Abstract
Phosphorylation on Ser 19 of the myosin II regulatory light chain by myosin light chain kinase (MLCK) regulates actomyosin contractility in smooth muscle and vertebrate nonmuscle cells. The smooth/nonmuscle MLCK gene locus produces two kinases, a high molecular weight isoform (long MLCK) and a low molecular weight isoform (short MLCK), that are differentially expressed in smooth and nonmuscle tissues. To study the relative localization of the MLCK isoforms in cultured nonmuscle cells and to determine the spatial and temporal dynamics of MLCK localization during mitosis, we constructed green fluorescent protein fusions of the long and short MLCKs. In interphase cells, localization of the long MLCK to stress fibers is mediated by five DXRXXL motifs, which span the junction of the NH2-terminal extension and the short MLCK. In contrast, localization of the long MLCK to the cleavage furrow in dividing cells requires the five DXRXXL motifs as well as additional amino acid sequences present in the NH2-terminal extension. Thus, it appears that nonmuscle cells utilize different mechanisms for targeting the long MLCK to actomyosin structures during interphase and mitosis. Further studies have shown that the long MLCK has twofold lower kinase activity in early mitosis than in interphase or in the early stages of postmitotic spreading. These findings suggest a model in which MLCK and the myosin II phosphatase (Totsukawa, G., Y. Yamakita, S. Yamashiro, H. Hosoya, D.J. Hartshorne, and F. Matsumura. 1999. J. Cell Biol. 144:735–744) act cooperatively to regulate the level of Ser 19–phosphorylated myosin II during mitosis and initiate cytokinesis through the activation of myosin II motor activity.
Keywords: myosin, phosphorylation, myosin light chain kinase, cell division, cytokinesis
Introduction
Smooth muscle and vertebrate nonmuscle myosin II are regulated by phosphorylation on the 20-kD regulatory light chain (RLC). The Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) phosphorylates the RLC primarily on Ser 19 and to a lesser extent on Thr 18 (Sellers et al. 1981), whereas protein kinase C (PKC) phosphorylates Ser 1/2 and Thr 9 (Ikebe et al. 1987). Phosphorylation on the MLCK sites promotes filament assembly in vitro (Suzuki et al. 1978), enhances the actin-activated ATPase activity of myosin II (Sellers et al. 1981), and is essential for the movement of actin filaments in vitro (Sellers et al. 1985). PKC phosphorylation directly inhibits the actin-activated ATPase of myosin II by reducing its affinity for actin and decreasing the rate of phosphorylation of the RLC by MLCK (Nishikawa et al. 1984b; Turbedsky et al. 1997). These findings establish that distinct phosphorylation events on the RLC control the activity of smooth muscle and vertebrate nonmuscle myosin II in vitro.
In higher eukaryotes, it is well-established that the phosphorylation state of the RLC is cell cycle dependent. In metaphase, the RLC is phosphorylated predominantly on Ser 1/2. However, as the cell cycle progresses through anaphase, the RLC is dephosphorylated on these sites and phosphorylated on Ser 19 (Satterwhite et al. 1992; Yamakita et al. 1994). Furthermore, a Ser 19–specific biosensor (DeBiasio et al. 1996) and antibodies specific for Ser 19 (Matsumura et al. 1998) demonstrate that Ser 19 phosphorylation immediately precedes the formation of the cleavage furrow. These findings suggest that myosin II activity is regulated temporally during mitosis, and the stimulation of myosin II motor activity by Ser 19 phosphorylation could provide the stimulus for the initiation of cytokinesis. Although cdc2 kinase has been implicated in phosphorylating Ser 1/2 (Satterwhite et al. 1992), the kinase that mediates phosphorylation of Ser 19 during mitosis has not been identified.
In nonmuscle cells, Ser 19 phosphorylation of myosin II correlates with stress fiber formation, capping of cell surface receptors, and cytokinesis (Majercik and Bourguignon 1988; Yamakita et al. 1994; Goeckeler and Wysolmerski 1995; DeBiasio et al. 1996; Matsumura et al. 1998). However, the role of MLCK in these processes remains largely uncharacterized. In fibroblasts, inhibition of MLCK by pAbs induces stress fiber disassembly and dephosphorylation of the RLC (Lamb et al. 1988). Similarly, a reduction in MLCK expression via antisense techniques causes cell rounding and decreased proliferation (Shoemaker et al. 1990). Finally, the inhibition of MLCK, either by an inhibitory peptide or through antisense expression, blocks the extension of lamellipodia and inhibits chemoattractant-stimulated cell locomotion (Walker et al. 1998; Kishi et al. 2000). Taken together, these observations directly implicate MLCK in the signaling pathways that mediate cell motility and cell morphology.
In vertebrates, the smooth/nonmuscle MLCK gene locus produces at least three proteins: high molecular weight (long MLCK) and low molecular weight (short MLCK) kinases with calculated masses of ∼210,000 D and 108,000–125,000 D, respectively (Fig. 1), and a catalytically inactive protein, called telokin, that lacks the kinase domain (Birukov et al. 1998). The long MLCK is identical to the short MLCK, with the exception of a novel NH2-terminal extension of 922–934 residues (Watterson et al. 1995; Garcia et al. 1997), whereas telokin is identical to the COOH-terminal domain of both MLCKs. The short MLCK is expressed in both smooth and nonmuscle tissues, whereas the long MLCK is expressed in a subset of these tissues and is the predominant isoform expressed in cultured nonmuscle cells (Gallagher et al. 1995).
Figure 1.
Schematic of the MLCK isoforms and MLCK truncations. The long MLCK is identical to the short MLCK, with the exception of a novel NH2-terminal extension of 934 residues. The hatched boxes represent the six IgG-C2 motifs present in the NH2-terminal extension, and the three IgG-C2 motifs present in the short MLCK. The black boxes represent the DXRXXL motifs that span the NH2-terminal extension/short MLCK junction.
To characterize the regulatory mechanisms mediating Ser 19 phosphorylation of the RLC during mitosis, we prepared green fluorescent protein (GFP) fusions of the two MLCK isoforms. In HeLa cells, the long MLCK demonstrated cell cycle–dependent localization to specific actomyosin-containing structures, which involved multiple molecular determinants, whereas the short MLCK distribution was uniform and diffuse. Furthermore, the activity of the long MLCK is cell cycle regulated, implicating MLCK as the kinase that mediates Ser 19 phosphorylation during cell division.
Materials and Methods
Cell Culture
PtK2 (CCL-56; American Type Culture Collection) cells were maintained in MEM containing 0.1 mM nonessential amino acids and 10% FBS. HeLa cells were maintained in DMEM containing 10% FBS. Rat thoracic aorta A10 cells (CRL-1476; American Type Culture Collection) were maintained in DMEM containing 10% FBS. Cells were grown at 37°C in a humidified incubator with a 5% CO2 atmosphere. HeLa cells were synchronized for 16 h in the presence of 250 or 50 ng/ml nocodazole, and mitotic cells were collected by selective detachment. Before lysis, nocodazole was removed by washing the cells two times with 10 ml of ice-cold PBS (1.5 mM KH2PO4, 8 mM Na2HPO4 · 7H2O, 2.6 mM KCl, 137 mM NaCl).
Antibodies
Chicken smooth muscle MLCK (short MLCK) was purified as described by Conti and Adelstein 1991. Rabbit pAbs to the short MLCK were prepared following the protocol of Fujiwara and Pollard 1976. Purified rabbit IgG antibodies were purchased from Sigma-Aldrich. The MLCK antibodies were purified by affinity chromatography on a column of Sepharose 4B (Sigma-Aldrich), activated with cyanogen bromide (March et al. 1974), and coupled with the chicken smooth muscle MLCK. The MLCK mAb (clone K36) was purchased from Sigma-Aldrich.
