Abstract
Activated epidermal growth factor receptors recruit various intracellular proteins leading to signal generation and endocytic trafficking. Although activated receptors are rapidly internalized into the endocytic compartment and subsequently degraded in lysosomes, the linkage between signaling and endocytosis is not well understood. Here we show that EGF stimulation of NR6 cells induces a specific, rapid and transient activation of Rab5a. EGF also enhanced translocation of the Rab5 effector, early endosomal autoantigen 1 (EEA1), from cytosol to membrane. The activation of endocytosis, fluid phase and receptor mediated, by EGF was enhanced by Rab5a expression, but not by Rab5b, Rab5c, or Rab5a truncated at the NH2 and/or COOH terminus. Dominant negative Rab5a (Rab5:N34) blocked EGF-stimulated receptor-mediated and fluid-phase endocytosis. EGF activation of Rab5a function was dependent on tyrosine residues in the COOH-terminal domain of the EGF receptor (EGFR). Removal of the entire COOH terminus by truncation (c'973 and c'991) abrogated ligand-induced Rab5a activation of endocytosis. A “kinase-dead” EGFR failed to stimulate Rab5a function. However, another EGF receptor mutant (c'1000), with the kinase domain intact and a single autophosphorylation site effectively signaled Rab5 activation. These results indicate that EGFR and Rab5a are linked via a cascade that results in the activation of Rab5a and that appears essential for internalization. The results point to an interdependent relationship between receptor activation, signal generation and endocytosis.
Keywords: signal transduction, endocytosis, Rab5, endosome, fusion
Introduction
Receptor signaling and receptor trafficking appear to be tightly linked cellular events. Both receptors with intrinsic tyrosine kinase activity (RPTK) (Sorkin 1998) and G protein–coupled receptors (Bunemann and Hosey 1999) have been shown to be internalized upon addition of ligand to cells. Receptor signaling cascades are initiated immediately after receptor ligation, which is followed by the internalization of receptor-ligand complexes. Internalization of signal transducing receptors appears to be mediated exclusively via coated vesicles, possibly via types of coated pits (Warren et al. 1997; Vickery and von Zastrow 1999). Signaling cascades associated with receptors have been shown to be actively involved in the assembly of coated vesicles (Wang and Moran 1996; Lohi et al. 1998; Wilde et al. 1999), suggesting that the activated receptor orchestrates its own internalization. After internalization, activated RPTK receptors continue to signal via their respective activated kinase domains (Haugh et al. 1999; Rizzo et al. 1999). Many questions about the physiologic rationale for the coupling of receptor signaling and receptor internalization have remained unanswered (Gruenberg and Maxfield 1995). Early workers suggested that internalization of receptor–ligand complexes provides a way to attenuate signaling responses by removing receptors from the cell surface (Wells et al. 1990). More recently, work from many labs has suggested that the nature of the signaling response to a ligand may be influenced by the quality of the internalization event. It may be possible for receptors to partner with one or more siblings in a given family of receptors (e.g., the Erb family) that in turn may affect their internalization rates and intracellular itinerary (Olayioye et al. 1998; Waterman et al. 1999). Moreover, the endosome may provide a staging platform for the assembly of signaling complexes, which are then putatively transported to different cellular destinations (Sorkin et al. 1993; Grimes et al. 1996).
The EGF receptor (EGFR) represents the prototype and best studied example of a RPTK receptor (Wells 1999). EGFR is actively internalized upon addition of EGF. Activation of the receptor stimulates a cascade of activities, all of which appear to be mediated by the kinase associated with the receptor (Wells 1999). A key downstream event after receptor ligation is the activation of the extracellular-regulated kinase/microtubule-activated protein (ERK/MAP) kinase pathway (Moghal and Sternberg 1999; Rizzo et al. 1999). Activation of the EGFR kinase leads to autophosphorylation of the receptor protein, and the recruitment of Eps15 (Torrisi et al. 1999) and the adapter AP-2 (Vieira et al. 1996; Sorkin 1998; Sorkina et al. 1999). A second activity revealed by recent studies indicates that activated EGF receptors stimulate SRC kinase, which in turn mediates phosphorylation of clathrin (Wilde et al. 1999). Clearly, these events prepare the path for rapid internalization and targeting of the receptor. The linkage between signaling and trafficking is partially met by the activation of proximal activities such as recruitment to the coated pit and activation of clathrin assembly processes (Vieira et al. 1996). However, little is known about the activation of more distal events (i.e., that require Rab5, a rate limiting GTPase required for endocytosis; Gruenberg and Maxfield 1995), and the linkage between proximal and distal events. In this paper, we explore the role of Rab5 and its isoforms (Lutcke et al. 1995; Alvarez-Dominguez and Stahl 1998; Chiariello et al. 1999) in two pathways known to be activated by EGFR. These include (a) activation of fluid phase endocytosis and (b) receptor-mediated endocytosis (i.e., EGF stimulated internalization of EGF–EGFR complexes). Our results indicate that both events are selectively stimulated by the Rab5a isoform, and inhibited by the dominant negative mutant of Rab5a (Rab5a:N34).
Materials and Methods
Cell Culture and Quiescence Protocol
NR6 mouse fibroblasts transfected with human EGFR wild type and mutants (Chen et al. 1994) were cultured in Corning tissue culture dishes in a 5% CO2 environment. All cell culture reagents were obtained from Life Technologies. The growth medium consisted of minimum essential medium (α-MEM), 26 mM sodium bicarbonate with 5% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM α-MEM nonessential amino acids, and the antibiotics penicillin, streptomycin, and G418 (350 μg/ml). Cells were growth arrested at subconfluence using restricted serum conditions without G418 (α-MEM, 26 mM sodium bicarbonate with 3% dialyzed fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and the antibiotics penicillin/streptomycin) for 16 h before experiments. Experiments were carried out in binding buffer [α-MEM, 13 mM HEPES, pH 7.4 at 37°C, with 0.3% dialyzed fetal bovine serum, 2 mM l-glutamine, including bovine serum albumin (1 mg/ml), penicillin, and streptomycin], in an air environment.
Construction of Recombinant Sindbis Viruses
cDNAs of Rab5a, Rab5b, Rab5c were subcloned into the unique XbaI restriction site of the Sindbis vector Toto1000:32J (Li and Stahl 1993). The cDNA of GFP-Rab5a, GFP-Rab5b, and GFP-Rab5c was subcloned as described by Roberts et al. 1999. The plasmid was then linearized by XhoI digestion and used as a template for in vitro transcription with SP6 RNA polymerase. The resulting RNA transcripts were used for transfection of confluent BHK-21 cell monolayers using a Lipofectin-mediated procedure (Life Technologies). Cells were maintained at 37°C, and the media containing released viruses were harvested 40 h after transfection. Virus titers were generally between 108 and 109 plaque-forming units per milliliter. Virus stocks were aliquoted and kept frozen at −80°C before use.
Uptake Assays
NR6 monolayers were mock infected or infected with the vector or recombinant viruses as described (Li et al. 1995). At 6 h after infection or as otherwise indicated, cells were washed twice with serum-free α-MEM, and HRP uptake was initiated by adding HRP (5 mg/ml) in α-MEM (1 ml) containing 0.2% bovine serum albumin and 13 mM Hepes, pH 6.8 at 37°C, either in the presence or absence of EGF. Cell lysates were assayed for HRP activity (Li et al. 1995).
