlamydomonas IFT88 EST clone is in Genbank (accession number AV395576). This EST sequence, which is from the 5′ end of the gene and overlaps the cDNA clones, was used to define the 5′ end of the cDNA sequence.
Four independent BAC clones (40-B3, 11-O21, 24-F2, and 27-M3) were found in the Genome Systems Chlamydomonas BAC library by Southern hybridization using the 365-bp fragment of IFT88 genomic DNA as a probe. The presence of the IFT88 gene in the clones was confirmed by Southern blotting.
Identification and Rescue of an IFT88 Mutant
DNA from each of the ∼400 transformants in our insertional mutant collection (Pazour et al. 1995, Pazour et al. 1998; Pazour and Witman 2000) was cut with PstI and analyzed by Southern blotting with the 365-bp fragment IFT88 genomic DNA fragment. This probe detected an ∼2.5-kb band in wild-type cells and all of the mutants except strain V79.
The motility/flagellar defect in V79 was rescued by transforming with BAC clones carrying the IFT88 gene. Transformation was performed by the glass bead method of Kindle 1990, and rescued cell lines were identified by restoration of their ability to swim. One rescued cell line was crossed to wild-type CC124 cells. Tetrads from this cross were dissected and analyzed by standard procedures (Levine and Ebersold 1960; Harris 1989) as described in Pazour et al. 1998. The flagellar phenotype was scored by light microscopy when the cells were in the early log phase of growth.
Electron Microscopy
Chlamydomonas cells were fixed in glutaraldehyde for EM (Hoops and Witman 1983) and processed as described in Wilkerson et al. 1995. Tissues of anesthetized mice were fixed in situ by brief cardiac perfusion with 2.5% gluteraldehyde in 100 mM cacodylate buffer. The kidneys were removed and a small amount of additional fixative was injected under the capsule of the kidney. The kidneys were placed in additional fixative for 1 h. At that time, the kidneys were sliced in half and further fixed for 2 d. The tissue was freeze fractured and metal impregnated as described in McManus et al. 1993.
Western Blotting
Whole cell extracts of wild-type and mutant cells were made by resuspending log-phase cells in SDS-sample buffer, heating at 50°C for 10 min, and repeatedly drawing the sample through a 26-gauge needle to shear the DNA. Proteins were separated by SDS-PAGE, blotted onto polyvinylidene difluoride membranes, and probed with antibodies as described in Pazour et al. 1998. Antibodies used included mAb57.1, mAb81.1, mAb139.1, and mAb172.1, which are mAbs against IFT particle proteins (Cole et al. 1998); FLA10N, which is specific for a kinesin-II motor subunit (Cole et al. 1998); DHC1b, which is specific for the heavy chain of DHC1b/DHC2 cytoplasmic dynein (Pazour et al. 1999); and B-5-1-2, which is specific for alpha tubulin (Piperno and Fuller 1985).
Chlamydomonas Culture
Chlamydomonas strains used in this work included: g1 (nit1, agg1, mt+) (Pazour et al. 1995), CC124 (nit1, nit2, mt−), and CC1390 (fla2, mt−). The latter two strains are available from the Chlamydomonas Genetics Center (Duke University, Durham, NC). Strains generated in the course of this work included: V79 (ift88-1::NIT1, mt+), which was generated by random insertional mutagenesis of g1, V79/40-B3#2.5 (ift88-1::NIT1, IFT88, mt+), generated by transformation of V79 with BAC clone 40-B3, and 3276.2 (ift88-1::NIT1), which is a progeny from a cross between V79/40-B3#2.5 and CC124.
Mouse Genotyping
DNA was purified by digesting mouse tails with proteinase K, extracting once with 50% phenol / 50% chloroform, once with chloroform, and then precipitating the DNA with ethanol. The genomic DNA was amplified using the RW450, RW451, and RW452 primer set described by Yoder et al. 1997. These primers amplified a 270-bp fragment from the wild-type locus and a 340-bp fragment from the mutant locus.
Digital Image Processing
Western and Southern blots were scanned from negative x-ray film with a Linotype-Hell Saphir Ultra 2 flatbed scanner and brought into Photoshop for cropping and contrast adjustment. Scanning EM micrographs were scanned from positive Polaroid film in the same way. Transmission EM negatives were scanned with a Polaroid Sprint Scan 45 and brought into Photoshop for cropping, contrast adjustment, and inversion from a negative to a positive image.
Results
Cloning and Sequencing of Chlamydomonas IFT88
To learn more about the structure and function of the proteins that make up the IFT particle, we cloned and sequenced the IFT88 protein, formerly known as p88 (Cole et al. 1998). To do this, Chlamydomonas IFT particles were purified from the matrix of isolated flagella by sucrose density gradient centrifugation and two-dimensional gel electrophoresis. IFT88 was cleaved by trypsin and two internal peptides were microsequenced (Cole et al. 1998), yielding the sequences AATNLAFLYFLEGETDQADKYSEMALK and SLFNEAAGIDPYCVEAIYNLGLVSQR. Degenerate PCR primers were designed from these sequences and used to amplify a fragment of genomic DNA. A cDNA library was screened with the genomic fragment and the resulting clones were sequenced by primer walking. Southern blots indicated that there is only one copy of the IFT88 gene in the Chlamydomonas genome.