Construction of MLCK-GFP Fusions
The avian full-length long MLCK was prepared from three overlapping clones: 7N-32-R-45D, which contains 47 nucleotides of the 5′ untranslated region and nucleotides 1–1276; 7N-32-R-45A, which contains nucleotides 1276–3075; and pC3/13, which contains nucleotides 2549–5718, the stop codon, and the 3′ untranslated region (Shoemaker et al. 1990; Watterson et al. 1995). The full-length long MLCK cDNA was constructed in pUC19 and the DNA sequence was confirmed by sequencing. To prepare the long MLCK-GFP fusion, full-length long MLCK was subcloned into the BamHI/EcoRI sites of pEGFP-N1 (CLONTECH Laboratories, Inc.). The final long MLCK-GFP construct contains seven additional amino acids at the COOH terminus (Gly-Asp-Pro-Pro-Val-Ala-Thr), followed by GFP. The avian short MLCK-GFP fusion and avian short MLCK-GFP fusion containing all five DXRXXL motifs were prepared in the same manner. The NH2-terminal extension (amino acid residues 1–934) and the NH2-terminal extension containing all five DXRXXL motifs were subcloned into the HindIII/BamH1 sites of pEGFP-C3 (CLONTECH Laboratories, Inc.). The final constructs contain GFP with eight additional amino acids (Tyr-Ser-Asp-Leu-Glu-Leu-Lys-Leu) and MLCK. The rabbit short MLCK-GFP construct was prepared as described in Lin et al. 1999.
Immunoblotting and Immunoprecipitation
For immunoblot analysis, cells were washed with PBS, scraped directly into ice-cold NP-40 lysis buffer (1% NP-40, 10 mM MOPS, pH 7.0, 5 mM EDTA, 0.5 mM EGTA, 1 mM DTT, 50 mM MgCl2, 300 mM NaCl, 1 mM PMSF, 50 μg/ml each of leupeptin, chymostatin, and pepstatin A), and centrifuged at 4°C for 5 min at 16,000 g. SDS-PAGE sample buffer was added to the supernatant, which was boiled and immediately loaded onto a 6% SDS-polyacrylamide gel for electrophoresis (Laemmli 1970). Proteins were transferred to nitrocellulose and reacted with antibodies to MLCK. Immunoreactive proteins were detected using the ECL chemiluminescent detection system (Amersham Pharmacia Biotech). To measure the relative amounts of the long MLCK and short MLCK in cells, autoradiograms were scanned on a Molecular Dynamics densitometer and quantified using the program ImageQuant v5.0.
The long MLCK was immunoprecipitated from PtK2 or HeLa cell extracts by the method of Gallagher et al. 1995. In brief, cells from a 100-mm dish were scraped into 350 μl of the NP-40 lysis buffer and clarified by centrifugation at 4°C for 15 min at 16,000 g. The supernatant was diluted 1:4 in 20 mM MOPS, pH 7.0, 10 mM magnesium acetate, and protease inhibitors (dilution buffer), as described above. Before the immunoprecipitation, rabbit nonimmune IgG or the MLCK mAb was bound to protein A–Sepharose (Sigma-Aldrich) in PBS (10 mM NaPO4, pH 7.5, 150 mM NaCl, 1 mM EGTA), containing 1 mg/ml BSA. The diluted supernatant was incubated with the antibody-protein A–Sepharose for 2 h at 4°C. Immune complexes were collected by centrifugation, washed three times in wash buffer (20 mM MOPS, pH 7.0, 10 mM magnesium acetate, 100 mM NaCl, 0.5 mM DTT), and used in phosphorylation assays.
For immunoprecipitation of MLCK-GFP fusion proteins, cells in 100-mm dishes were transfected with DNA encoding the long or short MLCK-GFP. After 40 h posttransfection, cells were scraped into 200 μl of the NP-40 lysis buffer containing a 1:100 dilution of phosphatase inhibitor cocktail 1 (microcystin LR, cantharidin, and [-]-p-bromotetramisole) and phosphatase inhibitor cocktail 2 (sodium vanadate, sodium molybdate, sodium tartrate, and imidazole) (Sigma-Aldrich) and clarified by centrifugation at 4°C for 15 min at 16,000 g. The supernatant was diluted 1:4 in dilution buffer, and the GFP fusions were immunoprecipitated with a GFP pAb (Santa Cruz Biotechnology, Inc.) coupled to protein A–Sepharose.
Interphase, metaphase, and cytokinetic HeLa cell extracts were prepared by resuspending cells in the NP-40 lysis buffer containing phosphatase inhibitor cocktails 1 and 2 (see above) (Sigma-Aldrich) and clarified by centrifugation at 4°C for 15 min at 16,000 g. To compare the activity of the endogenous long MLCK from HeLa interphase and metaphase extracts, the long MLCK was immunoprecipitated from equivalent amounts of cellular extract (0.5 mg for assays using the RLC as a substrate, and 1.0 mg for assays using heavy meromyosin [HMM] as a substrate), with the MLCK mAb coupled to protein A–Sepharose. Immunoprecipitates were washed and assayed as described above. In some experiments, immunoprecipitates were washed with phosphatase buffer (20 mM Tris-HCl, pH 8.2, 50 mM NaCl, 1 mM MnCl2, 2 mM MgCl2, 0.2 mM DTT, 0.2 mg/ml BSA) and treated with 5 U of calf intestinal alkaline phosphatase (GIBCO BRL) for 15 min at 30°C. After alkaline phosphatase treatment, MLCK immunoprecipitates were washed with wash buffer and assayed for activity. To assay the activity of the long MLCK during cytokinesis/early postmitotic spreading, synchronized cells were washed and replated without nocodazole. Approximately 65% of the cells had nearly completed cell division 85 min later and were in the early stages of postmitotic spreading (our unpublished observations). These cells were lysed, and the endogenous HeLa long MLCK were immunoprecipitated, as described above.
Phosphorylation Assays
Immunoprecipitates from PtK2 cells were resuspended in a kinase reaction mixture containing 10 mM MOPS, pH 7.0, 1 mM DTT, 4 mM MgCl2, 0.1 mM CaCl2, 1 μM calmodulin, 0.1 mM [γ32P]ATP, and 15 μM purified Xenopus RLCs (Bresnick et al. 1995) at 25°C. HeLa interphase and mitotic long MLCK immunoprecipitates were assayed as described above, except that 5 μM purified Xenopus RLCs or 2 μM chicken smooth muscle HMM (Sellers et al. 1981) was used in the reaction mix. Aliquots were removed at different intervals and spotted onto 1 × 1–cm P81 squares of phosphocellulose. The squares were washed 10 times with 100 ml of 75 mM phosphoric acid and measured for incorporation of 32P using an LKB 1219 Rackbeta scintillation counter. Control reactions lacking substrate indicated that autophosphorylation of the long MLCK was not detectable in these assays.
For the phosphorylation of myosin II, immunoprecipitates from PtK2 cells were resuspended in the kinase reaction mixture described above, except the mix contained 1 μM purified chicken smooth muscle myosin II at 30°C. The reaction was stopped by the addition of an equal volume of Laemmli sample buffer (1970), and the samples were boiled and then separated on a 12% SDS-polyacrylamide gel. The gel was stained, dried, and the RLC was measured for 32P incorporation.
GFP immunoprecipitates from HeLa cells were resuspended in a kinase reaction mixture, as described above, containing 5 μM purified Xenopus RLCs for 40 min at 25°C.