Receptor Internalization Studies
Mouse EGF (Life Technologies) was iodinated with 125I (NEN Life Science Products) using IODO-BEADS (Pierce Chemical Co.), according to the manufacturer's protocol. The specific activities of labeled ligands were typically 150,000 cpm/ng (600 Ci/mmol). Quiescent NR6 cells expressing different Rab constructs (Rab5, Rab7, Rab11, and mutants) were washed in binding buffer, incubated at 4°C for 3 h with 100 pM 125I-EGF, and warmed-up for different periods of time as indicated in each figure. Surface-bound and internalized ligand were discriminated essentially as described (Sorkin et al. 1991). In brief, unbound ligand was removed by washing the monolayer six times with ice-cold buffer containing (mM): 20 Hepes, 130 NaCl, 5 KCl, 0.5 MgCl2, 1 CaCl2, 1 mg/ml polyvinylpyrolidone, pH 7.4. Surface-bound ligand was then collected in ice-cold acid strip buffer (50 mM glycine-HCl, 100 mM NaCl, 1 mg/ml polyvinylpyrolidone, pH 3.0) for 2 min, and internalized ligand was released in 1 N NaOH overnight at room temperature. Nonspecific binding (<2%) was assessed in the presence of 200 nM unlabeled human EGF (Sigma-Aldrich) and subtracted from the total. Transferrin uptake was carried out essentially as reported earlier (D'Souza-Schorey et al. 1995).
Receptor Downregulation Assay
NR6 cells transfected with virus alone or with virus encoding Rab5 constructs were incubated for various times with 100 nM of EGF at 37°C, and then rinsed with cold medium. Surface EGF was then removed by mild acid/salt treatment as described above. Remaining cell surface binding sites were then quantified by incubating the cells with 100 pM 125I-EGF at 4°C for 2 h. Alternatively, NR6 cells were metabolically labeled overnight with 35S-methionine at 37°C in methionine-free medium containing 1% dialyzed calf serum. The cells were then transfected with virus alone or virus encoding different Rab5 constructs for 6 h. The cells were then washed three times and incubated in binding medium with or without 100 nM EGF for the indicated chase times. At the end of the chase, the cells were washed, lysed, and the EGFR was immunoprecipitated with anti–EGFR antibodies (Ab-5 monoclonal antibody; Oncogene Research Products). The immunoprecipitates were resuspended in sample buffer and separated in 7.5% SDS-PAGE. The gels were then soaked in enhancing solution, dried, and exposed to film (Eastman Kodak Co.) at −80°C. The relative amount of EGFR protein was determined by counting the excised bands in a γ counter.
Whole Cell Lysates and Western Blotting
Serum-starved NR6 cells were incubated with 100 mM EGF for the indicated times at 37°C. Cell monolayers were washed with phosphate-buffered saline containing 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, and lysed in ice-cold lysis buffer (1% Nonidet P-40, 10% glycerol, 50 mM Hepes, 100 NaCl, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, 5 mM EDTA, 1 mM Na F, 1 mM PMSF, 2 μg/ml pepstatin A, 2 μg/ml leupeptin, and 2 μg/ml aprotinin, pH 7.2). The lysate was vortexed and clarified by centrifugation at 16,000 g for 15 min at 4°C. Protein concentration in lysates was determined using the detergent-compatible protein assay (Bio-Rad Laboratories). Cell lysates (5 μl) were analyzed by SDS-PAGE, and the proteins were transferred to a nitrocellulose membrane using a semi-dry transfer apparatus (Bio-Rad Laboratories). The membranes were probed with different antibodies as indicated in each figure legend and the immunoblots were developed using the ECL reagents from Amersham Pharmacia Biotech.
Confocal Microscopy
NR6 cells, grown on glass coverslips and inverted on glass slides (made into a narrow flow cell by strips of vacuum grease; Heuser et al. 1993) were examined by confocal microscopy either in the presence or absence of 100 nM EGF in α-MEM containing 0.1% bovine serum albumin and 25 mM Hepes, pH 7.2. Time-lapsed confocal microscopy was carried out on a confocal microscope (MRC1024; Bio-Rad Laboratories) using a 63×, 1.4 numerical aperture bright-field objective and fluorescein filter sets.
Determination the Nucleotide Status of Rab5
To determine the ratio of GTP to GDP bound to Rab5a (GTP/GDP ratio), confluent (5 × 105 cells/dish) NIH3T3 cells expressing Rab5a were incubated in alpha-MEM phosphate-free medium for 3 h with 300 μCi of 32Pi (200 μCi/mmol; Amersham Pharmacia Biotech.). After incubation, the cells were stimulated with 100 nM EGF for 15 min. The cells were then lysed as described (Barbieri et al. 1998b), and Rab5 was immunoprecipitated using a 4F11 monoclonal antibody bound to the protein A-sepharose (Amersham Pharmacia Biotech.) for 10 min at 4°C in lysis buffer (Barbieri et al. 1998b). The beads were washed three times with wash buffer [20 mM Tris-Cl, 0.1% Nonidet P-40, 500 mM NaCl, 5 mM MgCl2,1 mM EGTA, 1 mM DTT, 1 mg/ml bovine serum albumin, 50 mM β-glycerophosphate, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2 μg/ml aprotinin (PMSF), and 2 μg/ml leupeptin] and three times with wash buffer containing 0.001% SDS. The bound nucleotides were then eluted in 12 μl of buffer (10 mM EDTA, 1 mM DTT, 0.1% SDS, 5 mM GDP, 5 mM GTP) for 3 min at 85°C. All the manipulations, from lysis of the sample to elution of the nucleotides bound to Rab5, were carried out at 4°C within 40 min. 4-μl samples were then spotted onto polyethyleneimine-cellulose TLC (Merck) plates, which were developed for 60 min in 0.75 M phosphate, pH 3.4. The samples were dried and placed in autoradiography cassettes. For visualization of the 32P-labeled GTP and GDP, films were exposed at −80°C for 24–36 h. A phosphoimager was used to determine the GTP/GDP ratio.
Results
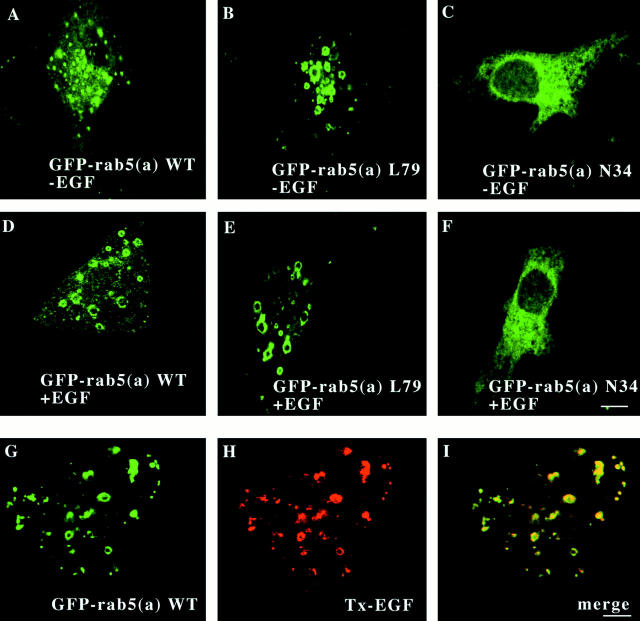
EGF stimulates endocytosis and the formation of large GFP-Rab5a positive endosomes. The initial stages of endocytosis are mediated by a series of fusion events that have been extensively studied both in vivo and in vitro. The GTPase Rab5 has been shown to be a key regulatory factor for these vesicle docking and fusion events (Barbieri et al. 1998a; McBride et al. 1999). Previous reports have shown that expression of Rab5a:L79, a GTPase-defective mutant, activates endocytosis and induces the formation of enlarged endosomes (Stenmark et al. 1994; Roberts et al. 1999). Moderately enlarged endosomes also occur in cells over-expressing Rab5a:wild type (Rab5a), but much smaller in size than in cells expressing Rab5a:L79. To analyze the effect of EGF receptor stimulation on Rab5 function, cells were transiently transfected with Sindbis virus encoding GFP-Rab5a, GFP-Rab5a:N34, and GFP-Rab5a:L79. The transfected cells were then incubated in the presence or absence of EGF as described in Materials and Methods. After incubation, the cells were fixed and studied by confocal microscopy. Fig. 1A and Fig. D, shows that the addition of EGF to cells expressing GFP-Rab5a results in a dramatic increase of the size of GFP-Rab5a–positive endosomes. The endosomes are similar in appearance to those observed when the Rab5a:L79 construct is expressed (Fig. 1 B). However, in cells expressing either GFP-Rab5a:L79 or GFP-Rab5a:N34, the addition of EGF did not result in any morphological change [Fig. 1B and Fig. E (Rab5a:L79), and C and F (Rab5a:N34)]. These results suggested that activation of the EGF receptor has a direct effect on Rab5a function. Moreover, the enlarged endosomal structures observed upon EGF receptor stimulation by EGF are accessible to EGF itself. We used Texas red (Tx)–EGF to monitor the internalization of EGF in cells expressing GFP-Rab5a. Tx-EGF was prebound to cells at 4°C. The cells were then incubated at 37°C for 5 min to induce internalization. In cells expressing GFP-Rab5a (Fig. 1 G), the internalized Tx-EGF was found in an enlarged vesicular compartment (H), with characteristics quite similar to those observed in cells expressing Rab5a:L79 (B). In contrast, cells expressing GFP-Rab5a:N34 internalized substantially less Tx-EGF. Confocal microscopy of the internalized Tx-EGF in the latter showed a diffuse punctuate staining pattern (data not shown). The merged image in Fig. 1G and Fig. H, shows that the Tx-EGF and GFP-Rab5a overlap substantially, if not completely (Fig. 1 I). After treatment of GFP-Rab5a–expressing cells with EGF, docking and fusion of two or more green endosomes were observed in all the preparations examined (data not shown; Barbieri et al. 1998a). Taken together, these data suggest that stimulation of EGFR regulates Rab5 function, which, in turn, affects the endocytic rate and fusion between endosomes.