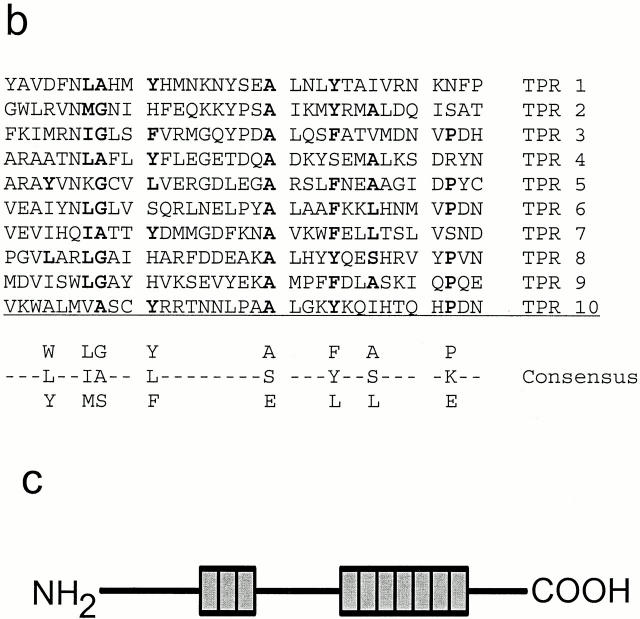
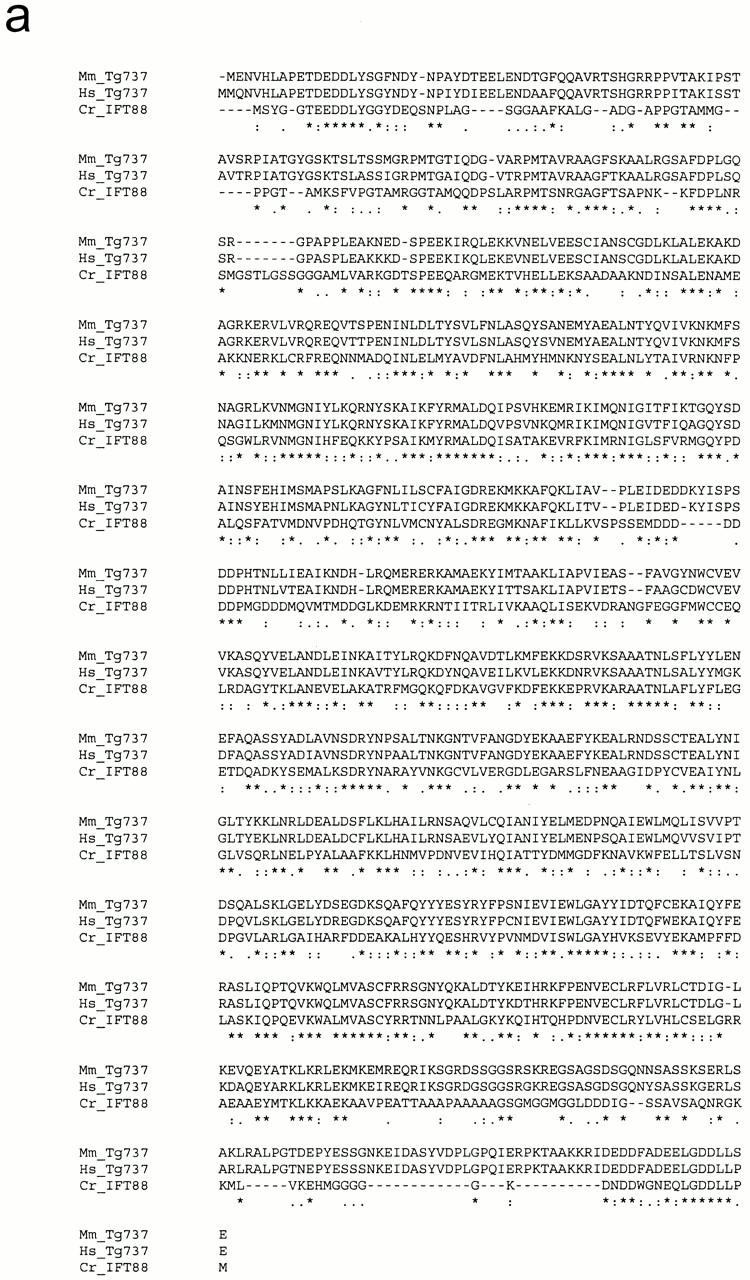
Sequence analysis showed that the IFT88 cDNA contains a 2,346-nucleotide open reading frame that is predicted to encode an 86.3-kD protein (Fig. 1 a) with a pI of 5.87. Perfect matches to both IFT88 tryptic peptides are found in the open reading frame of this cDNA, rigorously confirming that these clones encode the Chlamydomonas IFT88 protein. No discernible motifs were identified within the sequence except for the presence of 10 tetratricopeptide repeats (TPR) (Fig. 1 b). TPRs are degenerate 34–amino acid repeats (Lamb et al. 1995), present in tandem arrays of 3–16 U that are predicted to form amphipathic helices (Hirano et al. 1990). The first three TPR motifs are found closely spaced between amino acid residues 185–294. The other seven TPR motifs occur without spacing between amino acid residues 441–676 (Fig. 1 c).
Figure 1.
Sequence and structure of the Chlamydomonas IFT88 protein. (a) Chlamydomonas IFT88 is homologous to the mouse and human Tg737 proteins. The sequences of the Chlamydomonas (Cr_IFT88, accession number AF298884), mouse (Mm_Tg737, accession number AAB59705), and human (Hs_Tg737, accession number AAA86720) proteins were aligned using ClustalW (Thompson et al. 1994). (*) Complete conservation; :, highly conserved substitutions; and (.) semiconservative substitutions (below the alignment). (b) IFT88 contains 10 TPR repeats. Residues matching the TPR consensus sequence (bottom) are indicated by bold font. (c) The 10 TPR repeats (shaded boxes) are organized in a group of three in the NH2-terminal half of the protein and a group of seven in the COOH-terminal half of the protein.
Chlamydomonas IFT88 Is Homologous to a Mouse Polycystic Kidney Disease Gene
Blast searches with the Chlamydomonas IFT88 protein sequence indicate that it is very similar to the mouse (41% identical; BLAST E=e-148) and human Tg737 (40% identical; BLAST E=e-146) proteins. Mice with defects in Tg737 have severe polycystic kidney disease and die within a few weeks of birth. The protein also is homologous to proteins predicted by ESTs from zebra fish and swine and fragments of preliminary C. elegans and Drosophila melanogaster genomic sequence. IFT88 and Tg737 are likely to be functionally equivalent orthologs as the similarity between the Chlamydomonas and mammalian proteins is robust and distributed over the entire coding region and not just within the TPR domains (Fig. 1 a). 40% identity is very typical of the amount of similarity seen between other Chlamydomonas and mammalian orthologs that encode flagellar proteins (Pazour, G.J., B.L. Dickert, and G.B. Witman, manuscript in preparation).
IFT88 Is Required for Flagellar Assembly
To learn more about the function of IFT88 in cells, we searched our collection of Chlamydomonas insertional mutants (Pazour et al. 1995, Pazour et al. 1998; Pazour and Witman 2000) for a cell line with a defect in this gene. The insertional mutants were made by transforming cells with DNA carrying a selectable marker. In Chlamydomonas, transforming DNA is integrated randomly throughout the genome and disrupts genes at the site of integration. DNA was isolated from ∼400 insertional mutants having behavioral or motility defects and was screened by Southern blotting using a fragment of IFT88 genomic DNA as a probe. One cell line (V79) was identified that had an insertion in the IFT88 gene (Fig. 2 a). The fact that the single hybridizing band in wild-type cells was split into two bands in the mutant indicated that the selectable marker integrated into the gene within the region covered by the probe and did not result in a large deletion of the genome at the site of integration. The mutant allele was termed ift88-1.
Figure 2.