Transfections and Microscopy
Cells were transfected with the MLCK-GFP constructs using the calcium phosphate method or lipofectin (GIBCO BRL). Cells were fixed 48 h after transfection in freshly prepared 4% formaldehyde in PBS for 15 min at room temperature. After rinsing the cells in an excess of PBS containing 0.02% sodium azide, the coverslips were mounted in Pro-Long Antifade (Molecular Probes). Transfected HeLa cells were permeabilized according to the method of Lin et al. 1997. In brief, cells were cooled to 4°C, washed with ice-cold PBS, and extracted for 10 min with ice-cold 10 mM Tris-HCl, pH 7.0, 60 mM KCl, 125 mM sucrose, and 0.05% Triton X-100. Cells were washed three times with ice-cold 10 mM Tris-HCl, pH 7.0, 30 mM KCl, 5 mM MgCl2, and 1 μM CaCl2. Images were acquired using Espirit Image Analysis software (Life Sciences Resources) with an FKI 1000 interline 12-bit, cooled CCD camera (Life Sciences Resources) mounted on an Olympus IX70 microscope with a PlanApo 60×, 1.4 N.A. objective (Olympus) and HiQ bandpass filters (Chroma Technology Corp.). Images were imported into I.P. Lab Spectrum (Scanalytics) and deconvolved with Vaytek Power Hazebuster software. Images were processed using Adobe Photoshop® software (Adobe Systems).
Results
Expression of MLCK Isoforms in Cultured Cells
pAbs to the short MLCK confirmed previous observations that rat smooth muscle A10 cells express both the long and short MLCK isoforms (Fig. 2) (Gallagher et al. 1995). An MLCK mAb, whose epitope resides between residues 29 and 80 of the short MLCK (Gallagher et al. 1995), recognized both the long and short MLCKs in A10 cells (Fig. 2). In contrast, Gallagher et al. 1995 did not detect cross-reaction of the mAb with the long MLCK in A10 cells. Immunoblot analysis of detergent extracts from PtK2 and HeLa cells with the mAb demonstrated that both cell lines express the long MLCK (Fig. 2). We did not detect the short MLCK with the mAb in these extracts. However, immunoblots with our MLCK pAb revealed that HeLa cells also express low levels of the short MLCK. The apparent discrepancy in the reactivity of the mAb to the short compared with the long MLCKs is likely due to limited proteolysis and loss of the reactive epitope on the immediate NH2 terminus, as the short MLCK is known to be highly susceptible to degradation during purification (Adelstein and Klee 1981). Densitometric analysis indicates that in HeLa cells, the long MLCK is expressed at approximately threefold higher levels than the short MLCK.
Figure 2.
Immunoblot analysis of MLCK expression in cultured cell lines with smooth muscle MLCK (short MLCK) antibodies. The short MLCK has an apparent molecular mass of ∼130 kD on SDS-PAGE.
Phosphorylation of Myosin II by the Long MLCK
The avian long and short MLCKs have been reported to have specific activities of 0.8 μmol Pi/min·mg and 1.6 μmol Pi/min·mg, respectively (Kudryashov et al. 1999), and the apparent steady-state kinetic measurements indicate that the long and short MLCKs have comparable K m and V max values (Garcia, J.G.N. [Johns Hopkins University School of Medicine, Baltimore, MD], personal communication). Our results are consistent with these findings, as the substrate specificity of the long MLCK is identical to that observed for the short MLCK. The long MLCK primarily phosphorylates Ser 19 in a Ca2+/calmodulin-dependent manner, since phosphorylation is not detected in the presence of EGTA or with RLCs containing alanine substitutions at Ser 19 or at both Ser 19 and Thr 18 (Fig. 3 A). These findings agree with previous studies showing that phosphorylation at Thr 18 requires high concentrations of MLCK (Ikebe et al. 1986). In the context of the intact myosin II molecule, the long MLCK phosphorylated the RLC to a maximal extent of 0.89 mol phosphate/mol RLC in the presence of Ca2+/calmodulin. This was reduced to 0.034 mol phosphate/mol RLC in the presence of EGTA (Fig. 3 B). Immunoprecipitates with rabbit nonimmune IgG incorporated 0.032 mol phosphate/mol RLC. Immunoprecipitates of the long MLCK from HeLa cells also phosphorylated the wild-type RLC to 0.8 mol phosphate/mol RLC and HMM to 0.6 mol phosphate/mol RLC in a Ca2+/calmodulin-dependent manner.
Figure 3.

Phosphorylation of the RLC by the immunoprecipitated long MLCK. (A) Ca2+/calmodulin-dependent phosphorylation of the free RLC by the long MLCK immunoprecipitated from PtK2 cells. Symbols are as follows: •, wild-type RLC in the presence of Ca2+/calmodulin; ▵, wild-type RLC in the presence of Ca2+/calmodulin and 2 mM EGTA; ▪, T18AS19A RLC in the presence of Ca2+/calmodulin; and ▿, S19A RLC in the presence of Ca2+/calmodulin. Values represent the mean of five to six independent experiments. Error bars for assays using the T18AS19A and S19A RLCs or the wild-type RLC in the presence of EGTA are contained within the height of the symbol and cannot be seen. (B) Ca2+/calmodulin-dependent phosphorylation of myosin II by the long MLCK immunoprecipitated from PtK2 cells. SDS-polyacrylamide gels of myosin II stained with Coomassie blue and corresponding autoradiograms are shown. The 55- and 25-kD bands correspond to the heavy and light chains of the antibody, respectively. The molecular mass standards are in kilodaltons.
Distribution of the MLCK Isoforms in Interphase Cells
To study the localization of the long and short MLCKs in HeLa cells, we constructed GFP fusions for both MLCK isoforms. Immunoblot analysis with antibodies to GFP and MLCK demonstrated that the full-length long and short MLCK-GFP fusions are expressed in HeLa cells (Fig. 4 A). The immunoprecipitated long and short MLCK-GFPs incorporated 0.68–1.03 mol phosphate/mol RLC in the presence of Ca2+/calmodulin and 0.022 mol phosphate/mol RLC in the presence of EGTA (Fig. 4 B). Immunoprecipitates from untransfected cells incorporated 0.023 mol phosphate/mol RLC in the presence or absence of EGTA. In addition, the long and short MLCK-GFP fusions stoichiometrically phosphorylated myosin II in a Ca2+/calmodulin-dependent manner (data not shown). These results indicate that attachment of GFP to the COOH terminus of the avian MLCKs leaves the kinase activity intact, which has been observed previously for GFP fusions of the rabbit short smooth muscle MLCK (Lin et al. 1999).
Figure 4.

Expression and activity of the MLCK-GFP fusion proteins. (A) Whole cell lysates of HeLa cells expressing the long, short, and truncated MLCK-GFP fusions are shown. Lanes: 1, avian long MLCK; 2, avian short MLCK; 3, avian NH2-terminal extension; 4, avian NH2-terminal extension containing all five DXRXXL motifs; and 5, avian short MLCK containing all five DXRXXL motifs. (B) MLCK-GFP fusions were immunoprecipitated with a GFP pAb from untransfected, or long MLCK-GFP and short MLCK-GFP transfected, HeLa cells. Both the long and short MLCK-GFPs phosphorylated the RLC in a Ca2+/calmodulin-dependent manner.
Using the avian long MLCK-GFP construct, provided by our laboratory, and an antibody that recognizes only the long MLCK, Kudryashov et al. 1999 demonstrated that the long MLCK localizes primarily to stress fibers. We observed a similar localization for the long MLCK in HeLa cells (Fig. 5), and, in addition, found that the long MLCK localized to cortical actin filaments (arrows) and microspikes (arrowheads). In contrast, the short MLCK was primarily distributed throughout the cytoplasm, with some concentration in the lamellae. Even cells expressing low levels of the short MLCK displayed a predominantly cytoplasmic localization. The localization of the rabbit homologue of the short MLCK was indistinguishable from the avian short MLCK (Fig. 5). The localization of the long and short MLCK isoforms was distinct from that of GFP alone, which was found throughout the nucleus and cytoplasm, with no apparent concentration in stress fibers or lamellae. In chicken hepatoma DU24 cells, the long and short MLCKs had the same distribution as in HeLa cells (data not shown). When transfected cells were permeabilized with Triton X-100, only the long MLCK displayed significant association with stress fibers (Fig. 5). In permeabilized cells expressing the avian and rabbit short MLCKs, the diffuse cytoplasmic fluorescence disappeared, revealing a minor association of the short MLCKs with cortical stress fibers.
