Abstract
Rab5 regulates endocytic membrane traffic by specifically recruiting cytosolic effector proteins to their site of action on early endosomal membranes. We have characterized a new Rab5 effector complex involved in endosomal fusion events. This complex includes a novel protein, Rabenosyn-5, which, like the previously characterized Rab5 effector early endosome antigen 1 (EEA1), contains an FYVE finger domain and is recruited in a phosphatidylinositol-3-kinase–dependent fashion to early endosomes. Rabenosyn-5 is complexed to the Sec1-like protein hVPS45. hVPS45 does not interact directly with Rab5, therefore Rabenosyn-5 serves as a molecular link between hVPS45 and the Rab5 GTPase. This property suggests that Rabenosyn-5 is a closer mammalian functional homologue of yeast Vac1p than EEA1. Furthermore, although both EEA1 and Rabenosyn-5 are required for early endosomal fusion, only overexpression of Rabenosyn-5 inhibits cathepsin D processing, suggesting that the two proteins play distinct roles in endosomal trafficking. We propose that Rab5-dependent formation of membrane domains enriched in phosphatidylinositol-3-phosphate has evolved as a mechanism for the recruitment of multiple effector proteins to mammalian early endosomes, and that these domains are multifunctional, depending on the differing activities of the effector proteins recruited.
Keywords: endocytosis, Rab5, hVPS45, EEA1, Rabenosyn-5
Introduction
In eukaryotic cells, trafficking of membrane and proteins through the biosynthetic and endocytic pathways is subject to regulation by Rab GTPases (Mellman 1996). Different members of the Rab GTPase family have been identified to localize to distinct compartments of the endomembrane system (Simons and Zerial 1993). In several cases, these Rab GTPases have been demonstrated to regulate diverse functions that are associated with membrane trafficking, such as vesicle formation (Jones et al. 1993; McLauchlan et al. 1998), vesicle docking, and fusion events (Salminen and Novick 1987; Stenmark et al. 1995b; Mayer and Wickner 1997; Simonsen et al. 1998; Christoforidis et al. 1999a). Recently, in addition to their role in regulation of membrane tethering and docking of organelles, Rab GTPases have been implicated in the regulation of organelle association with, or movement upon, cytoskeletal networks within eukaryotic cells (Echard et al. 1998; Nielsen et al. 1999).
In the case of vesicle transport, a complex series of protein interactions ensures the coupling between the vesicle tethering, which is regulated by Rab GTPases through their effector proteins (Mayer and Wickner 1997; Christoforidis et al. 1999a; Guo et al. 1999), and membrane fusion, which occurs through priming and proper pairing of soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) molecules. However, the mechanism underlying the coupling of these two processes is still poorly understood. For the small GTPase Rab5, which regulates membrane traffic into and between early endosomes in mammalian cells, the coordination between vesicle tethering and SNARE function has been proposed to involve the organization of a specialized membrane domain on early endosomes (McBride et al. 1999; Sonnichsen et al. 2000). Consistent with this hypothesis, morphological studies have shown that Rab5 occupies a restricted membrane domain on endosomes that displays distinct biochemical features compared with the neighboring subcompartments occupied by Rab4 and Rab11 (Sonnichsen et al. 2000). The Rab5 GTPase forms this domain by recruiting the specific phosphatidylinositol (PI)-3-kinase isoform hVPS34, causing localized production of PI-3-phosphate (PI-3P) (Christoforidis et al. 1999b). The concomitant presence of Rab5 and PI-3P is necessary for the efficient recruitment of the Rab5 effector protein early endosome antigen 1 (EEA1) on early endosomes. EEA1, which serves as a membrane-tethering molecule, binds PI-3P through the interaction of a specialized zinc finger, called a FYVE finger (Simonsen et al. 1998). However, the recent demonstration that at least 20 proteins specifically interact with active Rab5 highlights the complexity of the downstream regulation of this GTPase (Christoforidis et al. 1999a), and raises the possibility that other effector proteins could be involved in regulation of endosomal fusion events.
Here, we identify and characterize a novel Rab5 effector complex that plays an important role in endosome fusion events. This complex contains two proteins. One is the previously described Sec1-like protein, hVPS45. The second is a novel protein that interacts directly with Rab5. We have named this protein Rabenosyn-5 to highlight its role as a link between the Rab5 GTPase and the syntaxin binding protein hVPS45: Rab + enono (“to link” in Greek) + syn (syntaxin-binding protein hVPS45). As with EEA1, Rabenosyn-5 contains a FYVE zinc finger, indicating that the generation of PI-3P is an important characteristic for the recruitment of multiple Rab5 effector proteins to the endosome.
Materials and Methods
Antibodies, Plasmids, and Other Reagents
Human anti-EEA1 serum (1:10,000) was a gift from Ban Hok Toh (Monash Medical School, Adelaide, Australia). Secondary antibody conjugates (HRP and fluorescently labeled) were purchased from Dianova. pGEM-Rab5Q79L (Stenmark et al. 1994), pGEX-syntaxin 7, and pGEX-syntaxin 13 (McBride et al. 1999) have been described previously. pGEX-syntaxin 6 was a gift from R. Piper (University of Iowa, Iowa City, IA) and pGEX-hVPS45 was a gift from R. Scheller (Stanford University, Stanford, CA). pCDNA3-syntaxin4ΔTM was a gift from A. Klip (Hospital for Sick Children, University of Toronto, Toronto, Canada). pGEX-syntaxin 4 was constructed by insertion of the syntaxin4ΔTM fragment from a pCDNA-syntaxin4ΔTM into the BamHI and EcoRI sites of pGEX-4T1 (Amersham Pharmacia Biotech). Full-length Rabenosyn-5 as well as fragments of Rabenosyn-5 were PCR amplified using primers against the corresponding cDNA sequence, and cloned into the EcoRI and HindIII restriction sites of the pGEM-myc4 vector (Stenmark et al. 1994). hVPS45 was PCR amplified and cloned into the XbaI–XhoI sites of pBluescript II KS (Stratagene).
Production of Antipeptide Antibodies against Rabenosyn-5 and hVPS45
A peptide, CRELKHTLAKQKGGTD, corresponding to the Rabenosyn-5 COOH-terminal sequence (Genosys Inc.), and two peptides, CQGRNWDPAQLSRTTQ and CSRESSQATSRSASRR, corresponding to internal and COOH-terminal sequences, respectively, were synthesized (Eurogentec). These peptides were conjugated to keyhole limpet hemocyanin (and mixed, in the case of the two hVPS45 peptides), and injected into rabbits. Antipeptide pAbs were affinity purified using the respective peptides immobilized on Sulfolink beads (Pierce Chemical Co.). Antibodies were eluted from the affinity column following standard procedures and equilibrated in PBS.
Gel Overlay Assay
This assay was a modification of the procedure of Horiuchi et al. 1997. Proteins were separated by two-dimensional SDS-PAGE, transferred to nitrocellulose (BA 85; Schleicher and Schuell), renatured by incubation at 4°C overnight, and washed as described previously (Horiuchi et al. 1997). The blot was incubated in binding buffer (12.5 mM Hepes/KOH, pH 7.4, 1.5 mM magnesium acetate, 75 mM potassium acetate, 1 mM DTT, 2 mg/ml BSA, 0.005% Triton X-100, 4 mM N-octylglycopyranoside) in the presence of 10 mg/ml glutathione S-transferase (GST)-Rab5 loaded with either GTPγS or GDP (Christoforidis and Zerial 2000). After washing with binding buffer, the blot was incubated for 1 h with 2.5 μg/ml anti-GST antibodies (Amersham Pharmacia Biotech) in binding buffer, washed again with binding buffer, and incubated for 1 h in binding buffer in 1:5,000 anti–sheep HRP-conjugated antibodies. After washing with binding buffer, the filter was incubated in chemiluminescence buffer (NEN Life Sciences Products) and exposed to x-ray film.
Amino Acid Sequence Determination and Rabenosyn-5 Cloning
Proteins were excised from gels and enzymatically digested (Schevchenko et al. 1996; Wilm et al. 1996). The tandem mass spectroscopy protein sequencing procedure was performed as described previously (Wilm et al. 1996; Wilm and Mann 1996). Peptides determined from Rabenosyn-5 were from bovine brain, and were used to identify corresponding expressed sequence tags (ESTs) using BLAST similarity searches (available at http://www.ncbi.nim.nih.gov/BLAST/). Four peptides (APEYIR, PPHPSNLR, YSATLFVQEK, and EQFEELK) were contained in an EST (sequence data available from EMBL/GenBank/DDBJ under accession no. W02080) that corresponded to the 5′ end of the Rabenosyn-5 gene. The remaining 3′ end of Rabenosyn-5 was then cloned and sequenced by screening a random primed HeLa cDNA plasmid library (Stenmark et al. 1995b) using a biotinylated primer, 5′-CGAGGCAGCATCAGCATGAGCAGTGTC-3′, with a ClonCapture cDNA selection Kit (CLONTECH Laboratories, Inc.).