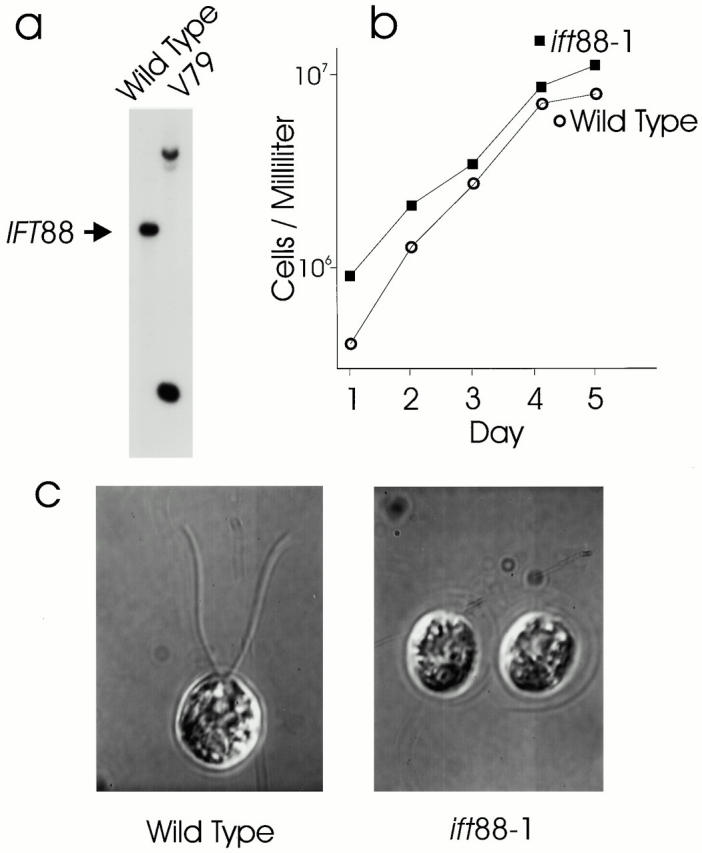
Phenotype of the ift88-1 mutant cells. (a) Southern blot showing that the V79 cell line has an insertion in the IFT88 gene. DNA was isolated from wild-type and V79 cells, digested with PstI, and analyzed by Southern blotting with a 365-bp fragment of IFT88 genomic DNA as a probe. The single band in wild-type cells is split into two bands in the mutant. (b) Disruption of IFT88 does not affect growth rate. Wild-type and ift88-1 mutant cells were grown in liquid culture and monitored as described in Pazour et al. 1998. (c) In contrast to wild-type cells that have two ∼10-μm-long flagella, flagella are not formed on ift88-1 cells. Cells were recorded by differential interference contrast microscopy as described in Pazour et al. 1998.
The ift88-1 cells grew at the same rate as wild-type cells, indicating that IFT88 is not required for processes essential for growth or cell division (Fig. 2 b). However, in contrast to wild-type cells that normally have two ∼10-μm long flagella extending from the anterior end of the cell body, the ift88-1 cells completely lack flagella (Fig. 2 c). Electron microscopic analysis of these cells showed that the basal bodies were structurally normal but the flagella did not extend beyond the transition zone (Fig. 3, arrows). In some cells, the membrane covering the flagellar tips was tightly apposed to the microtubules with no material between them and the membrane. In other cells, the flagellar stubs were slightly swollen and contained fragments of microtubules in random orientations. However, in contrast to the IFT mutants fla14 (Pazour et al. 1998) and dhc1b (Pazour et al. 1999), no accumulation of IFT particles was observed in any of the flagellar stubs.
Figure 3.
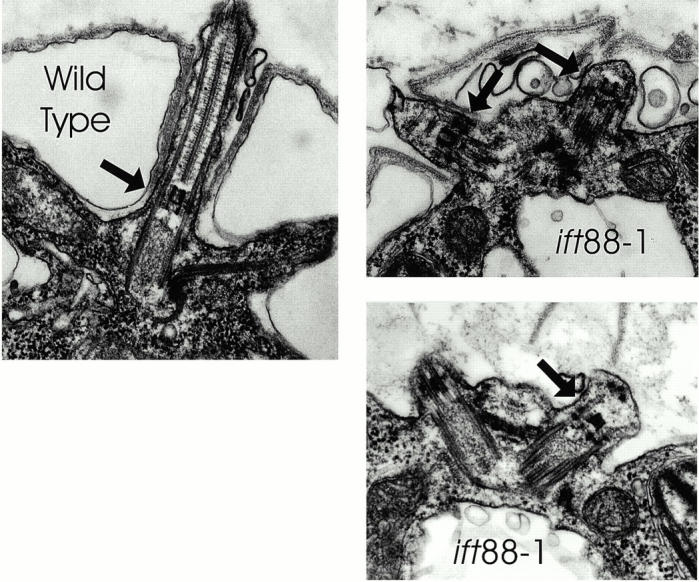
Ultrastructure of the ift88-1 flagella. The flagella on ift88-1 mutant cells are very short and the microtubules do not extend beyond the transition zone (arrows). The microtubules in wild-type cells start at the basal body, extend through the transition zone, and continue on to the flagellar tip. The wild-type flagellum shown here leaves the plane of section shortly after passing through the cell wall.
To determine the effect of the lack of IFT88 on the IFT particle and the IFT motors, we examined whole-cell extracts by Western blotting (Fig. 4). The IFT particle is made up of two large complexes. Complex A is composed of four to five proteins and includes IFT139, shown in Fig. 4. Complex B is composed of IFT88 and 10 other proteins including IFT172, IFT81, and IFT57, shown in Fig. 4. The complex A protein IFT139 is enriched in the mutant, suggesting that the gene may be upregulated in the mutant cells. The mutation has little or no effect on the levels of complex B proteins IFT172 and IFT81, but causes a significant decrease in IFT57, another complex B protein. The cellular levels of the IFT motors FLA10 kinesin-II and DHC1b are not affected by the ift88 mutation.
Figure 4.