Figure 5.
Distribution of the long and short MLCK isoforms in interphase cells. Fluorescence micrographs of HeLa cells transfected with the avian long MLCK-GFP, the avian short MLCK-GFP, the rabbit short MLCK-GFP, and GFP alone are shown. The top row shows representative images of intact cells. The bottom row shows representative images of Triton X-100–permeabilized cells. Arrows indicate cortical actin filaments; arrowheads indicate microspikes. Bar, 10 μm.
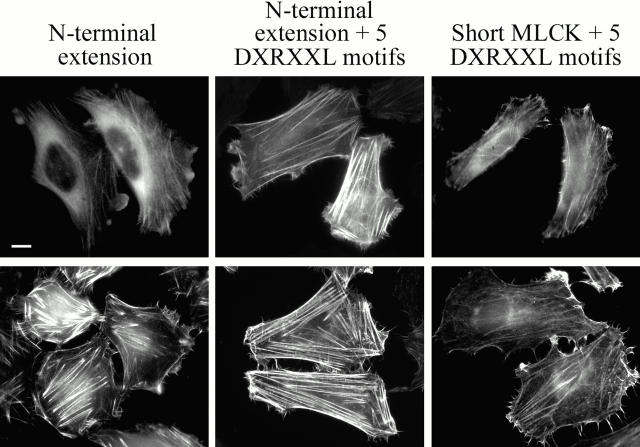
To identify domains involved in targeting the long MLCK to stress fibers, we expressed GFP-tagged MLCK truncations in HeLa cells. The long MLCK contains five DXRXXL motifs that span the junction of the NH2-terminal extension and the short MLCK, with two DXRXXL motifs in the COOH terminus of the NH2-terminal extension, and three DXRXXL motifs in the NH2 terminus of the short MLCK (see Fig. 1). The NH2-terminal extension, which contains two DXRXXL motifs, was localized to stress fibers with some diffuse cytoplasmic staining. However, when the NH2-terminal extension contained all five DXRXXL motifs, it concentrated predominantly on stress fibers with very little cytoplasmic localization (Fig. 6). After permeabilization with Triton X-100, the NH2-terminal extension and NH2-terminal extension containing all five DXRXXL motifs displayed prominent localization to stress fibers. Similarly, the short MLCK containing all five DXRXXL motifs was associated with stress fibers, which was more apparent in Triton X-100–permeabilized cells (Fig. 6). These data suggest that the five DXRXXL motifs are sufficient to target the long MLCK to stress fibers during interphase.
Figure 6.
Distribution of truncated MLCKs during interphase. Fluorescence micrographs are of HeLa cells transfected with the NH2-terminal extension, NH2-terminal extension containing all five DXRXXL motifs, and the avian short MLCK-GFP containing all five DXRXXL motifs. The top row shows representative images of intact cells. The bottom row shows representative images of Triton X-100–permeabilized cells. Bar, 10 μm.
Distribution of the MLCK Isoforms in Dividing Cells
In all stages of mitosis, the avian and rabbit short MLCKs were distributed uniformly throughout the cytoplasm and excluded from the cell cortex (Fig. 7). The localization of the short MLCKs was indistinguishable from that of GFP alone (Fig. 7), suggesting that the short isoforms do not target to specific cytoskeletal structures in dividing cells. In contrast, the long MLCK was consistently enriched in the cell cortex during metaphase, and concentrated in the cleavage furrow throughout anaphase and telophase (Fig. 7). The NH2-terminal extension alone did not localize to the cleavage furrow of dividing cells. However, the NH2-terminal extension construct containing the additional three DXRXXL motifs, normally found in the short MLCK, concentrated in the cleavage furrow and had a localization that was identical to that observed for the full-length long MLCK in all stages of mitosis (Fig. 8). The short MLCK containing all five DXRXXL motifs did not localize to the cleavage furrow (Fig. 8), suggesting that these motifs alone are insufficient to target the long MLCK to the contractile ring during mitosis. These observations indicate that nonmuscle cells utilize different mechanisms for targeting the long MLCK to specific actin-containing structures.
Figure 7.

Distribution of the long and short MLCK isoforms during mitosis. Fluorescence micrographs are of dividing HeLa cells transfected with either the avian long MLCK-GFP, the avian short MLCK-GFP, the rabbit short MLCK-GFP, or GFP alone. The images are representative of cells (from the top row) in metaphase, early anaphase, late anaphase, and telophase. Bar, 10 μm.
Figure 8.
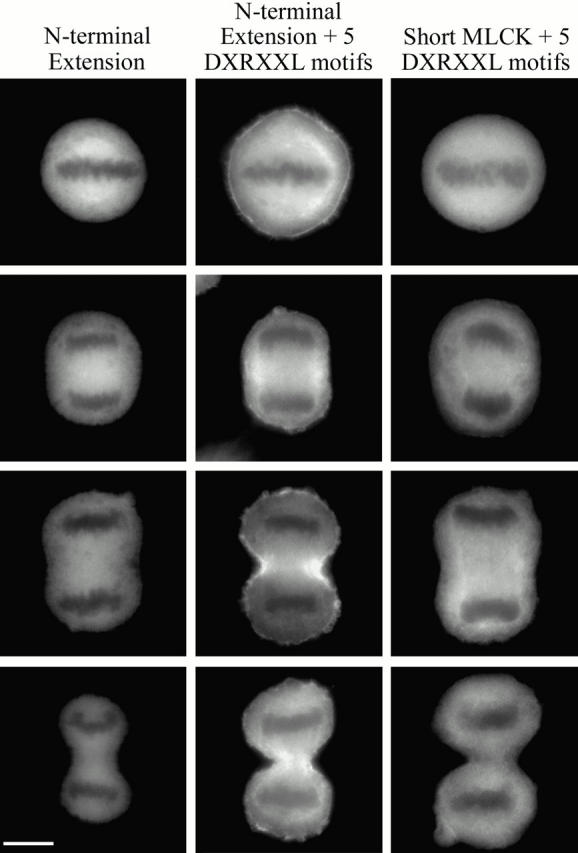
Distribution of truncated MLCKs during mitosis. Fluorescence micrographs are of HeLa cells transfected with the NH2-terminal extension, NH2-terminal extension containing all five DXRXXL motifs, and the avian short MLCK-GFP containing all five DXRXXL motifs. The images are representative of cells (from the top row) in metaphase, early anaphase, late anaphase, and telophase. Bar, 10 μm.
Deconvolution of conventional fluorescence micrographs revealed a rim of long MLCK staining underneath the plasma membrane in metaphase cells, which persisted through telophase (Fig. 9). This cortical layer of long MLCK was ∼0.6 μm thick and uniform along the cell circumference. We did not detect any enrichment of the short MLCK in the cell cortex, suggesting that this localization is specific for the long MLCK.
Figure 9.
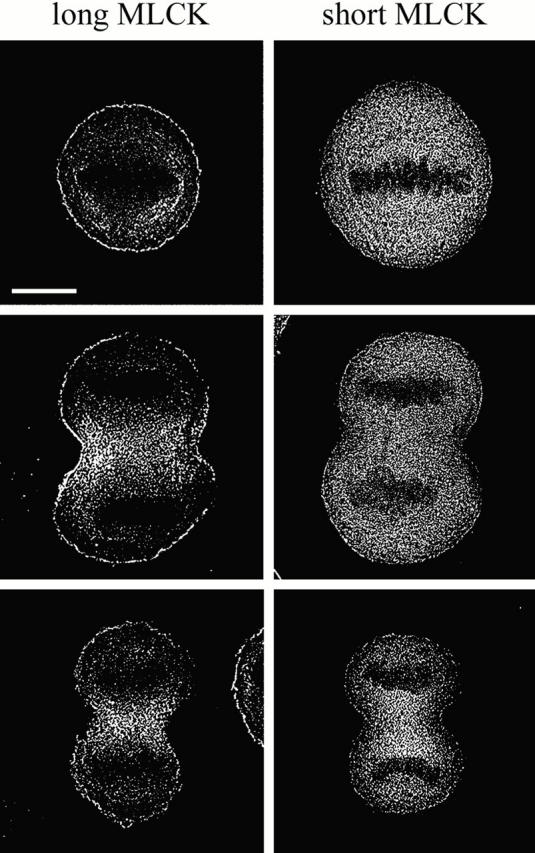
Deconvolved fluorescence micrographs of HeLa cells expressing the avian long or short MLCK-GFPs. The images are representative of cells in metaphase, anaphase, and telophase. Bar, 10 μm.