Cells, Transfection, and Cathepsin D Trafficking
HeLa cells were grown in MEM containing 5% heat-inactivated FCS, 5% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and nonessential amino acids. Stable transformed green fluorescent protein (GFP)-Rab5 A431 cells (Nielsen et al. 1999) were grown in DMEM containing 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 0.5 μg/ml G-418. For transient expression studies, HeLa cells were infected for 30 min with T7 RNA polymerase recombinant vaccinia virus and then transfected with plasmids containing cDNAs of interest, as described in Stenmark et al. 1995a. For cathepsin D trafficking studies, HeLa cells were allowed to express cDNA constructs for 2 h, starved for 15 min, and then labeled with [35S]methionine for 30 min, and chased with cold methionine for 0–4 h. Immunoprecipitation and analysis of 35S-labeled cathepsin D were performed as described previously (Press et al. 1998).
Confocal Immunofluorescence Microscopy
Cells grown on glass coverslips were processed for immunofluorescence as described previously (Stenmark et al. 1995b). Cells were mounted in moviol and examined on a confocal microscope (Microsystems LSM-510; ZEISS) using an Axioplan2 microscope with 63×/1.40 plan-Apochromat lens (ZEISS). Fluorescent images were collected at 2× zoom using the ZEISS LSM software package, and processed using Adobe Photoshop® v5.0. Quantification of the signal overlap was performed as described previously (Sonnichsen et al. 2000).
In Vitro Endosomal Fusion and Recruitment Assays
Early endosomes labeled with biotinylated transferrin or antitransferrin antibodies and clathrin-coated vesicles (CCVs) labeled with biotinylated transferrin were prepared from HeLa cells (Rubino et al. 2000). In vitro fusion assays were performed as in Horiuchi et al. 1997 and quantified using the ECL-Analyzer system from IGEN Inc. Recruitment of cytosolic proteins to early endosomes and liposomes was performed as described previously (Christoforidis et al. 1999b), using early endosomes from HeLa cells, or with liposomes (98% phosphatidylcholine [PC], 2% phosphoinositides; 1 mg/ml final concentration in 50 mM Hepes-KOH, pH 7.4, 110 mM KCl, 2 mM EGTA, 2 mM MgCl2) prepared as in Otter-Nilsson et al. 1999.
In Vitro Binding Assays
The GST-Rab5 affinity chromatography, and subsequent Superose-6 size-exclusion chromatography of Rab5 effector proteins, was performed as in Christoforidis and Zerial 2000. [35S]Methionine-labeled proteins were transcribed and translated in vitro using a TnT™ coupled transcription–translation kit (Promega). For Rab5 effector recruitment assays, in vitro– translated proteins were incubated with glutathione-sepharose beads complexed with GST-Rab5-GTPγS or GST-Rab5-GDP, as described in Christoforidis et al. 1999b, and then eluted using procedures as in Christoforidis and Zerial 2000. For hVPS45 recruitment to syntaxins, in vitro–translated hVPS45 was incubated with glutathione-sepharose beads complexed to GST-syntaxins (5 mg/ml) in binding buffer (50 mM Hepes/KOH, pH 8, 150 mM NaCl, 5 mM β-mercaptoethanol, 0.05% [vol/vol] Tween 20) overnight at 4°C. After incubation, beads were isolated and washed in binding buffer. GST-syntaxins, and associated proteins, were eluted with binding buffer containing 25 mM glutathione.
Results
Identification of a Novel Rab5 Effector Protein
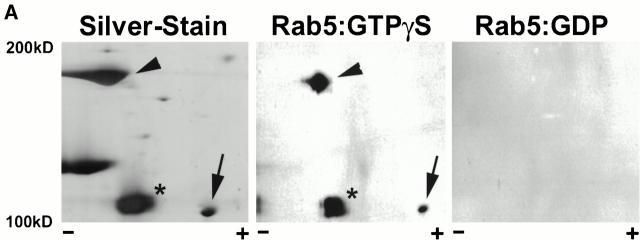
Although some previously characterized Rab5-interacting proteins bind to Rab5 directly (e.g., the PI-3-kinase catalytic subunit p110β), others (e.g., the regulatory subunit p85α) interact indirectly through binding to other Rab5 effectors (Christoforidis et al. 1999b). To identify those proteins in the Rab5-effector protein fraction that were capable of directly interacting with Rab5, we used a previously characterized gel-overlay assay (Horiuchi et al. 1997). In brief, proteins eluted from the Rab5 affinity column (Christoforidis et al. 1999b; Christoforidis and Zerial 2000) were separated by two-dimensional gel electrophoresis, transferred to nitrocellulose blots, and then probed for their ability to interact specifically with GST-Rab5 in the presence of GTPγS. The results from these experiments are displayed in Fig. 1 A. A significant fraction of the proteins from the Rab5 column eluate were capable of interacting directly with Rab5. Representative positions of the three most abundant Rab5-interacting proteins were determined by silver staining (Fig. 1 A, left). Two proteins, indicated by an arrowhead and an arrow, corresponded to EEA1 and Rabaptin-5, respectively. A third protein, indicated with an asterisk, corresponded to an unidentified protein with a molecular mass of 110 kD. This protein, as well as EEA1 and Rabaptin-5, interacted specifically with the GTP-associated form of GST-Rab5 (Fig. 1 A, middle), but not the GDP-associated form (Fig. 1 A, right). We isolated this protein band, subjected it to trypsin digestion, and sequenced the resultant peptide mixtures by nanoelectrospray tandem mass spectrometry (Wilm et al. 1996; Wilm and Mann 1996).
Figure 1.
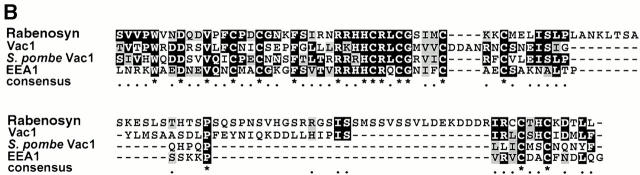
Rabenosyn-5 is a novel Rab5 effector protein with FYVE finger and C2H2 zinc finger domains. (A) Specific binding of Rab5 effector proteins to GST-Rab5-GTPγS. Rab5-interacting proteins purified by GST-Rab5 affinity chromatography were subjected to two-dimensional SDS-PAGE followed by silver staining (left), or transferred to nitrocellulose and gel-overlay assay with GST-Rab5-GTPγS (middle), or GST-Rab5-GDP (right). Positions of the known Rab5 effector proteins, EEA1 (arrowhead), and Rabaptin-5α (arrow) were determined by immunoblotting. The position of a novel 110-kD Rab5 effector, Rabenosyn-5, is marked with an asterisk. (B) Alignment of the FYVE finger domain of Rabenosyn-5 with other Rab effector proteins. The FYVE finger domains of human Rabenosyn-5 (sequence data available from EMBL/GenBank/DDBJ under accession no. AY009133), S. cerevisiae Vac1p (accession no. P32609), S. pombe Vac1p homologous protein (accession no. Z99162), and human EEA1 (accession no. S44243) were aligned using CLUSTALW. (C) Domain organization of Rabenosyn-5, Vac1p, and EEA1. Each protein is represented as a line; the relative lengths are proportional to the length of the coding sequence. Positions of C2H2, RING, and FYVE zinc fingers, and the NPF motif–containing domains are indicated. (D) The five NPF-containing motifs of Rabenosyn-5, and their consensus sequence. (E) Schematic diagram of the truncation mutations of Rabenosyn-5.