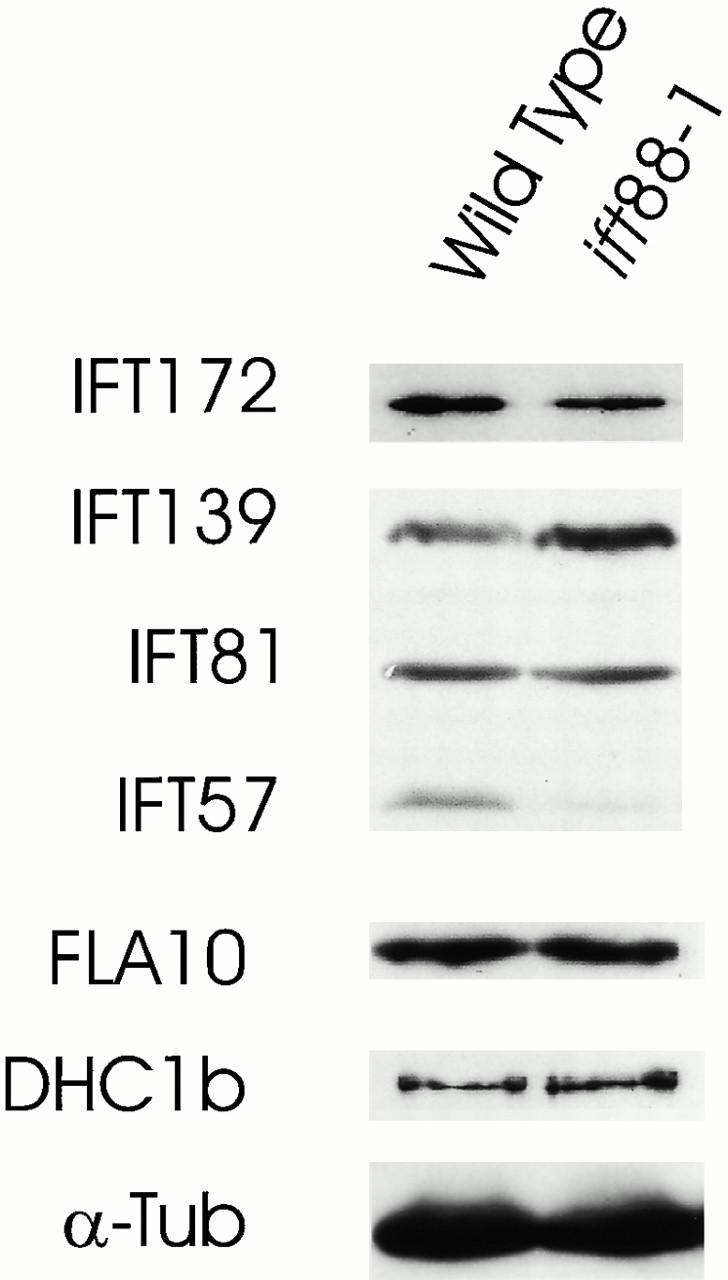
Western blot showing the effect of the ift88-1 mutation on the levels of IFT motor and particle proteins. Equal amounts of whole-cell extracts of ift88-1 (3276.2) and wild-type cells (CC124) were separated by SDS-PAGE, transferred to membrane and probed with antibodies to IFT particle proteins (IFT172, IFT139, IFT81, IFT57) and IFT motor proteins (FLA10, DHC1b). An antibody to α-tubulin (α-Tub) was used to confirm that equivalent amounts of wild-type and ift88-1 protein extracts were loaded on the gel.
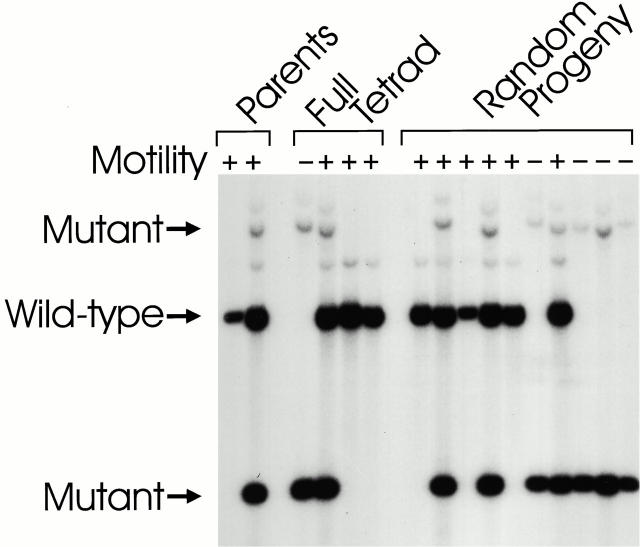
To be certain that the flagellar assembly defect is caused by the mutation in IFT88 and is not the result of another mutation elsewhere in the genome, we transformed the mutant cells with BAC clones carrying the IFT88 gene. Three independent BAC clones (40-B3, 24-F2, and 27-M3) complemented the flagellar defect. The complemented cell lines swam like wild-type cells and had IFT. One of the rescued cell lines was crossed to a wild-type cell line and 26 tetrads were dissected. All four products of one tetrad and a single product of the remaining 25 tetrads were analyzed by Southern blotting. Because the transformed copy of the IFT88 gene inserted at a site unlinked to the original locus, the inserted DNA segregated independently from the original gene, allowing offspring to carry zero, one, or two copies of the wild-type gene. Cells that carry at least one copy of wild-type IFT88 have normal flagella and motility, whereas those that carry no copies of wild-type IFT88 lack flagella and are nonmotile (Fig. 5). These data indicate that the flagellar defect is tightly linked to the ift88 mutation and is almost certainly the result of it.
Figure 5.
Presence of the IFT88 gene correlates with the wild-type phenotype in meiotic products. Strain V79 was transformed with a BAC clone containing the IFT88 gene. Transformed cells recovered the ability to swim and were enriched by taking inoculi from the top part of an unmixed culture. After enrichment, a pure culture of one of the transformants (V79/40-B3#2.5) was isolated and mated to a wild-type cell line (CC124) of the opposite mating type. Tetrads were dissected and the offspring scored for motility by light microscopy. DNA was isolated from the parents, the four products of one tetrad, and random single products of 10 additional tetrads. The DNA was analyzed by Southern blotting using a 386-bp fragment of IFT88 genomic DNA as a probe. Cells that swam normally carried at least one copy of the wild-type gene, whereas the nonmotile cells did not carry a copy of the wild-type gene.
Primary Cilia in the Kidney of Tg737 Mice Are Shorter than Normal
Primary cilia are present on many cells in the mammalian body (Wheatley 1995; Wheatley et al. 1996), and are particularly well developed in the kidney (Andrews and Porter 1974). Inasmuch as Chlamydomonas IFT88 is necessary for assembly of flagella and is homologous to mammalian Tg737, and because a defect in mouse Tg737 leads to kidney disease, it was of great interest to determine whether the defect in Tg737 affected formation of the primary cilia in the kidney. In wild-type rats, the cilia are ∼2.5-μm long and are found in the proximal tubule, the loop of Henle, the distal tubules, and the collecting ducts (Andrews and Porter 1974). In wild-type mice, these cilia are <5-μm long and similarly distributed (Flood and Totland 1977).