Cell Cycle Regulation of the Long MLCK
Ser 19 phosphorylation on the RLC of myosin II is first observed during anaphase (Yamakita et al. 1994; DeBiasio et al. 1996; Matsumura et al. 1998), suggesting that the activity of the kinases and phosphatases that mediate phosphorylation on this site are regulated during mitosis. To further investigate the role of MLCK during cytokinesis, we examined the activity of MLCK during the cell cycle. Immunoblot and densitometric analyses indicate that the long and short MLCK protein levels remained constant throughout the cell cycle (data not shown). The activity of the long MLCK was monitored across the cell cycle with the mAb that specifically immunoprecipitated the long MLCK from HeLa cells (Fig. 10 A). Analysis of the extract supernatants demonstrated that the immunoprecipitations were quantitative, bringing down all of the long MLCK present in the interphase, metaphase, and cytokinetic extracts (data not shown). In addition, immunoblot analysis and densitometry confirmed that equivalent amounts of the long MLCK were present in these immunoprecipitates (Fig. 10 B). At saturating calmodulin concentrations, the interphase long MLCK phosphorylated the RLC at a rate of 1.67 pmol phosphate/unit MLCK·min, whereas the metaphase long MLCK phosphorylated the RLC at a rate of 0.84 pmol phosphate/unit MLCK·min (Fig. 10 C and Table ). Using HMM as a substrate, we observed qualitatively similar differences in the activities of the interphase and metaphase long MLCKs with rates of 0.63 and 0.44 pmol phosphate/unit MLCK·min, respectively (Table ). Long MLCK immunoprecipitates from cells that had almost completed cytokinesis and were in the early stages of postmitotic spreading phosphorylated the RLC at a rate of 1.56 pmol phosphate/unit MLCK·min. These data demonstrate that the activity of the long MLCK is regulated in a cell cycle–dependent manner.
Figure 10.
The mitotic long MLCK displays decreased kinase activity towards the RLC. (A) The long MLCK was immunoprecipitated with the MLCK mAb and the immune complexes reacted with affinity-purified pAbs to MLCK. The bands at the bottom of the blot are the IgG heavy chains that cross-react with the secondary antibody used for chemiluminescent detection. (B) Immunoblot analysis confirmed that equivalent amounts of the long MLCK were present in the immunoprecipitates. (C) Symbols indicate the following: □, activity of the long MLCK from interphase extracts; ▪, activity of the long MLCK from interphase extracts treated with alkaline phosphatase; ○, activity of the long MLCK from mitotic extracts; and •, activity of the long MLCK from mitotic extracts treated with alkaline phosphatase. Values represent the mean and the SEM for three (with alkaline phosphatase) or eight (without alkaline phosphatase) independent experiments.
Table 1.
Cell Cycle–dependent Activity of the Long MLCK
| Interphase | Metaphase | Cytokinesis/earlypostmitotic spreading | ||||
|---|---|---|---|---|---|---|
| Phosphatase | − | + | − | + | − | + |
| RLC | 1.67 ± 0.08 | 1.57 ± 0.08 | 0.84 ± 0.07 | 1.57 ± 0.06 | 1.56 ± 0.03 | 1.59 ± 0.04 |
| HMM | 0.63 ± 0.04 | 0.63 ± 0.05 | 0.44 ± 0.02 | 0.67 ± 0.02 | ND | ND |
Units are pmol phosphate/unit MLCK·min. One unit of MLCK is defined as the amount of MLCK immunoprecipitated from 0.5 mg of extract. Values represent the mean for three to eight independent experiments.
To probe the regulatory mechanisms mediating the decreased activity of the metaphase long MLCK, we treated the immunoprecipitates with alkaline phosphatase and assayed for kinase activity. Alkaline phosphatase–treated immunoprecipitates from interphase and cytokinetic extracts phosphorylated the RLC at rates of 1.57 and 1.59 pmol phosphate/unit MLCK·min, respectively, which were comparable to the rates observed for the untreated kinase (Fig. 10 C and Table ). In contrast, the alkaline phosphatase–treated metaphase immunoprecipitate had a twofold increased kinase activity, with a rate of 1.57 pmol phosphate/unit MLCK·min for phosphorylation of the RLC (Fig. 10 C and Table ). These data indicate that a phosphorylation event modulates the activity of the long MLCK during mitosis.
Discussion
Expression and Activity of the Long MLCK
Based on immunological and biochemical characteristics, we have shown that epithelial cells express a long MLCK. In an in vitro activity assay, the long MLCK stoichiometrically phosphorylates both free wild-type RLC and intact myosin II in a Ca2+/calmodulin-dependent manner. Under the conditions of our assay, the long MLCK did not phosphorylate RLCs containing an alanine substitution at Ser 19, supporting Ser 19 as the primary site of phosphorylation by the long MLCK. However, in assays using higher concentrations of the long MLCK, we observed stoichiometries of ∼1.8 mol phosphate/mol RLC for myosin II (data not shown). These observations are consistent with in vitro studies demonstrating that higher concentrations of the tissue-purified short smooth muscle MLCK are needed to phosphorylate both Ser 19 and Thr 18 (Ikebe et al. 1986). Importantly, these findings demonstrate that the presence of the NH2-terminal extension does not effect the activity or specificity of the high molecular weight kinase.
Targeting of MLCK to Actin-containing Structures during Interphase
Previous studies using pAbs to the short smooth muscle MLCK have shown that MLCK localizes to stress fibers and the cytoplasm in interphase cells (de Lanerolle et al. 1981; Guerriero et al. 1981). As the antibodies, described by de Lanerolle et al. 1981, recognize both MLCK isoforms in COS cells (Klemke et al. 1997), and it is likely that the antibodies described by Guerriero et al. also recognize both MLCK isoforms, the individual localization of the long and short kinases could not be distinguished in these immunofluorescence studies. To study the localization of the MLCK isoforms and identify the determinants regulating MLCK localization in nonmuscle cells, we expressed GFP fusions of the MLCK isoforms. The Ca2+/calmodulin-dependent kinase activities of the long and short MLCK-GFP fusions were similar to that observed for the immunoprecipitated HeLa and PtK2 long MLCKs, as well as the tissue-purified chicken short smooth muscle MLCK. These observations indicate that the catalytic properties of the kinases are not effected by GFP; thus, the MLCK-GFP fusions are suitable reagents for assessing the localization of the MLCK isoforms in nonmuscle cells.
In interphase HeLa cells, the avian long MLCK localized to stress fibers, whereas both the avian and rabbit short MLCKs exhibited a diffuse cytoplasmic distribution. Importantly, the short MLCK displayed a diffuse distribution in cells expressing low levels of the kinase, demonstrating that this localization is not due to the saturation of endogenous kinase binding sites. This was confirmed in permeabilization studies, which showed that there is very little association of the short MLCK with stress fibers. Our results contrast with those of Lin et al. 1997, Lin et al. 1999, who found that the short MLCK bound to stress fibers in the A7r5 smooth muscle cell line in vivo and to stress fibers in permeabilized Swiss 3T3 fibroblasts. The lack of binding in epithelial cells may indicate that binding of the short MLCK to stress fibers is subject to different regulatory mechanisms in different cell types. However, in chicken hepatoma DU24 cells, we observed the same localization for the long and short MLCKs as observed in HeLa cells (data not shown). These observations demonstrate that the long and short MLCKs show a consistent localization pattern in diverse nonmuscle cell lines and suggest that this distribution pattern is a conserved feature of nonmuscle cells.