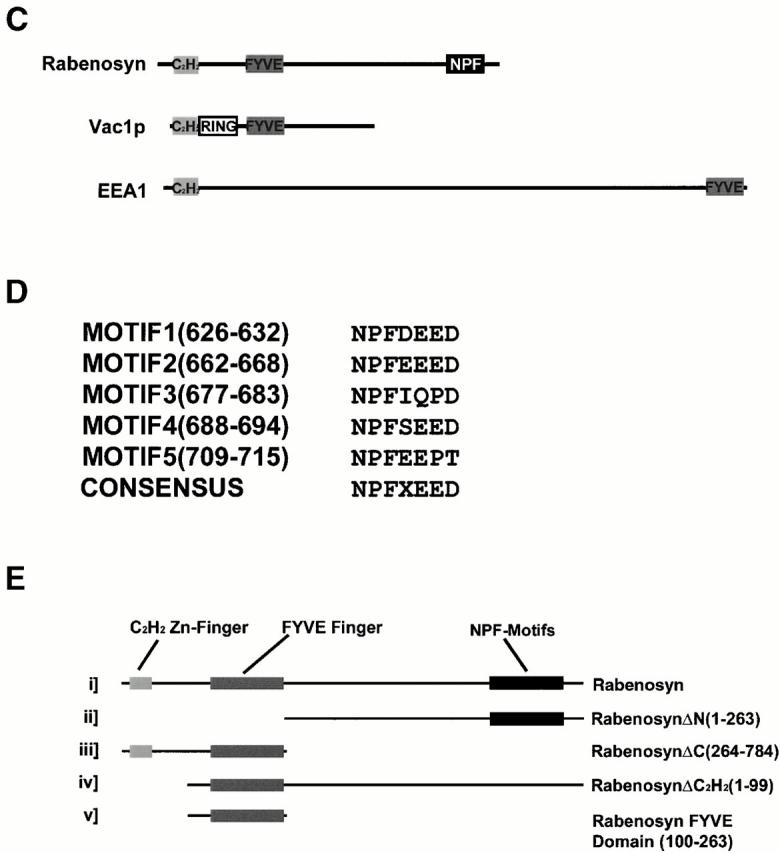
Several of the peptides from this 110-kD protein matched the deduced amino acid sequence of an EST (sequence data available from EMBL/GenBank/DDBJ under accession no. W02080). Using primers derived from the 3′ end of this insert, the entire coding region of the 110- kD protein was isolated from a random primed HeLa cDNA library (sequence data available from EMBL/GenBank/DDBJ under accession no. AY009133; see Materials and Methods). Computer predicted structural analysis of the open reading frame indicated that the protein was hydrophilic with no signal peptide or potential transmembrane domains. When we searched the GenBank nonredundant database using the BLAST program, we determined that this protein showed highest homology to the Saccharomyces cerevisiae protein Vac1p, a putative Vac1 homologue from Saccharomyces pombe and the human protein EEA1. However, in all cases, homology to the 110-kD protein, which we called Rabenosyn-5, was largely restricted to two predicted zinc finger domains, an NH2-terminal C2H2-type finger, and an internal FYVE finger domain (Fig. 1 B) (Stenmark et al. 1996; Stenmark and Aasland 1999). Although the domain organization within the NH2-terminal half of Rabenosyn-5 was more similar to Vac1p than EEA1, Rabenosyn-5 also showed features that distinguished it from Vac1p. Vac1p contained an additional RING zinc finger domain between the C2H2-type zinc finger and the FYVE finger (Fig. 1 C). Additionally, Rabenosyn-5 contains a significantly larger COOH-terminal region, displaying no apparent homology to Vac1p, that contains five copies of the amino acid motif NPF (Fig. 1 D). NPF-containing motifs have recently been identified as the core of a binding site for proteins containing Eps15 homology (EH) domains (Salcini et al. 1997) and are considered protein–protein interaction motifs. Therefore, Rabenosyn-5 is a novel protein and the second mammalian protein, after EEA1, that directly interacts with Rab5 and contains a FYVE finger domain (Mu et al. 1995; Simonsen et al. 1998).
Rabenosyn-5 Colocalizes with EEA1 on Rab5-positive Endosomes
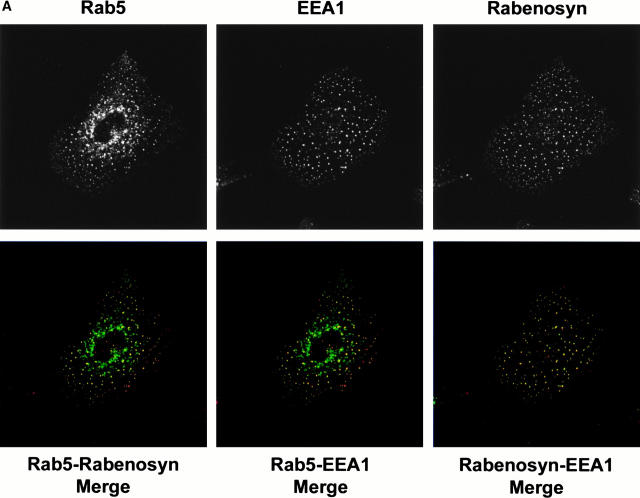
Because the FYVE finger domain plays an important role in targeting EEA1 to endosomes (Simonsen et al. 1998), we wanted to determine whether Rabenosyn-5 was localized to the same endosomes as EEA1. We performed triple labeling–confocal microscopy analysis to compare the localization of endogenous Rabenosyn-5 and EEA1 with each other and with Rab5 in A431 cells, which have stable expression of enhanced GFP (EGFP)-Rab5 (Nielsen et al. 1999). Cells were processed for immunofluorescence for Rabenosyn-5 and EEA1 (Fig. 2 A). Both Rabenosyn-5 and EEA1 showed significant overlap with one another and with EGFP-Rab5. When overlap of these proteins was quantitated (see Materials and Methods), ∼50% of EGFP-Rab5–positive structures colocalized with EEA1 or Rabenosyn-5, and ∼95% of EEA1 structures colocalized with Rabenosyn-5. We concluded that Rabenosyn-5 colocalized with Rab5-positive endosomes in vivo, as well as interacting with Rab5 in vitro (Fig. 1 A), and that these endosomes contain both EEA1 and Rabenosyn-5.
Figure 2.
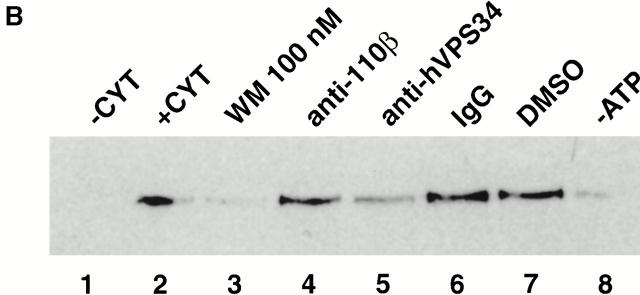
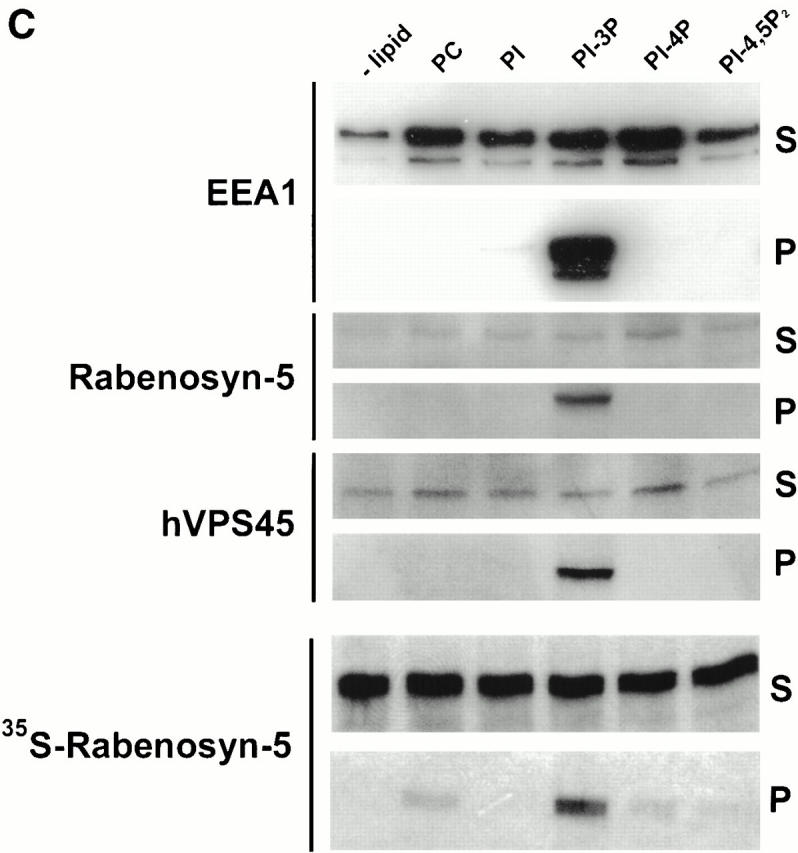
Localization of Rabenosyn-5 with early endosomes is PI-3-kinase dependent. (A) Rabenosyn-5 colocalizes with EEA1 on early endosomes in A431 cells. A431 cells expressing EGFP-Rab5 were processed for immunofluorescence and analyzed by laser scanning confocal microscopy to detect the extent of colocalization of EGFP-Rab5 fluorescence (top left), EEA1 was detected with human antiserum (top middle), and Rabenosyn-5 was detected with affinity-purified rabbit antibodies (top right). EGFP-Rab5 (green) colocalized significantly with Rabenosyn-5 (red; bottom left) and EEA1 (red; bottom middle); EEA1 (green) and Rabenosyn-5 (red) displayed complete colocalization (bottom right). (B) Recruitment of Rabenosyn-5 on early endosomes. Reactions containing early endosomes, cytosol (3 mg/ml), and an ATP-regenerating system were incubated for 30 min at 37°C (+CYT, lane 2), membranes were recovered by centrifugation, resuspended in SDS-PAGE buffer, and analyzed by immunoblotting with antibodies