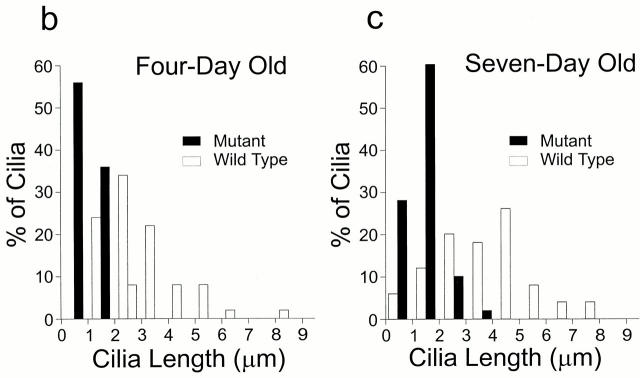
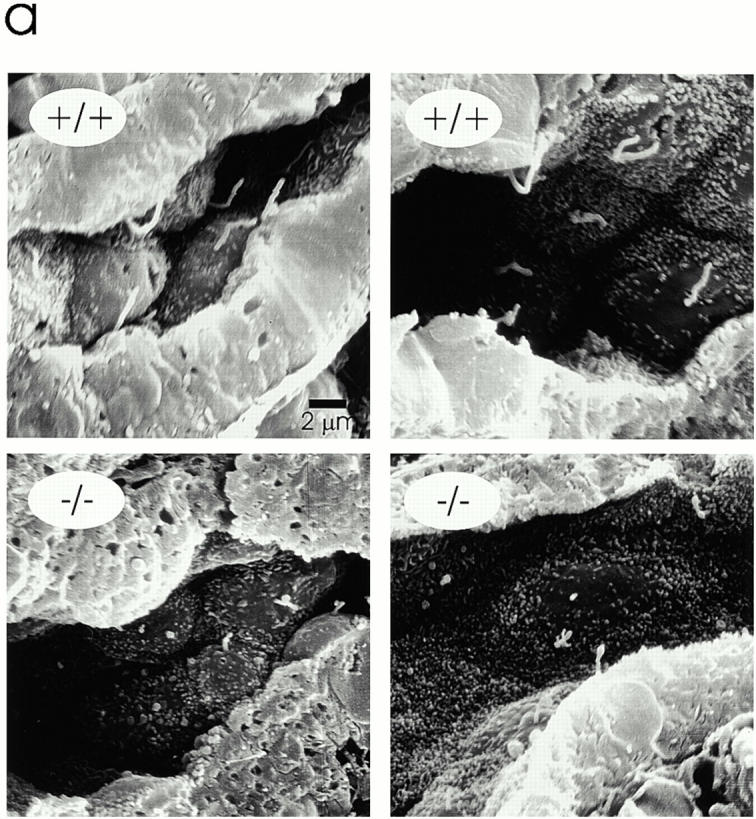
We obtained the hypomorphic Tg737-mutant mice from Oak Ridge National Laboratory and examined the kidneys of 4- and 7-d–old pups by scanning electron microscopy. Numerous monociliated cells were observed in the kidneys of both wild-type (Fig. 6 a, +/+) and homozygous mutant (Fig. 6 a, −/−) mice, but the cilia in the mutant kidneys were much shorter. To quantify this difference, the cilia lengths were measured from scanning electron micrographs taken from the tubules distal to the proximal tubule (Fig. 6b and Fig. c). The proximal tubule was avoided because it contains a thick brush border that can obscure a micron or more of cilia length. The tubules distal to the proximal tubule have only sparse microvilli that do not obscure cilia, and the cilia in these regions are uniform in length (Andrews and Porter 1974). These cilia in wild-type mice were 3.1 ± 1.4 and 3.5 ± 1.7 μm at 4 and 7 d, respectively, whereas these cilia in the mutant mice were 1.0 ± 0.6 and 1.3 ± 0.6 μm at 4 and 7 d, respectively. These values represent minimum lengths as it is difficult to accurately measure cilia that are lying at different angles in the tubules. However, the differences are quite large and are significant at the >99% level. Thus, Tg737 plays an essential role in assembly of the primary cilium in the mouse, just as IFT88 is essential for flagellar assembly in Chlamydomonas.
Figure 6.
Primary cilia in the kidney of Tg737 mutant mice are shorter than normal. (a) Kidneys from 4-d-old pups were fixed with glutaraldehyde, freeze fractured, metal impregnated, and examined by scanning electron microscopy. Numerous cilia were found on the epithelial cells in the tubules and collecting ducts of the wild-type mice (+/+). Cilia were also found in the homozygous mutant (−/−) pups, but they were usually <2-μm long and most were only short stubs. (b) Cilia length in kidneys of 4-d-old mice. Cilia were measured in scanning electron micrographs of tubules located distal to the proximal tubule. Wild-type cilia averaged 3.1 ± 1.4 μm (n = 50), whereas the mutant cilia averaged 1.0 ± 0.6 μm (n = 50). (c) Cilia length in kidneys of 7-d-old mice. The cilia lengths were measured in scanning electron micrographs of tubules located distal to the proximal tubule. Wild-type cilia averaged 3.5 ± 1.7 μm (n = 50), whereas the mutant cilia averaged 1.3 ± 0.6 μm (n = 50).
Discussion
The IFT88 Gene Is Required for Flagellar Assembly in Chlamydomonas
Chlamydomonas cells lacking the IFT88 gene do not assemble flagella, indicating that the IFT88 protein is required for flagellar assembly. This is the first Chlamydomonas IFT particle subunit to be shown to be required for ciliary assembly. Loss of IFT88 causes a substantial decrease in the amount of IFT57 relative to other IFT particle proteins in the cytoplasm, suggesting that IFT88 is important for assembly of at least a portion of the IFT particle. Thus, IFT may be blocked at a very early stage in the ift88-1 mutant. Consistent with this, IFT particles do not accumulate in the flagellar stubs of the ift88 mutant, in sharp contrast to the dramatic accumulation of apparently intact IFT particles in the flagella of mutants with defects in cytoplasmic dynein DHC1b/DHC2 (Pazour et al. 1999) or the dynein light chain LC8 (Pazour et al. 1998). Alternatively, IFT88 may have a vital role in the attachment of the IFT particle to its cargo or to the anterograde IFT motor FLA10-kinesin-II. In either case, loss of IFT88 would preclude flagellar assembly. It is also possible that IFT88 is essential for transduction of a signal that is necessary for flagellar assembly.