Figure 1.
EGF induces the formation of enlarged Rab5-positive endosomes. Cells expressing GFP-Rab5a (A and D), GFP-Rab5a:L79 (B and E), and GFP-Rab5a:N34 (C and F) were grown on coverslips and then were incubated in the presence or absence of 100 nM EGF for 15 min at 37°C. After stimulation, the cells were fixed with 2% paraformaldehyde, and then visualized by confocal microscopy. Cells expressing GFP-Rab5a (G–I) were incubated in the presence of 200 nM Texas red–EGF for 10 min at 37°C, fixed, and then visualized by confocal microscopy. Internalized Texas red–EGF (I) accumulated in the enlarged endosomes in cells expressing Rab5a (G). The yellow color indicates areas of colocalization of internalized Texas red–EGF and GFP-Rab5a (I). Optical sections viewed are 0.4 μM. Bars, 1 μM.
EGF Enhances GTP Loading of Rab5a and Membrane Recruitment of the Rab5a Interacting Protein, Early Endosomal Autoantigen 1
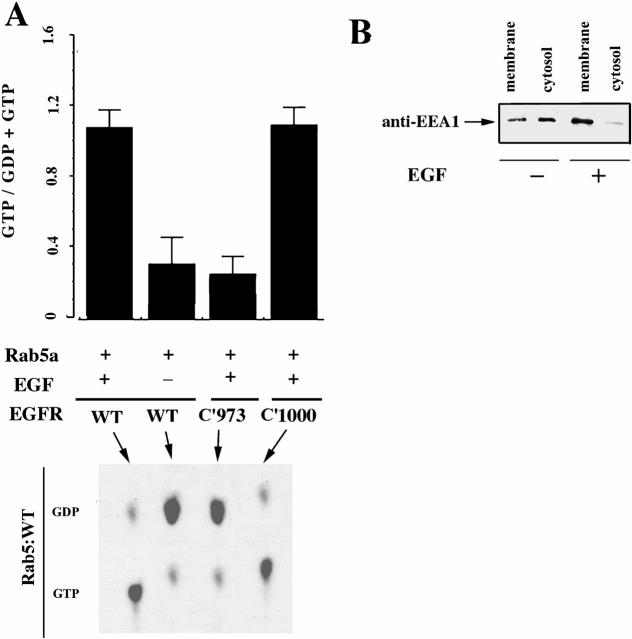
To further examine EGF activation of Rab5a, serum-starved cells were labeled with 32Pi to analyze the nucleotide status of Rab5a. Cells were then exposed to EGF (100 ng/ml) for 10 min. The cells were lysed and Rab5a was immunoprecipitated with monoclonal anti–Rab5 (4F11) antibody. 32P-guanine nucleotides, released from the Rab5a immunoprecipitates, were analyzed by TLC. The results in Fig. 2 A show that the GTP bound form of Rab5a is rapidly accumulated after exposure of the cells to EGF. Consistent with previous data, ∼90% of the Rab5a was in the GDP bound form in unstimulated cells. In stimulated cells, ∼80% of the Rab5a was found in the GTP bound form. Also shown in Fig. 2 A is the effect of EGF in cells expressing two truncated constructs of the EGF receptor, c'973, which is known not to be internalized, and c'1000, which is internalized. Activation of Rab5a in cells expressing these constructs is consistent with the conclusion that a fully active EGF receptor is required for Rab5a recruitment/activation. Representative data for one of these experiments, where 32P-labeled guanine nucleotides were released from the immunoprecipitated Rab5a and separated by TLC, is shown in Fig. 2 A (bottom). A second measure of the effect of EGF receptor activation on accelerated endocytosis is the status of early endosomal autoantigen 1 (EEA1), a cytosolic Rab5 interacting protein believed to be involved in the membrane recruitment of Rab5. Cells were incubated with EGF, and at 5 min the cells were lysed and membrane and cytosol fractions were prepared. The fractions were separated by SDS-PAGE and EEA1 was detected by Western blotting. As shown in Fig. 2 B, addition of EGF to NR6 cells, caused a rapid (5 min) shift of EEA1 from cytosol to the membrane.
Figure 2.
Membrane-cytosol redistribution of Rab proteins in the presence of EGF. (A) Cells expressing EGFR:WT (wild type) and mutants labeled with 32Pi, were transfected with GFP-Rab5a. Cells were incubated with 100 nM EGF for 15 min and the nucleotide status of Rab5 was analyzed as indicated in Materials and Methods (mean ± SD, n = 3.) (Bottom) The results from a typical experiment. (B) Cells expressing EGFR:WT were transfected with GFP-Rab5a. After transfection, the cells were incubated in the presence 100 nM EGF for 15 min and the membrane-cytosol distribution of EEA1 proteins was analyzed by Western blot. The experiment was repeated three times with similar results.