against Rabenosyn-5. Reactions were carried out in the absence of cytosol (−CYT, lane 1) or an ATP-regenerating system (−ATP, lane 8). Other reactions were carried with both cytosol and an ATP-regenerating system (lanes 2–7): alone (+CYT, lane 2); in the presence of 100 nM wortmannin (WM 100 nM, lane 3); function blocking antibodies against p110β (anti-110β, lane 4); hVPS34 (anti-hVPS34, lane 5); control nonspecific IgG (IgG, lane 6); or control concentration of DMSO (DMSO, lane 7). (C) Recruitment of Rabenosyn-5 on artificial liposomes. Reactions containing cytosol (5 mg/ml), or in vitro–translated, [35S]methionine-labeled Rabenosyn-5 were incubated for 15 min at room temperature with liposomes (100 μg total lipid) consisting of PC alone (100% total lipid), or PC mixed with PI (2% total lipid), PI-3P (2%), PI-4P (2%), or PI-4,5P2 (2%). Supernatants (S) and membrane pellets (P) were separated by centrifugation, resuspended in SDS-PAGE buffer (10% of total supernatants), and analyzed by immunoblotting with antibodies specific to Rabenosyn-5, EEA1, and hVPS45, or by fluorography to detect [35S]methionine-labeled Rabenosyn-5.
Rabenosyn-5 Is Targeted to Early Endosomes in a PI-3-kinase–dependent Manner through a FYVE Domain
Because EEA1 is coordinately recruited to endosomes by the action of Rab5 and the interaction of PI-3P with its FYVE domain (Simonsen et al. 1998), we wanted to determine if the FYVE finger domain of Rabenosyn-5 was responsible for targeting this protein to early endosomes in a PI-3-kinase–dependent manner. First, we tested the effect of the PI-3-kinase inhibitor, wortmannin, on recruitment of Rabenosyn-5 to endosomes using an in vitro recruitment assay. As for EEA1 (Simonsen et al. 1998; Christoforidis et al. 1999b), efficient recruitment of Rabenosyn-5 to endosomes was cytosol and ATP dependent (Fig. 2 B, lanes 1, 2, and 8). In the presence of wortmannin, Rabenosyn-5 was no longer efficiently recruited to endosomes (Fig. 2 B, compare lanes 2 and 3). Addition of anti-hVPS34 inhibitory antibodies, but not anti-110β, or nonspecific IgG, inhibited Rabenosyn-5 recruitment (Fig. 2 B, compare lanes 3, 4, and 5). This indicates that Rabenosyn-5 requires PI-3P to translocate from the cytosol to early endosomal membranes. To provide further support for the conclusion that Rabenosyn-5 binds endosomal membranes specifically via PI-3P, we prepared liposomes containing phosphatidylcholine and phosphoinositides (Fig. 2 C). Both cytosolic and in vitro–translated Rabenosyn-5, as well as EEA1, were efficiently recruited to liposomes containing PI-3P (Fig. 2 C). The recruitment of Rabenosyn-5 and EEA1 to PI-3P–containing liposomes was specific, as no significant association was observed with liposomes containing PI, PI-4P, or PI-4,5P2 (Fig. 2 C).
The FYVE finger of EEA1 has been demonstrated to overlap with one of two Rab5 interaction domains found in EEA1 by yeast two hybrid screening methods (Simonsen et al. 1998). Additionally, this domain is capable of recruitment to endosomes in vivo (Stenmark et al. 1996). To determine the regions of the Rabenosyn-5 protein responsible for its localization to endosomes, we constructed a series of truncation mutations of Rabenosyn-5 (see Fig. 1 E) and coexpressed them with Rab5Q79L in HeLa cells (Fig. 3). Full-length Rabenosyn-5 (see scheme in Fig. 1 E), and ΔCOOH-terminal, ΔC2H2, and the FYVE finger truncation mutants all efficiently colocalized with Rab5Q79L enlarged endosomes, whereas the ΔNH2-terminal deletion mutant did not. We conclude that the region (amino acids 100–263) containing the FYVE finger domain is capable of targeting Rabenosyn-5 to endosomes. These results suggest that, as for EEA1, both Rab5 interaction and PI-3P are required for localization of Rabenosyn-5 to early endosomes (Lawe et al. 2000).
Figure 3.
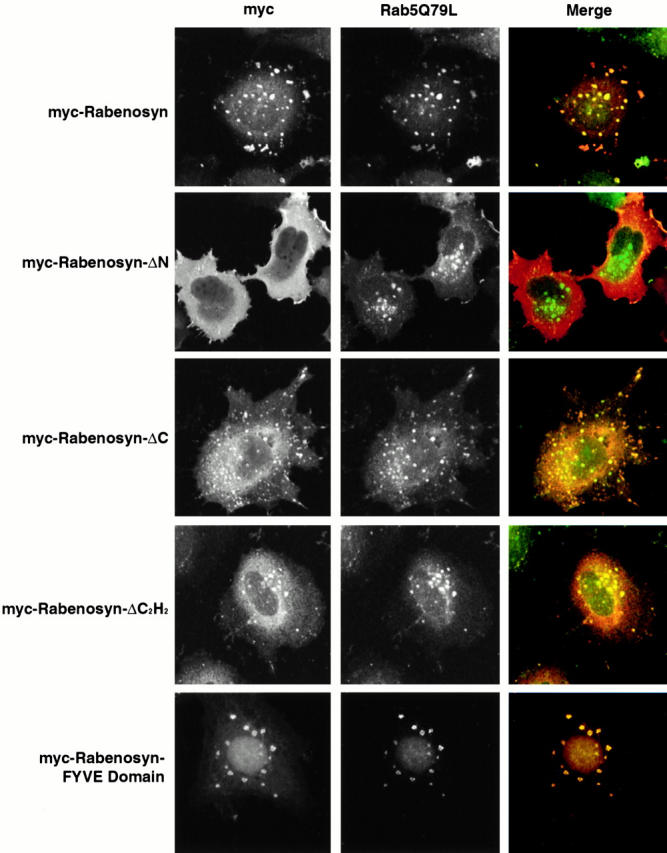
The Rabenosyn-5 FYVE domain is sufficient to target Rabenosyn-5 to early endosomes. HeLa cells coexpressing Rab5Q79L and myc-tagged truncation mutants of Rabenosyn-5 were processed for immunofluorescence and analyzed by laser scanning confocal microscopy for the extent of colocalization of myc-tagged truncations of Rabenosyn-5 (red, Merge) with Rab5Q79L-positive endosomal structures (green, Merge).