Tg737, the Mouse IFT88 Homologue, Is Required for Assembly of the Primary Cilia in Kidney
We have shown that IFT88 is highly similar to the mouse and human Tg737 proteins and that mice with defects in Tg737 have defective cilia in their kidneys. Tg737 was identified at Oak Ridge National Laboratory by random insertional mutagenesis of mice. Hypomorphic mutations in Tg737 cause kidney disease and death within a few weeks of birth. The phenotype of this mutation closely resembles human autosomal recessive polycystic kidney disease (ARPKD) in that the mice develop cystic kidneys and have hepatic biliary disease, which is also common in human patients with ARPKD (Moyer et al. 1994). The mice develop large cysts in their collecting ducts and are unable to concentrate urine (Yoder et al. 1996, Yoder et al. 1997). Null alleles of Tg737 have a more severe phenotype and cause the mice to die during midgestation (Murcia et al. 2000). The phenotype caused by the null Tg737 mutation closely resembles the phenotype of kinesin-II knockout mice (Nonaka et al. 1998; Marszalek et al. 1999, Takeda et al. 1999). Both the kinesin-II and Tg737 null mice have left–right asymmetry defects, lack cilia on the embryonic node, and die during midgestation. Our finding that IFT88 is required for ciliary assembly provides the first evidence that the lack of nodal cilia on embryos of Tg737 null mutant mice is a direct result of a defect in IFT.
Primary cilia are extremely widely dispersed throughout the mammalian body. The only cells that are known not to contain primary cilia are hepatocytes and differentiated cells of myeloid or lymphoid origin (Wheatley 1995; Wheatley et al. 1996). The primary cilia in kidney tubules and ducts (Andrews and Porter 1974) and hepatic biliary ducts (Motta and Fumagalli 1974) are unusually long and project into the lumens of these structures. The role of the primary cilia in the kidney or hepatic ducts is not known, but has been suggested to be sensory (Roth et al. 1988). The most studied primary cilia are the outer segments of rod and cone photoreceptor cells and the olfactory cilia in the nasal cavity. In these examples, the role of the primary cilia is clearly to serve as an appendage to concentrate sensory machinery. C. elegans also makes extensive use of primary cilia to detect osmolarity gradients and chemical signals (White et al. 1976; Lewis and Hodgkin 1977). Primary cilia on other cells may similarly have a sensory role. Supporting this idea, the somatostatin 3 receptor has recently been localized to primary cilia in the brain (Handel et al. 1999). Kidney epithelial cells sense multiple extracellular signals, including peptide hormones such as angiotensin and ions such as chloride (reviewed in Gunning et al. 1996). Whether any of these sensory receptors are localized to the primary cilia of the vertebrate kidney remains to be determined.
C. elegans homologues of the human polycystic kidney disease genes, PKD1 and PKD2, are localized to sensory cilia (Barr and Sternberg 1999). Humans with mutations in PKD1 and PKD2 develop kidney disease similar to that caused by Tg737 mutations in mice. PKD1 and PKD2 are transmembrane proteins that interact with each other (Qian et al. 1997; Tsiokas et al. 1997). PKD1 has a large extracellular domain that is thought to bind an unknown ligand (Hughes et al. 1995; The International Polycystic Kidney Disease Consortium 1995). PKD2 is homologous to calcium-regulated cation channels, suggesting that PKD2 is a cation channel (Chen et al. 1999). Further work will be necessary to determine if PKD1 or PKD2 are ever found on mammalian primary cilia.
TPR Repeats in IFT88 and Tg737 Suggest that these Proteins Are Involved in Protein–Protein Interactions
TPR repeats are degenerate 34 amino acid motifs (Lamb et al. 1995) that are present in tandem arrays in proteins. These arrays are predicted to form super helices (Hirano et al. 1990) with amphipathic grooves responsible for binding specific target proteins (Das et al. 1998). TPR domains have been found to mediate multiple simultaneous protein–protein interactions in such multiprotein complexes as molecular chaperones and the anaphase-promoting complex (reviewed in Blatch and Lassle 1999). In IFT88 and Tg737, there are three closely spaced TPR repeats in the amino-terminal half of the protein and another seven TPR repeats in the carboxyl-terminal half of the protein. These two separate TPR domains might serve to bind simultaneously to two separate target proteins. These target proteins could be axonemal subunits that are transported via IFT to the flagellar tip, where they are assembled (Piperno et al. 1996). The targets also could be membrane proteins such as receptors and channels, as IFT particles are tightly associated with the flagellar membrane (Kozminski et al. 1995; Pazour et al. 1998). Alternatively, IFT88 could be binding to other subunits of the IFT particle and holding it together. IFT57 is likely to be an interacting protein because it is destabilized in the absence of IFT88.
IFT Is a Conserved Mechanism in the Assembly and Maintenance of Cilia and Flagella
A strong body of evidence indicates that IFT is necessary for assembly and maintenance of all eukaryotic motile and sensory cilia. Previous work has shown that the anterograde motor, kinesin-II, is necessary for assembly and maintenance of cilia and flagella in diverse organisms that include green algae, ciliated protozoa, nematodes, echinoderms, and vertebrates (reviewed in Cole 1999; Marszalek and Goldstein 2000). The retrograde motor, cytoplasmic dynein DHC1b/DHC2, also has been shown to be required for assembly of Chlamydomonas flagella (Pazour et al. 1999; Porter et al. 1999) and Caenorhabditis sensory cilia (Signor et al. 1999; Wicks et al. 2000). Our initial report on the composition of the Chlamydomonas IFT particle proteins showed that IFT52 was homologous to C. elegans OSM-6 and that IFT172 was homologous to C. elegans OSM-1 (Cole et al. 1998). OSM-1 and OSM-6 are required for assembly of sensory cilia in worms (Perkins et al. 1986; Collet et al. 1998). The involvement of these two nematode proteins in IFT was recently confirmed when GFP-labeled OSM-6 and OSM-1 were both shown to undergo IFT in transformed C. elegans (Orozco et al. 1999; Signor et al. 1999). The work in this paper shows that the IFT particle protein IFT88 is required for ciliary assembly in Chlamydomonas and that the IFT88 homologue, Tg737, is required for assembly of primary cilia in the kidney of mice. Thus, evidence from a diverse group of eukaryotes shows that both the IFT motors and the IFT particle proteins are required for assembly and maintenance of cilia and flagella. This indicates that IFT is an ancient and conserved mechanism by which eukaryotic cilia and flagella are built and maintained.