EGF Stimulation of Fluid Phase Endocytosis Is Rab5a-dependent
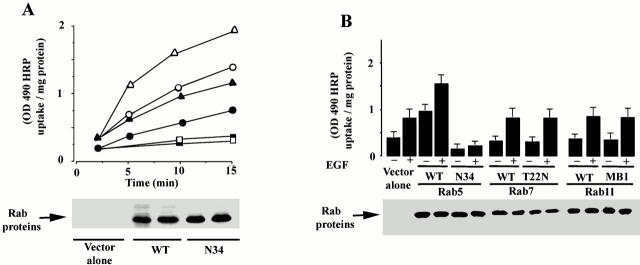
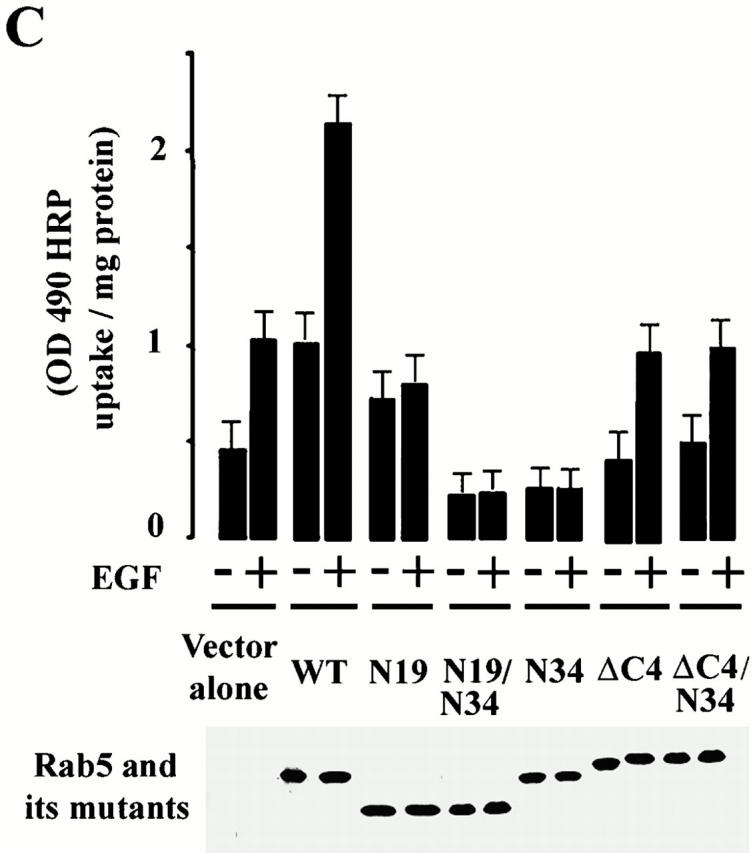
Another measure of the linkage between activation of the EGF receptor and Rab5a is fluid phase endocytosis (Fig. 3 A). EGF is well known to enhance the rate of fluid phase endocytosis. However, we found that EGF-stimulated fluid phase endocytosis was specifically increased by the expression of Rab5a. Both the initial rate of uptake and the accumulation of HRP over 15 min was substantially enhanced by the presence of GFP-Rab5a. Dominant negative Rab5a (GFP-Rab5a:N34) blocked both control endocytosis and EGF-induced fluid phase endocytosis. Western blots of cell lysates (Fig. 3, A–C) indicated that the constructs used were expressed to approximately the same level. As shown in Fig. 3 B, expression of GFP-Rab7 or GFP-Rab11 had no effect on the endocytic response to EGF either when expressed as wild-type proteins or as dominant negatives (GFP-Rab7:T22N or GFP-Rab11:MB1). Shown for comparison in Fig. 3 B are the pronounced effects of Rab5a, both dominant negative Rab5a:N34 and Rab5a, on EGF-stimulated fluid phase endocytosis. To further explore the role of Rab5a in EGF activation of endocytosis, a series of Rab5a mutants were employed. In Fig. 3 C, cells transfected with COOH-terminal (Rab5a:ΔC4) and NH2-terminal (Rab5a:ΔN19) truncation mutants of Rab5a, as well as Rab5a:N34, were stimulated with EGF. As expected, Rab5a:N34 had a dramatic inhibitory effect on the endocytosis of HRP. Both the NH2- and COOH-terminal deletion mutants of Rab5a were unable to facilitate the endocytic response to EGF. In spite of its inability to facilitate the response to EGF, Rab5a:ΔN19 was modestly active in stimulating endocytosis in the absence of EGF, as reported earlier (Li and Stahl 1993). Moreover, the endocytic effect of Rab5a:ΔN19 was abolished by the double mutant, Rab5a:ΔN19/N34. Rab5a:ΔC4, on the other hand, was both inactive in endocytosis and unable to support the endocytic effect of EGF. The double mutant Rab5a:ΔC4/N34 was also inactive. As expected, the inhibitory effect of Rab5a:N34 was abolished by COOH-terminal truncation. These results show that the NH2- and COOH-terminal domains as well the GTP-binding domain are required for optimal function of Rab5a in EGF-stimulated HRP endocytosis.
Figure 3.
HRP-stimulated EGF-dependent endocytosis requires the small GTPase Rab5. (A) Cells expressing EGFR:WT were either transfected with virus alone (control: ○, •), virus encoding Rab5 (▴, ▵), or virus encoding Rab5:N34 (□, ▪). After transient transfection, the amount of HRP internalized at 37°C for the indicated times was quantified in the presence (▵, ○, □) or absence (•, ▴, ▪) of EGF. This experiment was repeated at least three times with similar results. (B) Cells expressing EGFR were transfected with virus encoding Rab5, Rab5:N34, Rab11, Rab11:mB1, Rab7, or Rab7:N22. After transfection, the internalization of HRP (37°C for 10 min) was quantified in the presence or absence of 100 nM EGF. Values are mean ± SD, n = 3. (C) Cells expressing EGFR:WT were either transfected with virus alone, virus encoding Rab5, virus encoding Rab5:N34, virus encoding Rab5:N34/ΔN19, virus encoding Rab5:ΔN19, virus encoding Rab5:ΔC4, or virus encoding Rab5:ΔC4/N34. After transient transfection, the amount of HRP internalized at 37°C for 15 min was quantified in the presence or absence of 100 nM EGF. Values are mean ± SD, n = 3.
Effects of EGF on Receptor-mediated Endocytosis Are Rab5-dependent
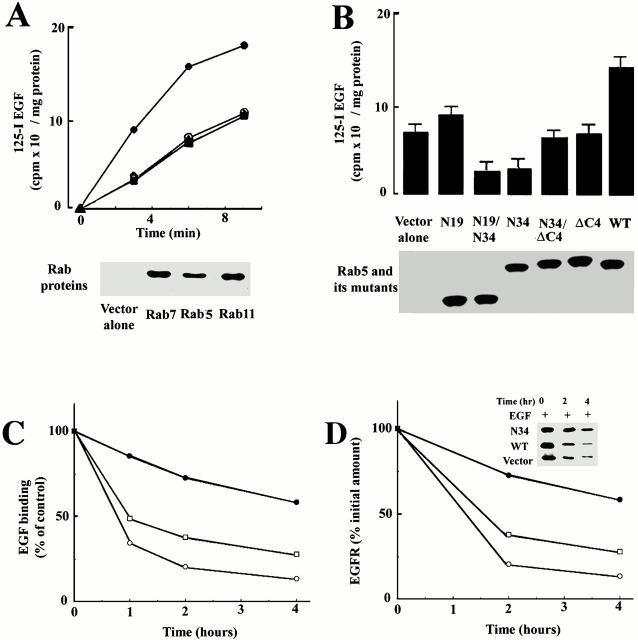
As shown above for fluid phase endocytosis, Rab5a is required for accelerated receptor-mediated endocytosis of 125I-EGF. After EGF binding, the uptake of 125I-EGF in cells expressing GFP-Rab5a was substantially accelerated (Fig. 4 A). Endocytosis of 125I-EGF was unaffected by expression of either GFP-Rab7 or GFP-Rab11 at 15 min of uptake. On the other hand, expression of Rab5a:N34 blocked 125I-EGF uptake (Fig. 4 B). Expression of the Rab11 and Rab7 dominant-negative mutants did not significantly interfere with EGF internalization at 15 min at 37°C (data not shown). At later time points, under conditions where internalized 125I-EGF was transported to the lysosomal compartment (data not shown), 125I-EGF accumulation was still strongly blocked by Rab5a:N34, but also partially inhibited by Rab7:N22. Similar to the requirement for an intact Rab5a molecule for the EGF-stimulated fluid-phase endocytosis, accelerated internalization of 125I-EGF also required intact Rab5a (Fig. 4 B). The accelerated internalization of 125I-EGF was not supported by Rab5a:ΔN19 nor by Rab5a:ΔC4. To examine more directly the effect of Rab5:N34 on the internalization and degradation of the EGF receptor, two additional experiments were carried out. In Fig. 4 C (left), the effect of Rab5:N34 expression on the loss of 125I-EGF binding in response to added EGF was quantified. Addition of EGF to cells is known to trigger internalization and degradation of the receptor. In Fig. 4 C, cells infected with the control virus or virus encoding Rab5a, rapidly lost cell surface 125I-EGF binding within ∼2 h after addition of EGF. When cells expressing Rab5a:N34 were examined with the same protocol, a binding capacity substantially delayed in the loss of EGF was recorded. At 2 h after EGF addition, there was only a 25% decrease in EGF binding capacity in cells expressing Rab5a:N34, whereas cells expressing Rab5a suffered an 80% loss in EGF binding. In Fig. 4 C (right), cells were metabolically labeled and radiolabeled EGF receptor was immunoprecipitated from cell lysates at different times after the addition of EGF to the cells. Cells infected with the control virus or virus encoding Rab5a rapidly lost radiolabeled receptor (see Fig. 4 C, inset) over the course of ∼2 h, whereas cells expressing Rab5a:N34 demonstrated only small losses in immunoprecipitable EGF receptor.