Rabenosyn-5 Associates with the Sec1 Homologue hVPS45
The data presented so far imply that Rab5-dependent endocytic membrane transport requires two FYVE finger–containing Rab5 effectors. We next sought to explore functional differences between these two proteins. In S. cerevisiae, Vac1p is thought to act in membrane trafficking as a complex with the Sec1 homologue Vps45p (Burd et al. 1997; Peterson et al. 1999; Tall et al. 1999). Given the similarities in domain order observed between the NH2 termini of Vac1p and Rabenosyn-5, and the large number of proteins recruited to the Rab5 affinity column (Christoforidis et al. 1999a), we wanted to determine if Rabenosyn-5 could be found in complex with other proteins, perhaps Sec1-like proteins. When Rab5 effector proteins were separated by size-exclusion chromatography, we observed that Rabenosyn-5 coeluted with a 65-kD protein (Fig. 4 A). Because this protein also eluted earlier than other proteins with higher apparent molecular weight, we suspected it might form a complex with Rabenosyn-5. Using mass spectroscopy protein sequencing techniques, we identified this protein as hVPS45, the human homologue of yeast Vps45p, and a Sec1-related protein (Pevsner et al. 1996).
Figure 4.
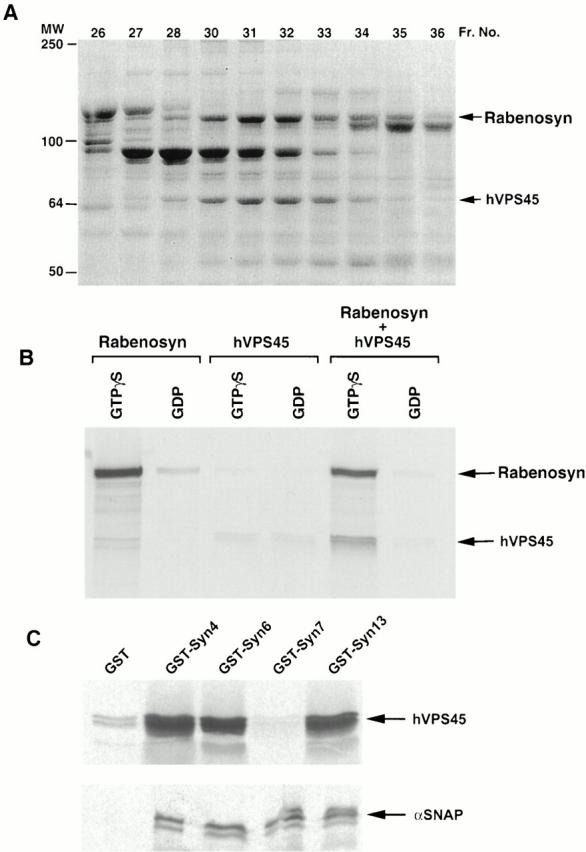
Rabenosyn-5 recruits the Sec1-like protein hVPS45 to Rab5. (A) SDS-PAGE analysis and Coomassie blue staining of Rab5-interacting proteins separated by Superose-6 size-exclusion chromatography. Fraction numbers are indicated at the top of each lane. MS/MS tandem mass spectroscopy sequencing identified Rabenosyn-5 and hVPS45 proteins. (B) Rabenosyn-5 recruits hVPS45 to GST-Rab5. Glutathione-sepharose beads loaded with GST-Rab5-GTPγS (GTPγS) or GST-Rab5-GDP (GDP) were incubated with [35S]methionine-labeled in vitro–translated Rabenosyn-5 alone (Rabenosyn), hVPS45 alone (hVPS45), or both Rabenosyn-5 and hVPS45 cotranslated together (Rabenosyn + hVPS45). Bound proteins were eluted and analyzed by SDS-PAGE followed by fluorography. (C) hVPS45 interacts with multiple syntaxin isoforms. Glutathione-sepharose beads loaded with GST-syntaxin fusion proteins (GST-syntaxin 4, GST-Syn4; GST-syntaxin 6, GST-Syn6; GST-syntaxin 7, GST-Syn7; and GST-syntaxin 13, GST-Syn13), or GST alone (GST), and incubated with [35S]methionine-labeled in vitro–translated hVPS45 (top), or α-SNAP (bottom). GST fusions and associated proteins were eluted and analyzed by SDS-PAGE followed by fluorography.
To determine whether Rabenosyn-5 and hVPS45 indeed form a complex, and if hVPS45 interacts directly or indirectly with Rab5, we performed an affinity-capture assay using glutathione beads containing GST-Rab5-GTPγS or GST-Rab5-GDP (Fig. 4 B). In vitro–translated Rabenosyn-5 alone, but not hVPS45 alone, preferentially interacted with GST-Rab5 in a GTP-specific manner (Fig. 4 B). Upon cotranslation of Rabenosyn-5 with hVPS45, hVPS45 was corecruited to GST-Rab5-GTPγS. This confirmed that Rabenosyn-5 was capable of forming a complex with hVPS45, and also indicated that Rabenosyn-5 was responsible for recruitment of hVPS45 to the GST-Rab5 column. Efficient recruitment of hVPS45, along with Rabenosyn-5, to PI-3P–containing liposomes (see Fig. 2 C) further indicated that these proteins are corecruited to endosomes, most likely in a complex.
hVPS45 Interacts with Multiple Endosomal Syntaxin Homologues
The observation that Rabenosyn-5 is found in complex with hVPS45 implies that this protein serves as a link between Rab5 regulation of endosomal fusion events and regulation of endosomal SNARE complex formation. As the yeast Vps45p can complex with different syntaxin isoforms, e.g., Pep12p and Tlg2p (Burd et al. 1997; Nichols et al. 1998; Abeliovich et al. 1999), hVPS45 was expected to interact with multiple syntaxins present on early endosomes. Besides syntaxin 6 (Tellam et al. 1997), hVPS45 was not known to interact with any other syntaxin family members. However, we have previously observed a requirement of syntaxin 13 for homotypic endosome fusion (McBride et al. 1999). Additionally, syntaxin 7 localizes to early endosomes in vivo (Prekeris et al. 1999). Therefore, we wanted to examine if hVPS45 could interact with these syntaxins. In vitro– translated hVPS45 was incubated with fusion proteins of GST-syntaxin 4, 6, 7, and 13, or GST alone. Fig. 4 C shows that hVPS45 interacted with GST-syntaxin 4, 6, and 13, but not GST-syntaxin 7 or GST. The lack of interaction of hVPS45 with GST-syntaxin 7 was not simply because this protein was inactive, since all GST-syntaxins, including syntaxin 7, bound α-SNAP (Fig. 4 C). We conclude that hVPS45 interacts with multiple syntaxins implicated in endocytic trafficking and/or TGN-endosome trafficking.
Rabenosyn-5 Is Required for Fusion of Endosomes, either Homotypically or with CCVs
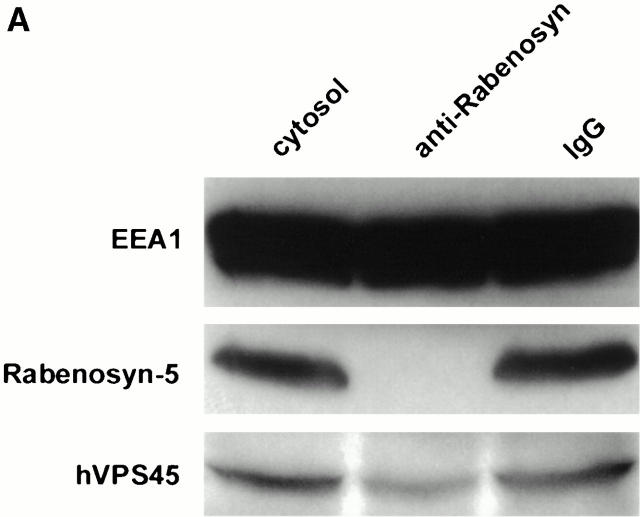
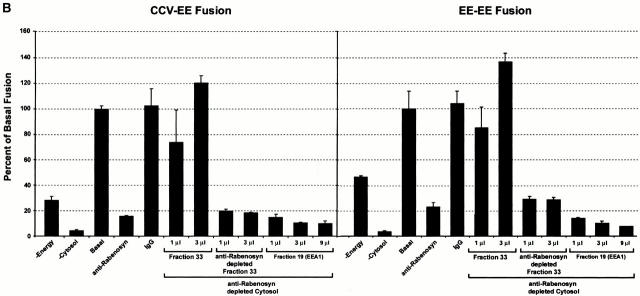
An established function of Rab5 is the regulation of fusion of plasma membrane–derived CCVs with early endosomes and homotypic early endosome fusion. Both processes require the activity of the PI-3-kinase hVPS34, which is essential for the membrane recruitment of EEA1 (Christoforidis et al. 1999b). Release of EEA1 from early endosomes after inhibition of PI-3-kinase activity was suggested as the reason for inhibition of endosome fusion (Li et al. 1995; Simonsen et al. 1998). The finding that Rabenosyn-5 is a Rab5 effector whose membrane association was also mediated by its FYVE finger domain raised the possibility that it might also play a role in endosomal fusion events. Therefore, we wanted to determine if Rabenosyn-5 was necessary for either homotypic endosome fusion, or fusion of endosomes with CCVs. Affinity-purified antibodies were used to quantitatively immunodeplete Rabenosyn-5 from the cytosol (Fig. 5 A). As a control, we verified that EEA1 levels in the cytosol were unaffected (Fig. 5 A). Additionally, hVPS45 levels were reduced, but not quantitatively depleted, indicating that not all cytosolic hVPS45 was associated with Rabenosyn-5. CCV–endosome fusion and homotypic endosome fusion were both inhibited by ∼80% in Rabenosyn-5 immunodepleted cytosol (Fig. 5 B; compare anti-Rabenosyn with IgG lanes). Control treatment of cytosol with nonspecific IgG did not significantly effect fusion (Fig. 5 B; compare Basal with IgG lanes). Inhibition of endosome fusion was rescued upon addition of a fraction from the Rab5 column eluate containing Rabenosyn-5 and hVPS45 (Fig. 5 B, Fraction 33). The specific presence of Rabenosyn-5 in the cytosol was required because (a) addition of purified EEA1 could not rescue endosome fusion (Fig. 5 B) (Christoforidis et al. 1999a), and (b) upon immunodepletion of Rabenosyn-5, fraction 33 failed to rescue the fusion activity. Loss of Rabenosyn-5 resulted in quantitative codepletion of hVPS45, but did not significantly reduce the levels of other proteins present in fraction 33 (data not shown). Because hVPS45 was not completely removed from the cytosol upon Rabenosyn-5 immunodepletion (Fig. 5 A), we conclude that Rabenosyn-5 is required for fusion of plasma membrane–derived CCVs with endosomes, and for homotypic endosome fusion.