IFT Is Likely to Play Important Roles in Many Disease States
In addition to polycystic kidney disease, discussed above, there are many other diseases that may involve IFT. This includes retinitis pigmentosa, which is a genetic disorder that causes destruction of photoreceptor cells, resulting in progressive vision loss. Transport of opsin and other components of the rod outer segment is very important in photoreceptor cells, as ∼10% of the outer segment is turned over each day. Transport from the inner segment to the outer segment occurs through the connecting cilium (reviewed in Besharse and Horst 1989). Kinesin-II and several IFT particle proteins are located in the connecting cilium of photoreceptor cells (Beech et al. 1996; Muresan et al. 1999; Whitehead et al. 1999; Pazour, G.J., D.G. Cole, J.A. Deane, S.A. Baker, B.L. Dickert, J.L. Rosenbaum, G.B. Witman, and J.C. Besharse, submittedmanuscript for publication). Moreover, Marszalek et al. 2000 showed that photoreceptor cells lacking kinesin-II accumulate opsin and arrestin in the inner segment, indicating that kinesin-II is involved in transport in photoreceptor cells. Therefore, IFT is likely to be an important transport mechanism in vertebrate photoreceptor cells, and mutations in IFT particle proteins are likely to cause vision defects.
Defects in IFT also are likely to affect motile cilia and flagella and be a cause of primary ciliary dyskinesia (PCD). PCD is a syndrome caused by defects in motile cilia and is characterized by male infertility, respiratory disease, and situs inversus. It is well known that defects in axonemal components cause PCD (Afzelius 1979; Pennarun et al. 1999). However, IFT particle proteins are highly expressed in the testis and lung, suggesting that they are involved in the assembly of motile sperm flagella and respiratory tract cilia (Pazour, G.J., unpublished results). Thus, a mutation in an IFT particle protein could lead to defects in sperm flagellar assembly and result in sperm with short disorganized tails, as have been described in some infertile human males (Baccetti et al. 1993). It is possible that mutations that prevent assembly of both motile and sensory cilia are so severe that the embryos terminate during gestation, as has been observed with mutations in the mouse kinesin-II motor subunits kif3a (Marszalek et al. 1999; Takeda et al. 1999) and kif3b (Nonaka et al. 1998).
Acknowledgments
We thank Ruth Hesselton, Greg Hendricks, Dennis Diener, Matthew Whitacre, Ben Lucker, John Leszyk, and Diane Casey for valuable assistance during this project.
This work was supported by National Institutes of Health grants GM30626 to G.B. Witman and GM14642 to J.L. Rosenbaum, by a grant from the University of Idaho Agricultural Experiment Station to D.G. Cole, by a Diabetes and Endocrinology Research Center Grant (DK 32520) at the University of Massachusetts Medical School, by an American Association of University Women International Fellowship (Y. Vucica), and by the Robert W. Booth Fund at the Greater Worcester Community Foundation (G.B. Witman).
Footnotes
Mr. Seeley's current address is Dartmouth Medical School, Department of Biochemistry, Hanover, NH 03755.
Ms. Vucica's current address is Cell Biology Group, School of Plant Science, The University of Tasmania, Hobart, Tasmania 7001, Australia.
Abbreviations used in this paper: IFT, intraflagellar transport; TPR, tetratricopeptide repeats.
References
- Afzelius B.A. The immotile-cilia syndrome and other ciliary diseases. Int. Rev. Exp. Pathol. 1979;19:1–43. [PubMed] [Google Scholar]
- Andrews P.M., Porter K.R. A scanning electron microscopic study of the nephron. Am. J. Anat. 1974;140:81–116. doi: 10.1002/aja.1001400107. [DOI] [PubMed] [Google Scholar]
- Baccetti B., Burrini A.G., Capitani S., Collodel G., Moretti E., Piomboni P., Renieri T. Notulae seminologicae. 2. The ‘short tail’ and ‘stump’ defect in human spermatozoa. Andrologia. 1993;25:331–335. [PubMed] [Google Scholar]
- Barr M.M., Sternberg P.W. A polycistic kidney-disease gene homologue required for male mating behaviour in C. elegans . Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Beech P.L., Pagh-Roehl K., Noda Y., Hirokawa N., Burnside B., Rosenbaum J.L. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. J. Cell Sci. 1996;109:889–897. doi: 10.1242/jcs.109.4.889. [DOI] [PubMed] [Google Scholar]
- Besharse J.C., Horst C.J. The photoreceptor connecting ciliuma model for the transition zone. In: Bloodgood R.A., editor. Ciliary and Flagellar Membranes. Plenum Publishing Corp; New York, NY: 1989. pp. 389–417. [Google Scholar]
- Blatch G.L., Lassle M. The tetratricopeptide repeata structural motif mediating protein–protein interactions. Bioessays. 1999;11:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Blyth H., Ockenden B.G. Polycystic disease of kidneys and liver presenting in childhood. J. Med. Genet. 1971;8:257–284. doi: 10.1136/jmg.8.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-Z., Vassilev P.M., Basora N., Peng J.-B., Nomura H., Segal Y., Brown E.M., Reeders S.T., Hediger M.A., Zhou J. Polycystin-l is a calcium-regulated cation channel permeable to calcium ions. Nature. 1999;401:383–386. doi: 10.1038/43907. [DOI] [PubMed] [Google Scholar]
- Cole B.R., Conley S.B., Stapleton F.B. Polycystic kidney disease in the first year of life. J. Pediatr. 1987;111:693–699. doi: 10.1016/s0022-3476(87)80244-5. [DOI] [PubMed] [Google Scholar]
- Cole D.G. Kinesin-II, coming and going. J. Cell Biol. 1999;147:463–466. doi: 10.1083/jcb.147.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.G., Diener D.R., Himelblau A.L., Beech P.L., Fuster J.C., Rosenbaum J.L. Chlamydomonas kinesin-II–dependent intraflagellar transport (IFT)IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet J., Spike C.A., Lundquist E.A., Shaw J.E., Herman R.K. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans . Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.K., Cohen P.W., Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5implications for TPR-mediated protein–protein interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood P.R., Totland G.K. Substructure of solitary cilia in mouse kidney. Cell Tissue Res. 1977;183:281–290. doi: 10.1007/BF00226625. [DOI] [PubMed] [Google Scholar]
- Gunning M.E., Ingelfinger J.R., King A.J., Brenner B.M. Vasoactive peptides and the kidney. In: Brenner B.M., editor. Brenner & Rector's The Kidney. W.B. Saunders Co; Philadelphia, PA: 1996. pp. 627–712. [Google Scholar]