Figure 4.
Endocytosis of EGF requires the small GTPase Rab5. (A) Cells expressing EGFR:WT were either transfected with virus alone (○), virus encoding Rab5a (•), virus encoding Rab7 (▴), or virus encoding Rab11 (▪). After transient transfection, the amount of 125I-EGF internalized at 37°C for the indicated times was quantified. This experiment was repeated at least four times, and the results were highly reproducible. (B) Cells expressing EGFR were transiently transfected with either virus alone, virus encoding Rab5, Rab5:N34, Rab5:N34/ΔN19, Rab5:ΔN19, Rab5:ΔC4, or Rab5:ΔC4N34. After transfection, uptake of 125I-EGF was carried out at 37°C for 10 min (mean ± SD, n = 3). (C) Cells transfected with virus alone or virus encoding Rab5:WT or Rab5:N34 were treated with EGF for different times. Surface EGFR was analyzed by 125I -EGF binding as described in Materials and Methods. This experiment was repeated at least three times, and the results were reproducible. (D) 35S-methionine–labeled NR6 cells transfected with virus alone, or virus encoding Rab5:WT or Rab5:N34, were treated with EGF. At the indicated times, aliquots were removed and EGFR was immunoprecipitated from cell lysates with anti–human EGFR antibody as described in Materials and Methods.
Truncated Forms of the EGF Receptor that Are Signaling-restricted Do Not Activate Endocytosis, Even in the Presence of Rab5a
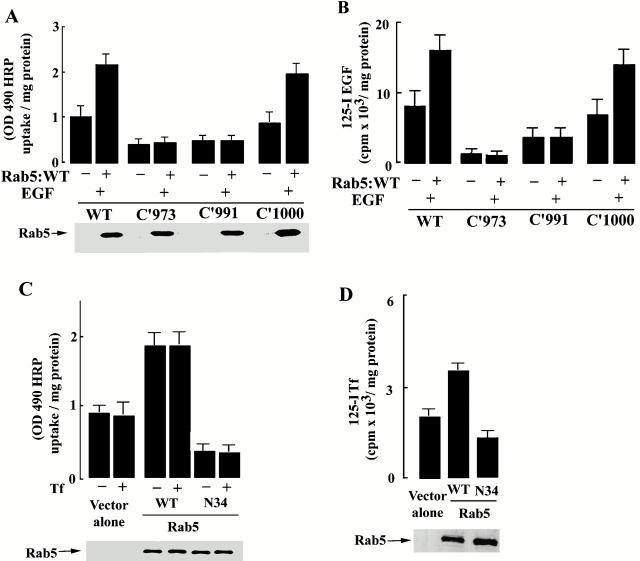
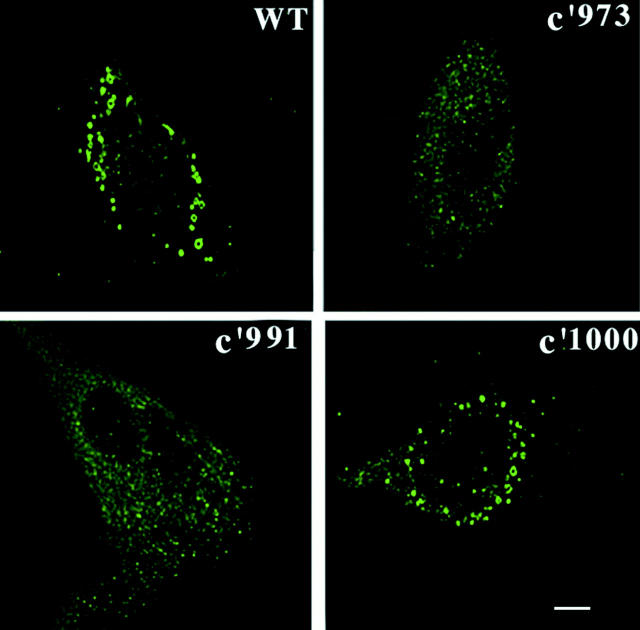
To identify the structural components of the EGF receptor that are responsible for enhanced endocytosis after EFG stimulation, several experiments were carried out with a series of EGF-receptor truncation mutants, as described in Fig. 5. Stable cell lines expressing the wild-type EGFR and mutant EGF receptors were transiently transfected with Sindbis virus encoding GFP-Rab5a. As shown in Fig. 5 A, the expression of Rab5a in cells expressing the wild-type EGFR caused a further increase in the fluid phase uptake of HRP after the addition of EGF (Fig. 5 A) and in the internalization of 125I-EGF (B). However, in cells expressing either EGFR:c'973 or EGFR:c'991, the expression of Rab5a did not have any effect on the uptake of HRP or 125I-EGF. Interestingly, in cells expressing EGFR c'1000, the endocytic effect of Rab5a expression was restored; i.e., cells expressing EGFR:c'1000 continue to accelerate fluid phase endocytosis and EGF internalization in response to Rab5a expression (Fig. 5A and Fig. B). As a control, we examined the effect of transferrin on fluid-phase endocytosis. Transferrin binds to the transferrin receptor and is internalized via clathrin-coated vesicles. As shown in Fig. 5 C, Rab5a increased (and Rab5a:N34 decreased) HRP endocytosis to the same extent in the presence or absence of transferrin. Rab5a and Rab5a:N34 also increased and decreased, respectively, the uptake of 125I transferrin, as shown earlier (Barbieri et al. 1998a) (Fig. 5 D). The addition of transferrin did not induce any change in the cytosol/membrane ratio of Rab5a (data not shown). Furthermore, no enlarged Rab5-positive endosomes were observed in cells stimulated with transferrin (data not shown). The effects of EGF in cells expressing the EGFR truncation mutants are confirmed at the morphological level in Fig. 6, where it is shown that incubation of cells expressing the wild-type EGFR and EGFR:c'1000, with EGF results in an increase in the size of GFP-Rab5a–positive endosomes. In contrast, the size of GFP-Rab5a–tagged endosomes remains unaffected by EGF stimulation in cells coexpressing GFP-Rab5a and EGFR:c'973 or EGFR:c'991 constructs (Fig. 6). These results indicate that residues in the tail of the EGFR are required for the regulation of Rab5 function, which, in turn, affect fluid-phase as well as EGF receptor–mediated endocytosis.
Figure 5.
Endocytosis of HRP and EGF require an intact cytoplasmic domain of the EGFR. Cells expressing either EGFR:WT, EGFR:c'973, EGFR:c'991, or EGFR:c'1000 were transfected with virus alone or virus encoding Rab5. After transient transfection, the amount of HRP (A) or 125I-EGF (B) internalized at 37°C was quantified as described in Materials and Methods (mean ± SD, n = 4). (C and D) Cells were transfected with virus alone, or virus encoding Rab5, or Rab5:N34 and the internalization of either HRP (C) or 125I-Tf (D) was carried out for 10 min at 37°C in the presence or absence of 6 μg/ml transferrin. Mean ± SD, n = 3.