Figure 5.
Requirement of Rabenosyn-5 for homotypic early endosome–early endosome, and heterotypic CCV–early endosome fusion. (A) Immunodepletion of Rabenosyn-5 from the cytosol. 100 μg of cytosol (cytosol), cytosol immunodepleted of Rabenosyn-5 (anti-Rabenosyn), or cytosol immunodepleted with nonspecific IgG (IgG) were suspended in SDS-PAGE buffer, and analyzed by immunoblotting with antibodies specific to EEA1, Rabenosyn-5, and hVPS45. (B) Fusion of CCVs loaded with biotinylated transferrin (donor) and early endosomes loaded with antitransferrin antibody (acceptor), or donor and acceptor loaded early endosomes was performed under standard conditions (see Materials and Methods). Reactions were carried out either in the absence of cytosol (−Cytosol), in the presence of untreated cytosol (Basal), in the presence of untreated cytosol but with no ATP-regenerating system (−Energy), in the presence of cytosol immunodepleted of Rabenosyn-5 (−Rabenosyn), or in the presence of cytosol treated with nonspecific IgG (IgG). Inhibition of fusion observed upon immunodepletion of Rabenosyn-5 could be rescued with Rab5 effector fractions containing Rabenosyn-5 (Fraction 33), but not with fractions containing EEA1 (Fraction 19). If Rabenosyn-5 was immunodepleted from fraction 33, the ability of this fraction to rescue fusion was abolished (anti-Rabenosyn depleted Fraction 33).
Rabenosyn-5, but Not EEA1, Plays a Role in Lysosomal Trafficking of Cathepsin D
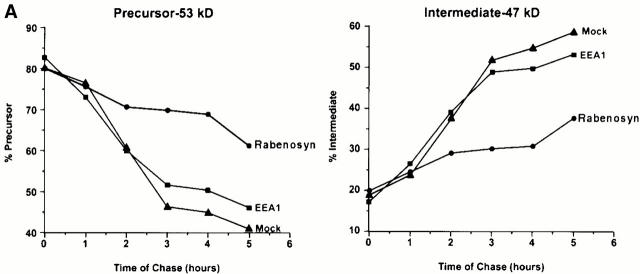
Vac1p was originally identified as a gene required for vacuole inheritance and vacuole protein sorting (Weisman and Wickner 1992; Burd et al. 1997). Since Rabenosyn-5 is similar to Vac1p and interacts with a Sec1 homologue, hVPS45, it is possible that Rabenosyn-5 might be involved in transport of newly synthesized lysosomal enzymes to lysosomes in mammalian cells through its function on the early endosome. To this end, we sought to examine whether Rabenosyn-5 is involved in trafficking of cathepsin D from the Golgi complex to lysosomes. Cathepsin D is synthesized as a 53-kD precursor, which is processed into a 47-kD intermediate form in endocytic compartments (Gieselmann et al. 1983). HeLa cells were transfected with expression vectors containing Rabenosyn-5, and as controls, EEA1 or empty vector. Cells were pulsed with [35S]methionine, chased with cold methionine for the given times (Fig. 6 A), collected, and subjected to quantitative immunoprecipitation with antibodies specific for cathepsin D. Fig. 6 A shows that overexpression of Rabenosyn-5 induced a delay in the processing of procathepsin D to its 47-kD intermediate. In contrast, overexpression of EEA1 had no effect when compared with mock-transfected cells. It is worth noting that overexpression of Rabenosyn-5 did not induce missorting of cathepsin D into the culture medium (data not shown) and, therefore, did not yield a full vps phenotype (Burd et al. 1997).
Figure 6.
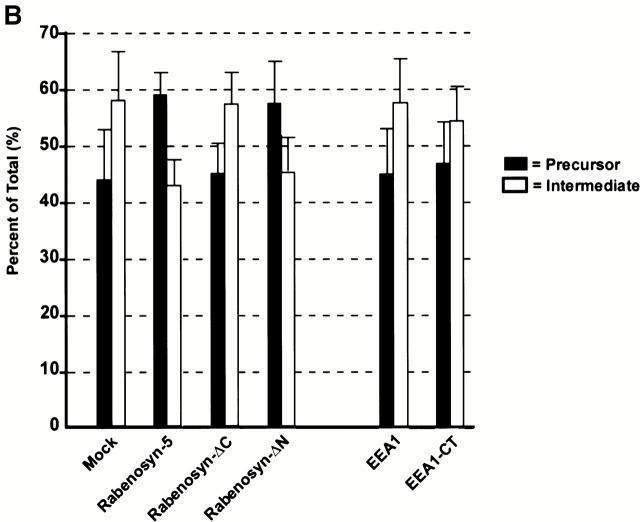
Rabenosyn-5 overexpression impairs cathepsin D trafficking. (A) Time course analysis of cathepsin D trafficking. HeLa cells overexpressing Rabenosyn-5, EEA1, or mock transfected were metabolically labeled with [35S]methionine for 30 min, chased with cold methionine for the indicated times, and then cellular cathepsin D was immunoprecipitated and relative quantities of precursor (left) or processed intermediate (right) cathepsin D were analyzed by SDS-PAGE followed by autoradiography. The signal was quantified by densitometric analysis of the autoradiograms. (B) Effect of overexpression of Rabenosyn-5 truncation mutants upon cathepsin D trafficking. Experiments were performed as described in A, except quantification of relative percentages of precursor cathepsin D (black bars) and processed intermediate cathepsin D (white bars) were performed after 4 h of chase time.
One trivial possibility to explain the phenotype induced by Rabenosyn-5 overexpression would be that the excess of FYVE fingers sequesters the PI-3P present on early endosomal membranes. However, this is unlikely given that EEA1 has no effect. To directly clarify this point, we investigated the effect of expressing truncation mutants (see scheme in Fig. 1 E) containing the FYVE finger and other portions of Rabenosyn-5 on cathepsin D processing (Fig. 6 B). Neither the ΔCOOH-terminal truncation mutant nor the Rabenosyn-5 FYVE finger domain (data not shown) had any effect upon processing. We also tested the effect of the dominant-negative, COOH-terminal FYVE finger domain of EEA1, which inhibits endosomal fusion (EEA1-CT; Simonsen et al. 1998; McBride et al. 1999). Overexpression of EEA1-CT had no effect upon cathepsin D processing (Fig. 6 B), ruling out the possibility that titration of PI-3P and Rab5-GTP causes the cathepsin D processing defect. However, as observed for full-length Rabenosyn-5, the ΔNH2-terminal truncation mutant inhibited cleavage of cathepsin D (Fig. 6 B). In control cells, after 4 h incubation, ∼58% of cathepsin D was converted to the intermediate 47-kD form and ∼42% remained as the 53-kD precursor (ratio r 47kD/53kD = 1.3), whereas in Rabenosyn-5 and Rabenosyn-ΔN overexpressing cells this proportion was inverted, with a majority (59 and 57%, respectively) remaining in the precursor form (r 47kD/53kD = 0.7). At present, the mechanism underlying this inhibition is unclear, but interestingly involves the most divergent region between Vac1p and Rabenosyn-5 that contains NPF motifs. In conclusion, the overexpression studies suggest that Rabenosyn-5, but not EEA1, is somehow involved in transport of cathepsin D from the Golgi complex to lysosomes, most likely at the level of the early endosomes.