Our studies with truncations of the long MLCK suggest that the five DXRXXL motifs that span the junction of the NH2-terminal extension and short MLCK (Fig. 1) are sufficient to target the long MLCK to stress fibers. Each DXRXXL motif is separated by 28–29 amino acids, suggesting that they may form a periodic structure that presents a continuous binding domain. Mutagenesis studies have shown that three motifs which contain DFRXXL in the NH2 terminus of the rabbit short MLCK mediate the binding of the kinase to smooth muscle myofilaments (Smith et al. 1999). Recent data also show that these motifs directly bind F-actin (Smith et al. 1999; our unpublished observations) and are consistent with the observation that the NH2 terminus of the short MLCK is required for binding to purified smooth muscle thin filaments (Lin et al. 1999). In addition, in vitro studies have demonstrated that the NH2-terminal extension alone can bind and bundle actin filaments (Kudryashov et al. 1999), which suggests that two copies of the DXRXXL motif (DFRXXL and DVRXXL) may be adequate for localization. The presence of five copies of this motif in the long MLCK suggests that the full-length protein may exhibit a higher affinity for stress fibers than the NH2-terminal extension alone. This is qualitatively supported by our fluorescence studies, which show more prominent stress fiber staining for the long MLCK and the NH2-terminal extension containing all five DXRXXL motifs (Fig. 5 and Fig. 6) than for the NH2-terminal extension containing only two DXRXXL motifs. However, we cannot discount that additional amino acid sequences or domains in the NH2-terminal extension contribute to the targeting of the long MLCK.
Cell Cycle Regulation of MLCK Localization and Activity
Previous studies have shown that MLCK localizes to the mitotic apparatus during metaphase and to the midbody in late telophase (Guerriero et al. 1981). However, these studies did not assess the localization of MLCK during cytokinesis. Our studies demonstrate, for the first time, that the long MLCK specifically localizes to the cleavage furrow of dividing cells. Importantly, the localization of the long MLCK during mitosis requires all five DXRXXL motifs, as well as additional amino acid sequences present in the NH2-terminal extension, since neither the short MLCK, containing all five DXRXXL motifs, nor the NH2-terminal extension, containing two DXRXXL motifs, localize to the furrow. These findings suggest that nonmuscle cells utilize different molecular mechanisms for targeting the long MLCK to stress fibers and to the cleavage furrow.
In addition to the DXRXXL sequence motifs, the long MLCK contains nine IgG-C2–type sequence motifs, with six IgG-C2 motifs in the NH2-terminal extension and three IgG-C2 motifs in the short MLCK (Fig. 1). The IgG-C2 motif is also found in neural adhesion molecules, the platelet-derived growth factor (PDGF) and Fc receptors, vertebrate titin, and the invertebrate minititins (Benian et al. 1989). The identification of this motif in cell surface molecules and proteins that span the sarcomere has led to the proposal that these domains are involved in protein–protein interactions (Benian et al. 1989; Olson et al. 1990; Gallagher et al. 1997). For cytoplasmic proteins involved in the dynamic remodeling of the cytoskeleton, such as the long MLCK, it seems likely that the IgG-C2–type motif could be involved in subcellular targeting. Specific interactions between modular scaffolding or anchoring proteins is a widely used strategy to restrict the intracellular localization of proteins to specific subcellular domains, allowing for the assembly of multicomponent complexes and the colocalization of specific enzyme–substrate pairs (Mochly-Rosen 1995; Dell'Acqua and Scott 1997). For example, the receptors for activated C kinase (RACKs) specifically bind and localize activated PKCs to distinct subcellular sites (Ron et al. 1994; Csukai et al. 1997). In addition, several A kinase–anchoring proteins, which bind the type II regulatory subunit of cAMP-dependent protein kinase (PKA), target PKA to several specific organelles, as well as the actin and microtubule cytoskeletons (Dell'Acqua and Scott 1997). The IgG-C2–type motif may provide such a targeting mechanism for the long MLCK.
Data presented here demonstrate that the activity of the long MLCK is cell cycle regulated with a twofold decrease in specific activity during mitosis. Treatment of the metaphase long MLCK with alkaline phosphatase restored the kinase activity to the same levels observed for the interphase and cytokinetic long MLCKs, indicating that phosphorylation is involved in regulating the activity of the kinase early in mitosis. Although these experiments do not distinguish between direct phosphorylation of the long MLCK and phosphorylation of a coprecipitating accessory molecule that modulates kinase activity, these results are consistent with in vitro studies showing that the short MLCK is a substrate for several kinases. These include PKA (Conti and Adelstein 1981), PKC (Ikebe et al. 1985; Nishikawa et al. 1985), cGMP-dependent protein kinase (PKG) (Nishikawa et al. 1984a), Ca2+/calmodulin-dependent protein kinase (CaM kinase II) (Ikebe and Reardon 1990), p21-activated kinase (PAK) (Sanders et al. 1999), and mitogen-activated protein (MAP) kinase (Klemke et al. 1997). In addition to the phosphorylation sites located in the COOH-terminal half of the protein, the long MLCKs also contain potential PKA, PKC, CaM kinase II, and casein kinase II phosphorylation sites in the NH2-terminal extension (Verin et al. 1998).
In vitro studies on the short MLCK strongly suggest that phosphorylation could downregulate the activity of the long MLCK. In the absence of calmodulin, PKC, PKA, and CaM kinase II phosphorylate the short MLCK on a serine residue in the calmodulin-binding domain (Conti and Adelstein 1981; Ikebe et al. 1985; Ikebe and Reardon 1990). Phosphorylation on this site increases the K calmodulin by 10-fold, thus desensitizing MLCK to activation by Ca2+/calmodulin. Importantly, in vivo activation of CaM kinase II in smooth muscle cells induces phosphorylation of the serine residue in the calmodulin-binding domain (Stull et al. 1990; Tansey et al. 1992), demonstrating that this phosphorylation event is physiologically significant. In nonmuscle cells, activation of PKA induces MLCK phosphorylation and is accompanied by stress fiber disassembly and dephosphorylation of the RLC (Lamb et al. 1988). These findings suggest that in nonmuscle cells a phosphorylation event downregulates MLCK activity. In addition to PKA-mediated inhibition of MLCK activity, PAK may also downregulate MLCK function in nonmuscle cells. In vitro, phosphorylation by PAK inhibits the activity of the short MLCK twofold, and overexpression of activated PAK results in decreased RLC phosphorylation and inhibition of cell spreading (Sanders et al. 1999). Interestingly, PAK phosphorylation of MLCK is reported to only effect the V max, not the K calmodulin, suggesting that the regulation of MLCK activity can also occur via phosphorylation on residues outside the calmodulin binding domain.
At present, little is known about the kinases that specifically phosphorylate the long MLCK during mitosis. Using the short MLCK as a substrate, Hosoya et al. 1991 identified a mitosis-specific MLCK kinase activity in HeLa extracts. Biochemical analyses and phosphopeptide maps demonstrate that the mitotic MLCK kinase is not PKA, PKG, PKC, or CaM kinase II. Candidate kinases that could inhibit the activity of the long MLCK early in mitosis include cdc2 kinase and cdc2-regulated kinases. The long and short MLCKs each contain a single consensus cdc2 kinase phosphorylation site corresponding to residues 1229–1233 and 1216–1220 in the human and avian long MLCKs, respectively. Future studies directed at identifying the phosphorylated residues on the mitotic MLCK, as well as the functional consequences of phosphorylation, will be required to establish the molecular basis for the regulation of MLCK activity during mitosis.