Figure 6.
Formation of enlarged Rab5-positive endosomes requires an intact cytoplasmic domain of the EGFR. Cells expressing either EGFR:WT, EGFR:c'973, EGFR:c'991, or EGFR:c'1000 were transfected with virus encoding GFP-Rab5a. After transient transfection, the cells were incubated with EGF and the formation of enlarged endosomes were visualized by confocal microscopy as described in Materials and Methods. This experiment was repeated three times and the results were highly reproducible.
Stimulation of Endocytosis by EGF Is Rab5 Isoform-specific
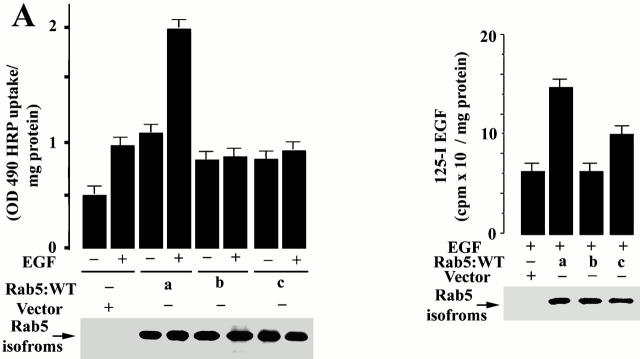
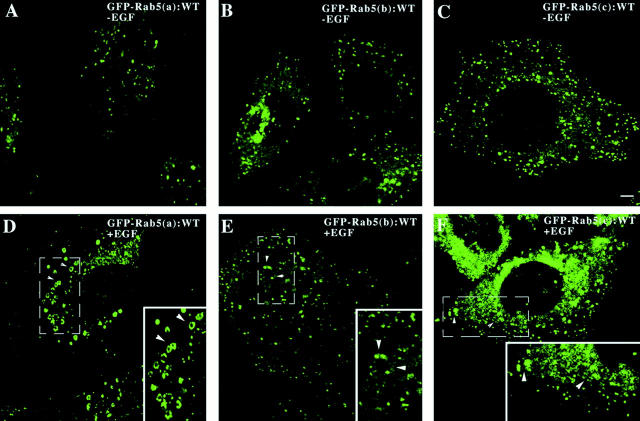
To determine the specificity of the activation of Rab5 by EGFR, we explored the possible role of the other two Rab5 isoforms, Rab5b and Rab5c. Three isoforms of Rab5 have been described (Lutcke et al. 1995; Chiariello et al. 1999). Rab5a is the most studied and best characterized isoform. All of the isoforms have been shown to be active in endocytosis after transient expression (Lutcke et al. 1995). To determine whether the activation of the other isoforms (Rab5b and Rab5c) were also regulated by EGF, cells expressing Rab5b and Rab5c were incubated with EGF and stimulated endocytosis was recorded. As shown in Fig. 7 A, expression of all three Rab5 isoforms increased the uptake of HRP under nonstimulated conditions. Surprisingly, whereas Rab5a was a potent enhancer of HRP endocytosis in cells stimulated by EGF, Rab5b and Rab5c were inactive. Furthermore, EGF stimulation did not change the cytosolic/membrane distribution of Rab5b and Rab5c (data not shown). To complement the studies on fluid-phase endocytosis, we next investigated the uptake of 125I-EGF in cells expressing Rab5a, Rab5b, and Rab5c. In Fig. 7 B, expression of Rab5a specifically increased the rate of 125I-EGF endocytosis. In contrast, the expression of the Rab5b and Rab5c isoforms did not substantially increase the rate of the EGF endocytosis. Morphological analysis show that enlarged endosomes were only observed in EGF-stimulated cells expressing the Rab5a isoform (Fig. 8). Fig. 8A and Fig. D, shows the effect of EGF in cells expressing GFP-Rab5a. Fig. 8B and Fig. E, shows the effects of EGF in cells expressing GFP Rab5b; Fig. 8C and Fig. D, shows the effects of EGF in cells expressing GFP-Rab5c. Fig. 8D–F (insets), shows more clearly the differential effects of EGF and Rab5a in producing large endosomes. Rab5b is virtually inactive; i.e., unable to enhance this effect of EGF. Rab5c is only modestly active.
Figure 7.
EGFR specifically regulates Rab5a function. (A) Cells expressing EGFR:WT were transfected with virus alone or virus encoding Rab5a, Rab5b, or Rab5c, and the internalization of HRP in the presence or absence of 100 nM EGF was carried out for 10 min at 37°C (mean ± SD, n = 3). (B) Cells expressing EGFR:WT were transfected as in A. After transfection, the uptake of 125I-EGF was carried out at 37°C for 10 min as described in Materials and Methods. Mean ± SD, n = 3.
Figure 8.
The formation of enlarged endosomes by EGF is specific for the Rab5a isoform. Cells expressing EGFR:WT were transfected with virus encoding GFP-Rab5a (A and D), GFP-Rab5b (B and E), or GFP-Rab5c (C and F), and the visualization of the formation of enlarged endosomes were analyzed as described in Materials and Methods. This experiment was repeated three times and the results were highly reproducible.
Discussion
While many cell-surface receptors (Hopkins and Trowbridge 1983; Hanover et al. 1984; Lamaze et al. 1993) are thought to internalize and recycle constitutively; e.g., transferrin and low density lipoprotein receptors (Goldstein et al. 1985; Hopkins et al. 1985), others, such as many tyrosine kinase growth-factor receptors and G protein–coupled receptors, are internalized only after binding to their respective agonists. Moreover, many signal-transducing receptors, internalized by ligand addition, are degraded via transport to lysosomes (Beguinot et al. 1984). A direct linkage between receptor activation and receptor internalization was revealed by the studies of Sorkin and colleagues (Sorkin 1998; Sorkina et al. 1999), beginning with the report that the EGF receptor, upon activation with EGF, interacts with adapter complexes (Sorkin and Carpenter 1993). Recent work has shown that activation of the EGF receptor leads to activation of Eps15, a substrate for EGF-receptor kinase (Benmerah et al. 1995; van Delft et al. 1997; Torrisi et al. 1999). Eps15 appears to be an organizing molecule mediating many of the functions associated with RPTK growth-factor responses in eucaryotic cells (Torrisi et al. 1999). G protein–linked receptor internalization is promoted via the effects of arrestin, which enhances clathrin-coated pit localization of this class of receptors (Bunemann and Hosey 1999; Premont et al. 1999) and several reports have suggested that G protein–linked receptors need not be internalized to generate signals (Bunemann and Hosey 1999; Luttrell et al. 1999; Premont et al. 1999). However, for the tyrosine kinase–linked receptors, results to date suggest that receptor internalization and receptor signaling are linked (Sorkin 1998; Torrisi et al. 1999).
In this paper, we explore EGF-receptor activation of the GTPase Rab5 and the linkage between signaling and endocytic trafficking. Rab5 is the rate-limiting GTPase for endocytosis (Bucci et al. 1992; Li et al. 1994; Stenmark et al. 1994). The dominant-negative mutant of Rab5, Rab5:N34, reduces endocytic rate and blocks in vitro endosome fusion (Li et al. 1994). Rab5 is activated by guanine nucleotide exchange, a process that is coupled to Rab5 recruitment from the cytosol to the membrane fraction (McBride et al. 1999; Ullrich et al. 1994). Downstream effectors of Rab5 include EEA1 and Syntaxin among other proteins (Simonsen et al. 1999; McBride et al. 1999) and a direct interaction among Rab5, EEA1, and Syntaxin (Simonsen et al. 1999) strengthens earlier work suggesting a linkage between RabGTPases and SNARES (soluble N-ethylmaleimide–sensitive factor attachment protein receptor; Sogaard et al. 1994). Several proteins have been identified that act as guanine nucleotide exchange factors and GTPase-activating proteins for Rab5, including Rabex5 (Horiuchi et al. 1997), VPS9 (Hama et al. 1999), and tuberin (Xiao et al. 1997), respectively. Moreover, recent work indicates that EEA1, a FYVE domain containing early endosome protein, which specifically binds three phosphorylated phosphatidylinositols, is required for the tethering of Rab5 to the membrane (Stenmark and Aasland 1999). A major unanswered question is how Rab5 is recruited and activated since it is known that multiple upstream events activate endocytosis (Li et al. 1997). In this context, it is interesting that addition of EGF to cells stimulated the membrane recruitment of EEA1. It is possible that this represents an early event in Rab5 recruitment and/or activation.