Discussion
Rab5 specifically interacts with PI-3-kinases in a GTP-dependent manner (Christoforidis et al. 1999b), resulting in localized synthesis of PI-3P. This mechanism is important not only for membrane docking and fusion, but also for the minus end–directed motility of endosomes along microtubules (Nielsen et al. 1999). In identifying Rabenosyn-5, we have established that this system is exploited not only to recruit EEA1 to the early endosome membrane (Simonsen et al. 1998), but also to coordinate the recruitment of multiple FYVE finger–containing Rab5 effectors within the same membrane environment (Sonnichsen et al. 2000). In support of this, Rabenosyn-5 displays almost complete overlap of localization with EEA1. Although these two Rab5 effectors share a role in the same transport steps (homotypic endosome fusion and fusion of CCVs to endosomes), they clearly perform distinct functions. First, except for the presence of the FYVE finger and Rab5-binding domains, Rabenosyn-5 and EEA1 possess very different structural features (see Fig. 1 C). Second, whereas Rabenosyn-5 associates with the Sec1 homologue hVPS45, EEA1 does not appear to associate with Sec1-like proteins. Third, the addition of EEA1 cannot rescue endosome fusion inhibited by immunodepletion of Rabenosyn-5. Finally, Rabenosyn-5 overexpression inhibits cathepsin D processing, though neither EEA1 nor its COOH-terminal domain has any effect.
It is well established that the function of SNAREs in membrane transport is subject to regulation by Rab proteins and their effectors (Novick and Zerial 1990). Rab effectors mediate initial docking of vesicles to their target compartment, which must be synchronized with the priming of SNAREs and generation of trans-paired SNARE complexes, ultimately resulting in lipid bilayer fusion (Weber et al. 1998). SNARE priming and pairing is a multistep process that must be coordinated with membrane tethering. EEA1 has recently been demonstrated to associate with oligomeric structures containing NSF and Rabaptin-5/Rabex-5 on endosomal membranes, and interacts directly with syntaxin 13 in vitro (McBride et al. 1999). Through EEA1 (Christoforidis et al. 1999a), membrane tethering can be spatially and temporally coupled to SNARE priming by NSF. However, SNAREs have been shown to bind several regulatory proteins in cis that modulate their ability to form complexes with other SNAREs (e.g., tomosyn) (Fujita et al. 1998). Among them, Sec1-like proteins are thought to serve as negative regulators of SNARE pairing by sequestering syntaxin molecules (Pevsner et al. 1994; Yang et al. 2000). For syntaxins to assemble into membrane fusion–competent complexes, these proteins must first be removed. Recent studies of the structure of the Sec1–syntaxin 1A complex raise the possibility that binding of other proteins, possibly Rab effectors, to Sec1 could trigger conformational changes causing Sec1 to “present” the syntaxin molecule to other SNARE complex members (Misura et al. 2000). We propose that for endosomal SNAREs, this function would be contributed by Rabenosyn-5 through its interaction with hVPS45. Rabenosyn-5 could confer similar changes to endosomal syntaxins and present them to EEA1, or the SNARE priming machinery, NSF and α-SNAP. In the case of syntaxin 13, this would then allow for efficient pairing of this protein to EEA1, thus leading to a transition from endocytic membrane docking to fusion. Sec1p has also been shown to bind preassembled SNARE complexes, suggesting that it may play an active role in SNARE-dependent membrane docking and fusion (Carr et al. 1999). In this case, by presenting hVPS45 to trans-paired v-t-SNAREs, Rabenosyn-5 would thus stabilize the fusion complex. Importantly, both Rabenosyn-5 and EEA1 are recruited to PI-3P–enriched Rab5 endosomal subcompartments (Sonnichsen et al. 2000) by FYVE finger domains. If SNAREs flow along the pathway of membrane traffic between the Golgi complex, plasma membrane, and endosomes mostly in an inhibited conformation, upon arrival in the Rab5 domain, the concomitant presence of the two Rab5 effectors would ensure local activation of these molecules and their engagement in fusion-competent complexes. It is also not excluded that Rabenosyn-5 itself may directly participate together with EEA1 in endosome membrane docking and fusion.
In yeast, the connection between Rab GTPases and SNARE function in prevacuolar membrane trafficking has been attributed to the action of a single protein, Vac1p. Vac1p was found in complex with Pep12p and Vps45, upon isolation of these proteins from cells with mutant NSF (Sec18) (Burd et al. 1997), and these proteins are implicated in proper sorting and trafficking of proteins from the TGN to the vacuole (Piper et al. 1994; Halachmi and Lev 1996; Burd et al. 1997). Vac1p also interacts with the yeast homologue of Rab5, Vps21p/Ypt51p (Horazdovsky et al. 1994; Singer-Krüger et al. 1994; Peterson et al. 1999). Due to the common features between EEA1 and Vac1p, (both are Rab5 effectors and have a FYVE finger), EEA1 has been considered as the mammalian homologue of Vac1p (Peterson et al. 1999). This view needs to be reexamined in light of our data. With respect to domain organization, Rabenosyn-5 shares more homology to Vac1p than EEA1 and, like Vac1p, it complexes with hVPS45. Therefore, regarding SNAREs, Rabenosyn-5 may exhibit similar functions as Vac1p. Indeed, we have found that overexpression of Rabenosyn-5 results in inhibition of cathepsin D processing, suggesting a role for Rabenosyn-5 in trafficking of newly synthesized proteins through early endosomes en route to lysosomes. This is not due to a general perturbation of endosome function, as recycling of transferrin to the plasma membrane is not inhibited under the same conditions (De Renzis, S., and M. Zerial, unpublished data).
Exactly which step along this transport route is affected and by what mechanism are not clear at present. It is possible that Rab5 and Rabenosyn-5 may regulate the influx of vesicles not only from the plasma membrane, but also from the Golgi complex towards early endosomes, as proposed for Vac1p in yeast. Given its ability to interact with syntaxin 6 (Simonsen et al. 1999), in addition to syntaxin 13 (McBride et al. 1999), EEA1 may also participate in the same transport reaction, despite the fact that no effect was observed in our experiments. Alternatively, Rabenosyn-5 may not only participate in the biosynthetic transport to endosomes, but may also carry out additional functions on the early endosome that are critical for lysosomal enzyme sorting and transport. In this respect, it is interesting to note that expression of the COOH-terminal region of Rabenosyn-5, to which Vac1p does not display homology, inhibited cathepsin D processing. This region contains several NPF motifs, which mediate interactions with proteins containing EH domains. Many EH domain–containing proteins have been implicated in endocytic trafficking and signaling pathways (Di Fiore et al. 1997), raising the possibility that Rabenosyn-5 interaction with as yet unidentified EH domain–containing partner(s) may be responsible for the observed cathepsin D trafficking defects. There also appear to be important differences between yeast and mammalian proteins and their site(s) of action in the pathways. In mammalian cells, transport of lysosomal hydrolases from the Golgi complex to lysosomes is thought to intersect the endocytic pathway at the level of the early endosome (Ludwig et al. 1991; Press et al. 1998). In S. cerevisiae, Vac1p and Vps21p/Ypt51p are thought to participate in transport from the Golgi complex to a prevacuolar compartment (PVC), the postulated equivalent of a mammalian late endosome (Vida et al. 1993; Gerrard et al. 2000). However, direct evidence that these proteins play a role in fusion of Golgi-derived vesicles to PVCs has not been provided. Furthermore, whereas Rab5 regulates plasma membrane to early endosome transport in mammalian cells, Vps21p/Ypt51p is thought to function between early endosomes and PVCs in yeast (Gerrard et al. 2000). Using a specific in vitro fusion assay, we have shown that, like Rab5, Rabenosyn-5 is required for fusion of plasma membrane–derived CCVs with the early endosomes. To date, such function has not been attributed to Vac1p. In yeast, Vps45p interacts with Tlg2p, in addition to Pep12p (Nichols et al. 1998). These interactions have been demonstrated to reflect involvement of Vps45p in distinct trafficking pathways to vacuoles (Abeliovich et al. 1999). Given that hVPS45 interacts with several different syntaxin homologues, including syntaxin 6, 13, and 4, the role of Rabenosyn-5 and hVPS45 may, therefore, include all trafficking steps involving these components, e.g., sorting between the vacuole and lysosomes, recycling to the plasma membrane, and integration of these trafficking pathways in highly polarized cells.