Biological Significance of MLCK Regulation during Cell Division
In higher eukaryotes, phosphorylation of the RLC on specific residues varies with time during mitosis. During metaphase, the RLC is phosphorylated predominantly on Ser 1/2, which has an inhibitory effect on myosin II's ATPase activity. However, by anaphase, the RLC is dephosphorylated on these sites and phosphorylated on Ser 19, which stimulates the motor and perhaps assembly properties of myosin II (Satterwhite et al. 1992; Yamakita et al. 1994; DeBiasio et al. 1996; Matsumura et al. 1998). These observations indicate that myosin II activity is regulated temporally during mitosis, and suggest that phosphorylation on Ser 19 of the RLC could provide the stimulus for contraction of the cleavage furrow.
Consistent with this hypothesis is emerging evidence that the kinases and phosphatases mediating phosphorylation on Ser 19 of the RLC are themselves regulated during mitosis. During prophase and metaphase, phosphorylation on Ser 430 of the myosin binding subunit (MBS) of the myosin II phosphatase enhances its binding to myosin II, as well as its phosphatase activity (Totsukawa et al. 1999). In conjunction with increased phosphatase activity, mitosis-specific phosphorylation and inhibition of MLCK's kinase activity would allow for a net decrease in Ser 19 phosphorylation on the RLC and inhibition of myosin II's contractile activity early in mitosis. Reduced phosphorylation on Ser 19 during prophase would promote the disassembly of stress fibers that is observed in cells that have entered mitosis (Fig. 11).
Figure 11.
Model for the regulation of RLC phosphorylation during mitosis. The different stages of mitosis are shown in the cartoon diagram on top. The rows below depict the activities of the myosin II phosphatase, MLCK, and myosin II. See text for details.
Conversely, inhibition of the myosin phosphatase and activation of MLCK at later stages of mitosis would favor increased levels of Ser 19 phosphorylation and the activation of myosin II motor activity required for cytokinesis. This proposal is supported by the observation that during cytokinesis, the MBS is no longer phosphorylated on Ser 430 (Totsukawa et al. 1999). Subsequent phosphorylation of the MBS on Thr 695 by Rho kinase, which is known to inhibit phosphatase activity (Feng et al. 1999), could downregulate the phosphatase activity late in mitosis. The activation of MLCK would occur after dephosphorylation of the inhibitory sites and the binding of Ca2+/calmodulin. The involvement of a Ca2+ signal in regulating cell division is supported by studies in Xenopus and zebrafish embryos, which show that localized Ca2+ transients correlate spatially and temporally with the initiation of furrowing (Chang and Meng 1995; Muto et al. 1996; Webb et al. 1997). Although a localized Ca2+ transient has not been detected in vertebrate nonmuscle cells, a sustained elevation in intracellular Ca2+ is observed, which begins in anaphase and is sustained through telophase and daughter cell separation (Tombes and Borisy 1989). Propagation of a Ca2+ signal via calmodulin is supported by the recent finding that calmodulin is enriched at the cell equator just before the onset of cytokinesis (Li et al. 1999). In addition, a biosensor that detects Ca2+/calmodulin–target protein complexes (Torok and Trentham 1994) has shown that during anaphase, only the calmodulin population localized at the cell equator is activated (Li et al. 1999). Taken together, these observations suggest that the myosin II phosphatase and MLCK act cooperatively to increase the level of activated myosin II for cytokinesis (Fig. 11).
Acknowledgments
We are grateful to Dr. Jim Sellers for providing us with chicken smooth muscle HMM, and we thank Dr. Steven Almo for helpful comments, and Dr. Thomas Pollard for his support in the production of the MLCK pAb.
This work was supported by the American Heart Association (A.R. Bresnick) and the National Institutes of Health (J.T. Stull).
Footnotes
A. Poperechnaya and O. Varlamova contributed equally to this work.
Abbreviations used in this paper: CaM kinase II, Ca2+/calmodulin-dependent protein kinase; GFP, green fluorescent protein; HMM, heavy meromyosin; MBS, myosin binding subunit; MLCK, myosin light chain kinase; PAK, p21-activated kinase; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; RLC, regulatory light chain.
References
- Adelstein R., Klee C. Purification and characterization of smooth muscle myosin light chain kinase. J. Biol. Chem. 1981;256:7501–7509. [PubMed] [Google Scholar]
- Benian G.M., Kiff J.E., Neckelmann N., Moerman D.G., Waterston R.H. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans . Nature. 1989;342:45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- Birukov K.G., Schavocky J.P., Shirinsky V.P., Chibalina M.V., Van Eldik L.J., Watterson D.M. Organization of the genetic locus for chicken myosin light chain kinase is complexmultiple proteins are encoded and exhibit differential expression and localization. J. Cell. Biochem. 1998;70:402–413. [PubMed] [Google Scholar]
- Bresnick A.R., Wolff-Long V.L., Baumann O., Pollard T.D. Phosphorylation on threonine-18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry. 1995;34:12576–12583. doi: 10.1021/bi00039a012. [DOI] [PubMed] [Google Scholar]
- Chang D.C., Meng C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J. Cell Biol. 1995;131:1539–1545. doi: 10.1083/jcb.131.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M.A., Adelstein R.S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3′:5′ cAMP-dependent protein kinase. J. Biol. Chem. 1981;256:3178–3181. [PubMed] [Google Scholar]
- Conti M.A., Adelstein R.S. Purification and properties of myosin light chain kinases. Methods Enzymol. 1991;196:34–37. doi: 10.1016/0076-6879(91)96006-d. [DOI] [PubMed] [Google Scholar]
- Csukai M., Chen C.H., De Matteis M.A., Mochly-Rosen D. The coatomer protein beta′-COP, a selective binding protein (RACK) for protein kinase C epsilon. J. Biol. Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R.S., Feramisco J.R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc. Natl. Acad. Sci. USA. 1981;78:4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasio R.L., LaRocca G.M., Post P.L., Taylor D.L. Myosin II transport, organization, and phosphorylationevidence for cortical flow/solation-contraction coupling during cytokinesis and cell locomotion. Mol. Biol. Cell. 1996;7:1259–1282. doi: 10.1091/mbc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua M.L., Scott J.D. Protein kinase A anchoring. J. Biol. Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- Feng J., Ito M., Ichikawa K., Isaka N., Nishikawa M., Hartshorne D.J., Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T.D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J. Cell Biol. 1976;71:848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P.J., Garcia J.G., Herring B.P. Expression of a novel myosin light chain kinase in embryonic tissues and cultured cells. J. Biol. Chem. 1995;270:29090–29095. doi: 10.1074/jbc.270.49.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P.J., Herring B.P., Stull J.T. Myosin light chain kinases. J. Muscle Res. Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- Garcia J.G., Lazar V., Gilbert-McClain L.I., Gallagher P.J., Verin A.D. Myosin light chain kinase in endotheliummolecular cloning and regulation. Am. J. Respir. Cell Mol. Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- Goeckeler Z.M., Wysolmerski R.B. Myosin light chain kinase–regulated endothelial cell contractionthe relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero V., Jr., Rowley D.R., Means A.R. Production and characterization of an antibody to myosin light chain kinase and intracellular localization of the enzyme. Cell. 1981;27:449–458. doi: 10.1016/0092-8674(81)90386-x. [DOI] [PubMed] [Google Scholar]
- Hosoya H., Yamashiro S., Matsumura F. Mitosis-specific phosphorylation of myosin light chain kinase. J. Biol. Chem. 1991;266:22173–22178. [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Phosphorylation of smooth myosin light chain kinase by smooth muscle Ca2+/calmodulin-dependent multifunctional protein kinase. J. Biol. Chem. 1990;265:8975–8978. [PubMed] [Google Scholar]