Using the EGF receptor as a model, we show that EGF stimulation of receptor-mediated endocytosis and fluid-phase endocytosis is Rab5-dependent and is coupled to Rab5 activation. On the other hand, the dominant negative mutant of Rab5a (Rab5a:N34) blocks both processes. At the light microscope level, we took advantage of GFP-tagged Rab5 molecules to trace the effect of EGF on the endocytic pathway. EGF stimulates the formation of enlarged endosomes that can be monitored in living cells. Multiple in vivo endosome fusion events are readily observed in the EGF stimulated preparation (Roberts et al. 1999; Barbieri, M.A., and P.D. Stahl, unpublished data). The effect of Rab5 on enhancing EGF endocytosis was specific. Expression of Rab7 and Rab11, as wild-type or dominant-negative constructs, were unable to enhance the endocytic effects of EGF. An important set of experiments linking the EGF pathway to activation of Rab5 is found in Fig. 2. Here, addition of EGF to serum-starved cells results in GTP loading of Rab5; green fluorescent protein (GFP) Rab5 and endogenous Rab5 behave virtually the same in this assay, confirming the use of the GFP constructs for these experiments.
The EGF receptor has served as a model for signal transduction studies and many truncation and deletion mutants have been characterized. As shown in Fig. 2, Fig. 5, and Fig. 6, the effect of Rab5a on enhancing the endocytic effect depends on signal-transduction pathways elicited by the EGF tyrosine kinase. Truncation mutants of EGFR, which do not undergo autophosphorylation or activate PLCγ and other SH2-containing effectors, also fail to synergize with Rab5 in elevating endocytosis. Thus, a signal transduction pathway activated by EGF tyrosine kinase appears to mediate activation of Rab5. This pathway may involve phosphatidylinositol 3-kinase and protein kinase B since wortmannin and expression of dominant-negative PKB/akt, respectively, substantially block the EGF effect on endocytosis (data not shown).
What is the relationship of the EGF receptor to Rab5 in the context of signal transduction? Interaction of ligands with cell-surface EGF receptors initiates (a) the recruitment of cytosolic proteins, (b) the generation of activated effector molecules, and (c) the internalization and, presumably, the correct intracellular targeting of occupied receptors. Thus it was very interesting that expression of dominant-negative Rab5a (Rab5a:N34) resulted in an abrogation of the effect of EGF, not only on fluid-phase endocytosis, but also the EGF receptor–mediated endocytosis. The effect of Rab5a:N34 is specific. Expression of Rab7 and Rab11, as wild-type or dominant-negative constructs, were unable to enhance the early endocytic effects of EGF. By what mechanism does Rab5:N34 block EGF signal transduction? McLauchlan et al. 1998 have suggested that Rab5 is incorporated into nascent-coated vesicles. Others have failed to detect Rab5 in coated vesicles (Fischer von Mollard et al. 1994; our unpublished observations), although the difference in results may have to due with methodology. It is possible that the incorporation of Rab5 or a Rab5-interacting protein into an EGF-receptor/macromolecular complex at the membrane precedes and is required for the formation of a clathrin-coated vesicle. In the former case, dominant-negative Rab5a, trapped in the GDP form, would fail to be recruited to the membrane, thereby inhibiting the EGF receptor–dependent coated vesicle assembly. In the latter scenario, Rab5a:N34 would sequester a factor that is needed for assembly of an EGF-receptor macromolecular complex. The latter would be consistent with the hypothesis that Rab5 may be incorporated into newly formed endosomes after their formation but before docking and fusion. Carter et al. 1993 suggested some years ago that a GTPase may play an early role in coated vesicle formation based on inhibition by guanosine 5′-[γ-thio]triphosphate, a nonhydrolyzable analogue of GTP, of the sequestration phase of coated vesicle formation in vitro. As shown in Fig. 5B and Fig. C, Rab5a and Rab5a:N34 had robust effects on transferrin receptor internalization, which further supports the idea that the dominant-negative Rab5a disrupts early events. A second signaling pathway, further delineated by this study, is the pathway that leads from EGF receptor activation to stimulation of fluid-phase endocytosis. Many studies have demonstrated the effect of EGF and other growth factors on fluid-phase endocytosis. Indeed, West et al. 1989 have attributed a portion of the fluid phase endocytic effect of EGF to macropinocytosis. Since wortmannin and Rab5a:N34 block EGF activation of fluid-phase endocytosis, it is likely that EGF activates fluid-phase endocytosis by a novel signaling pathway. Earlier work with activated Ras (RasV12) demonstrated that activation of endocytosis via Ras used the phosphatidylinositol 3-kinase and PKB/akt pathways (Barbieri et al. 1998b; Li et al. 1997).
Lastly, an intriguing set of observations concerns the specific effects of the Rab5a isoform in EGF-stimulated endocytosis and signal transduction. The effect of EGF on endocytosis and the selective effects of the Rab5 isoforms can be seen clearly by light confocal microscopy. EGF stimulates endocytosis and the formation of large endosomes, which are GFP-Rab5a positive. This effect is Rab5 isoform-specific and the endosomes that are formed are both GFP-Rab5 and Tx-EGF positive (Fig. 2). Rab5a very strongly facilitates the formation of large endosomes after EGF stimulation, whereas Rab5b is virtually inactive. Rab5c is intermediate between Rab5a and Rab5b. Rab5 exists as three closely related molecules in mammalian cells. All three isoforms are ubiquitously expressed in endosomal membranes and all enhance endocytosis when overexpressed in a variety of cells. Singer-Kruger et al. 1994 cloned three isoforms of Rab5-like GTPases in yeast, Ypt51p, Ypt52p Ypt55p, and demonstrated that all play a role in endocytosis and targeting to the vacuole. They envisioned each of these closely related Rab5 GTPases playing key, possibly sequential, roles along a complex pathway. Based on work suggesting a role in host defense (Alvarez-Dominguez and Stahl 1998) in and phagocytosis (Alvarez-Dominguez and Stahl 1999), we showed that Rab5a is selectively induced in mammalian macrophages by interferon. Chiariello et al. 1999 recently showed differential phosphorylation of the Rab5 isoforms. Selective induction of the Rab5 isoforms, as demonstrated by the recent studies using DNA microchips to catalog gene induction by serum (Iyer et al. 1999), coupled with the aforementioned effects of interferon and the selective post-translational modification of the Rab5 GTPases, highlight the point that the three Rab5 isoforms most likely have different functions. These findings also anticipate the identification of alternate, perhaps parallel, endocytic pathways.
Acknowledgments
We thank Libby Peters and Lori LaRose for the excellent technical assistance.
This work was supported by National Institutes of Health grants GM42259, AI35884, and AI20015 to P.D. Stahl and GM54739 to A. Wells.
Footnotes
Abbreviations used in this paper: EEA1, early endosomal autoantigen 1; EGFR, EGF receptor; GFP, green fluorescent protein; RPTK, receptors with intrinsic tyrosine kinase activity; Tx, Texas red.