In conclusion, our results suggest that in contrast to budding yeast, which centers on Vac1p, the mammalian early endocytic system has evolved at least two proteins, Rabenosyn-5 and EEA1, to cope with the regulation of SNARE priming and complex formation in vesicular trafficking on early endosomes. Future work should shed light on the function of this machinery in the entry and sorting of proteins in early endosomes of mammalian cells.
Acknowledgments
We are grateful to Drs. A. Klip, R. Piper, H. Stenmark, and R. Scheller for providing antibodies and plasmids. We would also like to thank A. Giner for technical assistance. Special thanks to Dr. H. McBride and S. De Renzis for valuable discussions and critical reading of the manuscript.
E. Nielsen, S. Christofordis, and M. Miaczynska were recipients of European Molecular Biology Organization (EMBO) Long-term, Max-Planck, and Human Frontier Science Program Fellowships, respectively. This work was supported by the Max Planck Gesellschaft and by grants from the Human Frontier Science Program (RG-432/96), European Union-Training and Mobility of Researchers (EU TMR) (ERB-CT96-0020), and Biomed (BMH4-97-2410) (M. Zerial).
Footnotes
S. Christoforidis' present address is Laboratory of Biological Chemistry, Medical School, University of Ioannina, 45110 Ioannina, Greece.
Abbreviations used in this paper: CCV, clathrin-coated vesicle; EEA1, early endosome antigen 1; EGFP, enhanced GFP; EH, E15 homology; EST, expressed sequence tag; GFP, green fluorescent protein; GST, glutathione S-transferase; NSF, N-ethylmaleimide–sensitive factor; P, phosphate; PC, phosphatidylcholine; PI, phosphatidylinositol; PVC, prevacuolar compartment; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor.
References
- Abeliovich H., Darsow T., Emr S.D. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE–Sec1p complex composed of Tlg2p and Vps45p. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:6005–6016. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G., Peterson M., Cowles C.R., Emr S.D. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C.M., Grote E., Munson M., Hughson F.M., Novick P.J. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S., Zerial M. Purification and identification of novel Rab effectors using affinity chromatography. Methods. 2000;20:403–410. doi: 10.1006/meth.2000.0953. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., McBride H.M., Burgoyne R.D., Zerial M. The Rab5 effector EEA1 is a core component of endosome docking Nature 397 1999. 621 625a [DOI] [PubMed] [Google Scholar]
- Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S.C., Waterfield M.D., Backer J.M., Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors Nat. Cell Biol 1 1999. 249 252b [DOI] [PubMed] [Google Scholar]
- Di Fiore P.P., Pelicci P.G., Sorkin A. EHa novel protein–protein interaction domain potentially involved in intracellular sorting. Trends Biochem. Sci. 1997;22:411–413. doi: 10.1016/s0968-0004(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Echard A., Jollivet F., Martinez O., Lacapere J.J., Rousselet A., Janoueix-Lerosey I., Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Shirataki H., Sakisaka T., Asakura T., Ohya T., Kotani H., Yokoyama S., Nishioka H., Matsuura Y., Mizoguchi A., Scheller R.H., Takai Y. Tomosyna syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Gerrard S.R., Bryant N.J., Stevens T.H. VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol. Biol. Cell. 2000;11:613–626. doi: 10.1091/mbc.11.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J. Cell Biol. 1983;97:1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for sec4p, targeting secretory vesicles to sites of exocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi N., Lev Z. The Sec1 familya novel family of proteins involved in synaptic transmission and general secretion. J. Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Horazdovsky B.F., Busch G.R., Emr S.D. VPS21 encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Lippé R., McBride H.M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jones S.M., Crosby J.R., Salamero J., Howell K.E. A cytosolic complex of p62 and rab6 associates with TGN38/41 and is involved in budding of exocytic vesicles from the trans-Golgi network. J. Cell Biol. 1993;122:775–788. doi: 10.1083/jcb.122.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawe D.C., Patki V., Heller-Harrison R., Lambright D., Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- Li G., D'Souza-Schorey C., Barbieri M.A., Roberts R.L., Klippel A., Williams L.T., Stahl P.D. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T., Griffiths G., Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J. Cell Biol. 1991;115:1461–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J. Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Misura K.M., Scheller R.H., Weis W.I. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Mu F., Callaghan J.M., Steele-Mortimer O., Stenmark H., Parton R.G., Campbell P.L., McCluskey J., Yeo J.P., Tock E.P.C., Toh B.H. EEA1, an early endosome-associated protein. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Holthuis J.C., Pelham H.R. The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur. J. Cell Biol. 1998;77:263–268. doi: 10.1016/s0171-9335(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J.M., Hyman A.A., Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1990;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Otter-Nilsson M., Hendriks R., Pecheur-Huet E.I., Hoekstra D., Nilsson T. Cytosolic ATPases, p97 and NSF, are sufficient to mediate rapid membrane fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2074–2083. doi: 10.1093/emboj/18.8.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.R., Burd C.G., Emr S.D. Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol. 1999;9:159–162. doi: 10.1016/s0960-9822(99)80071-2. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S.C., Braun J.E., Calakos N., Ting A.E., Bennett M.K., Scheller R.H. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S.C., Hyde P.S., Scheller R.H. Mammalian homologues of yeast vacuolar protein sorting (vps) genes implicated in Golgi-to-lysosome trafficking. Gene. 1996;183:7–14. doi: 10.1016/s0378-1119(96)00367-8. [DOI] [PubMed] [Google Scholar]
- Piper R.C., Whitters E.A., Stevens T.H. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur. J. Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Prekeris R., Yang B., Oorschot V., Klumperman J., Scheller R.H. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol. Biol. Cell. 1999;10:3891–3908. doi: 10.1091/mbc.10.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press B., Feng Y., Hoflack B., Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino M., Miaczynska M., Lippe R., Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- Salcini A.E., Confalonieri S., Doria M., Santolini E., Tassi E., Minenkova O., Cesareni G., Pelicci P.G., Di Fiore P.P. Binding specificity and in vivo targets of the EH domain, a novel protein–protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P.J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Lippé R., Christoforidis S., Gaullier J.-M., Brech A., Callaghan J., Toh B.-H., Murphy C., Zerial M., Stenmark H. EEA1 links phosphatidylinositol 3-kinase function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Gaullier J.M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B., Stenmark H., Düsterhöft A., Philippsen P., Yoo J.-S., Gallwitz D., Zerial M. Role of three Rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Aasland R. FYVE-finger proteins—effectors of an inositol lipid. J. Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R.G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Bucci C., Zerial M. Expression of Rab GTPases using recombinant vaccinia virus Methods Enzymol. 257 1995. 155 164a [DOI] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion Cell 83 1995. 423 432b [DOI] [PubMed] [Google Scholar]
- Stenmark H., Aasland R., Toh B.H., D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc- binding FYVE finger. J. Biol. Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- Tall G.G., Hama H., DeWald D.B., Horazdovsky B.F. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol. Biol. Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam J.T., James D.E., Stevens T.H., Piper R.C. Identification of a mammalian Golgi Sec1p-like protein, mVps45. J. Biol. Chem. 1997;272:6187–6193. doi: 10.1074/jbc.272.10.6187. [DOI] [PubMed] [Google Scholar]
- Vida T.A., Huyer G., Emr S.D. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J. Cell Biol. 1993;121:1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Solner T., Rothman J.E. SNAREpinsminimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weisman L.S., Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J. Biol. Chem. 1992;267:618–623. [PubMed] [Google Scholar]
- Wilm M., Mann M. Analytical properties of the nano electrospray ion source. Anal. Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- Wilm M., Shevchenko A., Houthaeve T., Breit S., Scweigerer L., Fotsis T., Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379:466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- Yang B., Steegmaier M., Gonzalez L.C., Jr., Scheller R.H. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]