- Handel M., Schulz S., Stanarius A., Schreff M., Erdtmann-Vourliotis M., Schmidt H., Wolf G., Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Harris E.H. The Chlamydomonas Sourcebook 1989. Academic Press, Inc; San Diego, CA: pp. 780 pp [DOI] [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and holeessential repeats in S. pombe nuclear protein nuc2+ Cell. 1990;60:319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Hoops H.J., Witman G.B. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J. Cell Biol. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Ward C.J., Peral B., Aspinwall R., Clark K., San Millan J.L., Gamble V., Harris P.C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- Kindle K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii . Proc. Nat. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K.G., Beech P.L., Rosenbaum J.L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K.G., Johnson K.A., Forscher P., Rosenbaum J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Nat. Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J.R., Tugendreich S., Hieter P. Tetratrico peptide repeat interactionsto TPR or not to TPR? Trends Biochem. Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Levine R.P., Ebersold W.T. The genetics and cytology of Chlamydomonas . Annu. Rev. Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Lewis J.A., Hodgkin J.A. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans . J. Comp. Neurol. 1977;172:489–510. doi: 10.1002/cne.901720306. [DOI] [PubMed] [Google Scholar]
- Marszalek J.R., Ruiz-Lozano P., Roberts E., Chien K.R., Goldstein L.S.B. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl. Acad. Sci. USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek J.R., Goldstein L.S. Understanding the functions of kinesin-II. Biochim. Biophys. Acta. 2000;1496:142–150. doi: 10.1016/s0167-4889(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Marszalek J.R., Liu X., Roberts E.A., Chui D., Marth J.D., Williams D.S., Goldstein L.S. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- McManus W.R., McMahon D.J., Oberg C.J. High-resolution scanning electron microscopy of milk productsa new sample preparation procedure. Food Struct. 1993;12:475–482. [Google Scholar]
- Motta P., Fumagalli G. Scanning electron microscopy demonstration of cilia in rat intrahepatic bile ducts. Z. Anat. Entwickl. Gesch. 1974;145:223–226. doi: 10.1007/BF00519634. [DOI] [PubMed] [Google Scholar]
- Moyer J.H., Lee-Tischler M.J., Kwon H.-Y., Schrick J.J., Avner E.D., Sweeney W.E., Godfrey V.L., Cacheiro N.L., Wilkinson J.E., Woychik R.P. Candidate gene associated with a mutation causing recessive polycistic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- Murcia N.S., Richards W.G., Yoder B.K., Mucenski M.L., Dunlap J.R., Woychik R.P. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development (Camb.). 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Muresan V., Lyass A., Schnapp B.J. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J. Neurosci. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Tanaka Y., Okada Y., Takada S., Harada A., Kanai Y., Kido M., Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking kif3b motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Orozco J.T., Wedaman K.P., Signor D., Brown H., Rose L., Scholey J.M. Movement of motor and cargo along cilia. Nature. 1999;398:674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- Pazour G.J., Dickert B.L., Witman G.B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Sineschekov O.A., Witman G.B. Mutational analysis of the phototransduction pathway of Chlamydomonas reinhardtii . J. Cell Biol. 1995;131:427–440. doi: 10.1083/jcb.131.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Wilkerson C.G., Witman G.B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J. Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Witman G.B. Forward and reverse genetic analysis of microtubule motors in Chlamydomonas . Methods. 2000;In press doi: 10.1006/meth.2000.1081. [DOI] [PubMed] [Google Scholar]
- Pennarun G., Escudier E., Chapelin C., Bridoux A.M., Cacheux V., Roger G., Clement A., Goossens M., Amselem S., Duriez B. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am. J. Hum. Genet. 1999;65:1508–1519. doi: 10.1086/302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L.A., Hedgecock E.M., Thomson J.N., Culotti J.G. Mutant sensory cilia in the nematode Caenorhabditis elegans . Dev. Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Mead K., Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1Fla10 to reach the distal part of the flagella in Chlamydomonas . J. Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M.E., Bower R., Knott J.A., Byrd P., Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas . Mol. Biol. Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F., Germino F.J., Cai Y., Zhang X., Somlo S., Germino G.G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J.L., Cole D.G., Diener D.R. Intraflagellar transportthe eyes have it. J. Cell Biol. 1999;144:385–388. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K.E., Rieder C.L., Bowser S.S. Flexible-substratum technique for viewing cells from the sidesome in vivo properties of primary (9+0) cilia in cultured kidney epithelia. J. Cell Sci. 1988;89:457–466. doi: 10.1242/jcs.89.4.457. [DOI] [PubMed] [Google Scholar]
- Signor D., Wedaman K.P., Orozco J.T., Dwyer N.D., Bargmann C.I., Rose L.S., Scholey J.M. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans . J. Cell Biol. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Yonekawa Y., Tanaka Y., Okada Y., Nonaka S., Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3Anew insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J. Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Polycystic Kidney Disease Consortium Polycystic kidney diseasethe complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL Wimproving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L., Kim E., Arnould T., Sukhatme V.P., Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Nat. Acad. Sci. USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D.N. Primary cilia in normal and pathological tissues. Pathobiology. 1995;63:222–238. doi: 10.1159/000163955. [DOI] [PubMed] [Google Scholar]
- Wheatley D.N., Wang A.M., Strugnell G.E. Expression of primary cilia in mammalian cells. Cell Biol. Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans . Phil. Trans. R. Soc. Lond. B Biol. Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- Whitehead J.L., Wang S.Y., Bost-Usinger L., Hoang E., Frazer K.A., Burnside B. Photoreceptor localization of the KIF3A and KIF3B subunits of the heterotrimeric microtubule motor kinesin II in vertebrate retina. Exp. Eye Res. 1999;69:491–503. doi: 10.1006/exer.1999.0724. [DOI] [PubMed] [Google Scholar]
- Wicks S.R., de Vries C.J., van Luenen H.G.A.M., Plasterk R.H.A. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans . Dev. Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- Wilkerson C.G., King S.M., Koutoulis A., Pazour G.J., Witman G.B. The 78,000 Mr intermediate chain of Chlamydomonas outer arm dynein is a WD-repeat protein required for arm assembly. J. Cell Biol. 1995;129:169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B.K., Richards W.G., Sommardahl C., Sweeney W.E., Michaud E.J., Wilkinson J.E., Avner E.D., Woychik R.P. Functional correction of renal defects in a mouse model for ARPKD through expression of the cloned wild-type Tg737 cDNA. Kidney Int. 1996;50:1240–1248. doi: 10.1038/ki.1996.433. [DOI] [PubMed] [Google Scholar]
- Yoder B.K., Richards W.G., Sommardahl C., Sweeney W.E., Michaud E.J., Wilkinson J.E., Avner E.D., Woychik R.P. Differential rescue of the renal and hepatic disease in an autosomal recessive polycystic kidney disease mouse mutant. Am. J. Pathol. 1997;150:2231–2241. [PMC free article] [PubMed] [Google Scholar]