References
- Alvarez-Dominguez C., Stahl P.D. Interferon-gamma selectively induces Rab5a synthesis and processing in mononuclear cells. J. Biol. Chem. 1998;273:33901–33904. doi: 10.1074/jbc.273.51.33901. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dominguez C., Stahl P.D. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- Barbieri M.A., Hoffenberg S., Roberts R., Mukhopadhyay A., Pomrehn A., Dickey B.F., Stahl P.D. Evidence for a symmetrical requirement for Rab5:GTP in in vitro endosome–endosome fusion J. Biol. Chem 273 1998. 25850 25855a [DOI] [PubMed] [Google Scholar]
- Barbieri M.A., Kohn A.D., Roth R.A., Stahl P.D. Protein kinase B/akt and rab5 mediate Ras activation of endocytosis J. Biol. Chem 273 1998. 19367 19370b [DOI] [PubMed] [Google Scholar]
- Beguinot L., Lyall R.M., Willingham M.C., Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc. Natl. Acad. Sci. USA. 1984;81:2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Gagnon J., Begue B., Megarbane B., Dautry-Varsat A., Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Bunemann M., Hosey M.M. G-protein coupled receptor kinases as modulators of G-protein signalling. J. Physiol. 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.L., Redelmeier T.E., Woollenweber L.A., Schmid S.L. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J. Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Gupta K., Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J. Cell Biol. 1994;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M., Bruni C.B., Bucci C. The small GTPases Rab5a, Rab5b and Rab5c are differentially phosphorylated in vitro . FEBS Lett. 1999;453:20–24. doi: 10.1016/s0014-5793(99)00686-9. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Li G., Colombo M.I., Stahl P.D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Walch-Solimena C., Takei K., Daniels L., Khoklatchev A., De Camilli P., Sudhof T.C., Jahn R. Localization of Rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur. J. Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- Goldstein J.L., Brown M.S., Anderson R.G.W., Russel D.W., Schneider W.J. Receptor-mediated endocytosisconcepts emerging from the LDL receptor system. Annu. Rev. Cell. Biol. 1985;1:1–19. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Grimes M.L., Zhou J., Beattie E.C., Yuen E.C., Hall D.E., Valletta J.S., Topp K.S., LaVail J.H., Bunnett N.W., Mobley W.C. Endocytosis of activated TrkAevidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Maxfield F.R. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hama H., Tall G.G., Horazdovsky B.F. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J. Biol. Chem. 1999;274:15284–15291. doi: 10.1074/jbc.274.21.15284. [DOI] [PubMed] [Google Scholar]
- Hanover J.A., Willingham M.C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Schooler K., Wells A., Wiley H.S., Lauffenburger D.A. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J. Biol. Chem. 1999;274:8958–8965. doi: 10.1074/jbc.274.13.8958. [DOI] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C.R., Trowbridge I.S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A-431 cells. J. Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C.R., Miller K., Beardmore J.M. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptorsa comparison of constitutive and ligand-induced uptake. J. Cell Sci. 1985;3:173–186. doi: 10.1242/jcs.1985.supplement_3.17. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Lippe R., McBride H.M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Iyer V.R., Eisen M.B., Ross D.T., Schuler G., Moore T., Lee J.C.F., Trent J.M., Staudt L.M., Hudson J., Jr., Boguski M.S. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Baba T., Redelmeier T.E., Schmid S.L. Recruitment of epidermal growth factor and transferrin receptors into coated pits in vitrodiffering biochemical requirements. Mol. Biol. Cell. 1993;4:715–727. doi: 10.1091/mbc.4.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Stahl P.D. Structure–function relationship of the small GTPase rab5. J. Biol. Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- Li G., Barbieri M.A., Colombo M.I., Stahl P.D. Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J. Biol. Chem. 1994;269:14631–14635. [PubMed] [Google Scholar]
- Li G., D'Souza-Schorey C., Barbieri M.A., Roberts R.L., Stahl P.D. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., D'Souza-Schorey C., Barbieri M.A., Cooper J.A., Stahl P.D. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J. Biol. Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- Lohi O., Poussu A., Merilainen J., Kellokumpu S., Wasenius V.-M., Lehto V.-P. EAST, an epidermal growth factor receptor- and Eps15-associated protein with Src homology 3 and tyrosine-based activation motif domains. J. Biol. Chem. 1998;273:21408–21415. doi: 10.1074/jbc.273.33.21408. [DOI] [PubMed] [Google Scholar]
- Lutcke B.C., Steele-Mortimer O., Olkkonen V.M., Dupree P., Chiariello M., Bruni C.B., Simons K., Zerial M. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. doi: 10.1016/0014-5793(95)00477-q. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Daaka Y., Lefkowitz R.J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- McBride H.M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Moghal N., Sternberg P.W. Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell. Biol. 1999;11:190–196. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- Olayioye M.A., Graus-Porta D., Beerli R.R., Rohrer J., Gay B., Hynes N.E. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol. Cell. Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R.T., Claing A., Vitale N., Freeman J.L., Pitcher J.A., Patton W.A., Moss J., Vaughan M., Lefkowitz R.J. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA. 1999;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M.A., Shome K., Vasudevan C., Stolz D.B., Sung T.-C., Frohman M.A., Watkins S.C., Romero G. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent Raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 1999;274:1131–1139. doi: 10.1074/jbc.274.2.1131. [DOI] [PubMed] [Google Scholar]
- Roberts R.L., Barbieri M.A., Pryse K.M., Chua M., Morisaki J.H., Stahl P.D. Endosome fusion in living cells overexpressing GFP-rab5. J. Cell Sci. 1999;112:3667–3675. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Gaullier J.M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B., Stenmark H., Dusterhoft A., Philippsen P., Yoo J.S., Gallwitz D., Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M., Tani K., Ye R.R., Geromanos S., Tempst P., Kirchhausen T., Rothman J.E., Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Sorkin A. Endocytosis and intracellular sorting of receptor tyrosine kinases Front. Biosci 3 1998. 729 738(Online). [DOI] [PubMed] [Google Scholar]
- Sorkin A., Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Waters C., Overholser K.A., Carpenter G. Multiple autophosphorylation site mutations of the epidermal growth factor receptor. Analysis of kinase activity and endocytosis. J. Biol. Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- Sorkin A., Eriksson A., Heldin C.-H., Westermark B., Claesson-Welsh L. Pool of ligand-bound platelet-derived growth factor γ-receptors remain activated and tyrosine-phosphorylated after internalization. J. Cell Physiol. 1993;156:373–382. doi: 10.1002/jcp.1041560221. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Bild A., Tebar F., Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J. Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Aasland R. FYVE-finger proteins—effectors of an inositol lipid. J. Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R.G., Steele-Mortimer O., Lutcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M.R., Lotti L.V., Belleudi F., Gradini R., Salcini A.E., Confalonieri S., Pelicci P.G., Di Fiore P.P. Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell. 1999;10:417–434. doi: 10.1091/mbc.10.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O., Horiuchi H., Bucci C., Zerial M., Stenmark H. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- van Delft S., Schumacher C., Hage W., Verkleij A.J., van Bergen en Henegouwen P.M.P. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J. Cell Biol. 1997;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery R.G., von Zastrow M. Distinct dynamin-dependent and independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J. Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze C., Schmid S.L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;27:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wang Z., Moran M.F. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Warren R.A., Green F.A., Enns C.A. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- Waterman H., Alroy I., Strano S., Seger R., Yarden Y. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:3348–3358. doi: 10.1093/emboj/18.12.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. Molecules in focus EGF receptor. Int. J. Biochem. Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- Wells A., Welsh J.B., Lazar C.S., Wiley H.S., Gill G.N., Rosenfeld M.G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- West M.A., Bretscher M.S., Watts C. Distinct endocytotic pathways in epidermal growth factor–stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A., Beattie E.C., Lem L., Riethof D.A., Liu S.H., Mobley W.C., Soriano P., Brodsky F.M. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- Xiao G.H., Shoarinejad F., Jin F., Golemis E.A., Yeung R.S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]