- Ikebe M., Inagaki M., Kanamaru K., Hidaka H. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J. Biol. Chem. 1985;260:4547–4550. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D.J., Elzinga M. Identification, phosphorylation and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J. Biol. Chem. 1986;260:36–39. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D.J., Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J. Biol. Chem. 1987;262:9569–9573. [PubMed] [Google Scholar]
- Kishi H., Mikawa T., Seto M., Sasaki Y., Kanayasu-Toyoda T., Yamaguchi T., Imamura M., Ito M., Karaki H., Bao J. Stable transfectants of smooth muscle cell line lacking the expression of myosin light chain kinase and their characterization with respect to the actomyosin system. J. Biol. Chem. 2000;275:1414–1420. doi: 10.1074/jbc.275.2.1414. [DOI] [PubMed] [Google Scholar]
- Klemke R.L., Cai S., Giannini A.L., Gallagher P.J., de Lanerolle P., Cheresh D.A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashov D.S., Chibalina M.V., Birukov K.G., Lukas T.J., Sellers J.R., Van Eldik L.J., Watterson D.M., Shirinsky V.P. Unique sequence of a high molecular weight myosin light chain kinase is involved in interaction with actin cytoskeleton. FEBS Lett. 1999;463:67–71. doi: 10.1016/s0014-5793(99)01591-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N.J., Fernandez A., Conti M.A., Adelstein R., Glass D.B., Welch W.J., Feramisco J.R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J. Cell Biol. 1988;106:1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.J., Heim R., Lu P., Pu Y., Tsien R.Y., Chang D.C. Dynamic redistribution of calmodulin in HeLa cells during cell division as revealed by a GFP-calmodulin fusion protein technique. J. Cell Sci. 1999;112:1567–1577. doi: 10.1242/jcs.112.10.1567. [DOI] [PubMed] [Google Scholar]
- Lin P.J., Luby-Phelps K., Stull J.T. Binding of myosin light chain kinase to cellular actin-myosin filaments. J. Biol. Chem. 1997;272:7412–7420. doi: 10.1074/jbc.272.11.7412. [DOI] [PubMed] [Google Scholar]
- Lin P.J., Luby-Phelps K., Stull J.T. Properties of filament-bound myosin light chain kinase. J. Biol. Chem. 1999;274:5987–5994. doi: 10.1074/jbc.274.9.5987. [DOI] [PubMed] [Google Scholar]
- Majercik M.H., Bourguignon L.Y. Insulin-induced myosin light-chain phosphorylation during receptor capping in IM-9 human B-lymphoblasts. Biochem. J. 1988;252:815–823. doi: 10.1042/bj2520815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S.C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal. Biochem. 1974;60:149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Ono S., Yamakita Y., Totsukawa G., Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteinsa theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Muto S., Inoue T., Okano H., Mikoshiba K. Calcium waves along the cleavage furrows in cleavage-stage Xenopus embryos and its inhibition by heparin. J. Cell Biol. 1996;135:181–190. doi: 10.1083/jcb.135.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T.M., Adelstein R.S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase J. Biol. Chem 259 1984. 8429 8436a [PubMed] [Google Scholar]
- Nishikawa M., Sellers J.R., Adelstein R.S., Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase J. Biol. Chem 259 1984. 8808 8814b [PubMed] [Google Scholar]
- Nishikawa M., Shirakawa S., Adelstein R.S. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J. Biol. Chem. 1985;260:8978–8983. [PubMed] [Google Scholar]
- Olson N.J., Pearson R.B., Needleman D.S., Hurwitz M.Y., Kemp B.E., Means A.R. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc. Natl. Acad. Sci. USA. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Chen C.H., Caldwell J., Jamieson L., Orr E., Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase Ca homolog of the beta subunit of G proteins. Proc. Natl. Acad. Sci. USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L.C., Matsumura F., Bokoch G.M., de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Satterwhite L.L., Lohka M.J., Wilson K.L., Scherson T.Y., Cisek L.J., Corden J.L., Pollard T.D. Phosphorylation of myosin II regulatory light chain by cyclin-p34cdc2a mechanism for the timing of cytokinesis. J. Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J.R., Pato M.D., Adelstein R.S. Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J. Biol. Chem. 1981;256:13137–13142. [PubMed] [Google Scholar]
- Sellers J.R., Spudich J.A., Sheetz M.P. Light chain phosphorylation regulates the movement of smooth muscle myosin on actin filaments. J. Cell Biol. 1985;101:1897–1902. doi: 10.1083/jcb.101.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker M.O., Lau W., Shattuck R.L., Kwiatkowski A.P., Matrisian P.E., Guerra-Santos L., Wilson E., Lukas T.J., Van Eldik L.J., Watterson D.M. Use of DNA sequence and mutant analyses and antisense oligodeoxynucleotides to examine the molecular basis of nonmuscle myosin light chain kinase autoinhibition, calmodulin recognition, and activity. J. Cell Biol. 1990;111:1107–1125. doi: 10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L., Su X., Lin P., Zhi G., Stull J.T. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J. Biol. Chem. 1999;274:29433–29438. doi: 10.1074/jbc.274.41.29433. [DOI] [PubMed] [Google Scholar]
- Stull J.T., Hsu L.C., Tansey M.G., Kamm K.E. Myosin light chain kinase phosphorylation in tracheal smooth muscle. J. Biol. Chem. 1990;265:16683–16690. [PubMed] [Google Scholar]
- Suzuki H., Onishi H., Takahashi K., Watanabe S. Structure and function of chicken gizzard myosin. J. Biochem. (Tokyo) 1978;84:1529–1542. doi: 10.1093/oxfordjournals.jbchem.a132278. [DOI] [PubMed] [Google Scholar]
- Tansey M.G., Word R.A., Hidaka H., Singer H.A., Schworer C.M., Kamm K.E., Stull J.T. Phosphorylation of myosin light chain kinase by the multifunctional calmodulin-dependent protein kinase II in smooth muscle cells. J. Biol. Chem. 1992;267:12511–12516. [PubMed] [Google Scholar]
- Tombes R.M., Borisy G.G. Intracellular free calcium and mitosis in mammalian cellsanaphase onset is calcium modulated, but is not triggered by a brief transient. J. Cell Biol. 1989;109:627–636. doi: 10.1083/jcb.109.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok K., Trentham D.R. Mechanism of 2-chloro-(epsilon-amino-Lys75)-[6-[4-(N,N-diethylamino)phenyl]-1,3,5-triazin-4-yl]calmodulin interactions with smooth muscle myosin light chain kinase and derived peptides. Biochemistry. 1994;33:12807–12820. doi: 10.1021/bi00209a012. [DOI] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hosoya H., Hartshorne D.J., Matsumura F. Activation of myosin phosphatase targeting subunit by mitosis-specific phosphorylation. J. Cell Biol. 1999;144:735–744. doi: 10.1083/jcb.144.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbedsky K., Pollard T.D., Bresnick A.R. A subset of protein kinase C phosphorylation sites on the myosin II regulatory light chain inhibits phosphorylation by myosin light chain kinase. Biochemistry. 1997;36:2063–2067. doi: 10.1021/bi9624651. [DOI] [PubMed] [Google Scholar]
- Verin A.D., Gilbert-McClain L.I., Patterson C.E., Garcia J.G. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am. J. Respir. Cell Mol. Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- Walker J.W., Gilbert S.H., Drummond R.M., Yamada M., Sreekumar R., Carraway R.E., Ikebe M., Fay F.S. Signaling pathways underlying eosinophil cell motility revealed by using caged peptides. Proc. Natl. Acad. Sci. USA. 1998;95:1568–1573. doi: 10.1073/pnas.95.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D.M., Collinge M., Lukas T.J., Van Eldik L.J., Birukov K.G., Stepanova O.V., Shirinsky V.P. Multiple gene products are produced from a novel protein kinase transcription region. FEBS Lett. 1995;373:217–220. doi: 10.1016/0014-5793(95)01048-j. [DOI] [PubMed] [Google Scholar]
- Webb S.E., Lee K.W., Karplus E., Miller A.L. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev. Biol. 1997;192:78–92. doi: 10.1006/dbio.1997.8724. [DOI] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J. Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]