Abstract
The yeast a-factor receptor (Ste3p) is subject to two mechanistically distinct modes of endocytosis: a constitutive, ligand-independent pathway and a ligand-dependent uptake pathway. Whereas the constitutive pathway leads to degradation of the receptor in the vacuole, the present work finds that receptor internalized via the ligand-dependent pathway recycles. With the a-factor ligand continuously present in the culture medium, trafficking of the receptor achieves an equilibrium in which continuing uptake to endosomal compartments is balanced by its recycling return to the plasma membrane. Withdrawal of ligand from the medium leads to a net return of the internalized receptor back to the plasma membrane. Although recycling is demonstrated for receptors that lack the signal for constitutive endocytosis, evidence is provided indicating a participation of recycling in wild-type Ste3p trafficking as well: a-factor treatment both slows wild-type receptor turnover and results in receptor redistribution to intracellular endosomal compartments. Apparently, a-factor acts as a switch, diverting receptor from vacuole-directed endocytosis and degradation, to recycling. A model is presented for how the two Ste3p endocytic modes may collaborate to generate the polarized receptor distribution characteristic of mating cells.
Keywords: endocytosis, Saccharomyces cerevisiae, pheromones, cell surface, endosomes
Introduction
Recycling of cell surface receptors in mammalian cells allows the receptor protein to participate in multiple rounds of ligand binding and internalization (Ciechanover et al. 1983; Goldstein et al. 1985). Liganded receptor is internalized to the recycling endosome where the low lumenal pH of this compartment leads to the dissociation of ligand and receptor. Whereas ligand generally is routed onward in the endocytic system to the lysosome for degradation, receptor is recycled back to the cell surface. For some receptors, notably the low density lipoprotein receptor and the transferrin receptor, recycling is the obligate routing pathway. For others, e.g., the EGF receptor, a mixed outcome awaits the internalized liganded receptor, with some of the receptors being reused via recycling and some being degraded through transport to the lysosome. Recycling may also be used for receptor resensitization. The ligand-induced phosphorylation of the β2-adrenergic receptor together with the subsequent binding of β-arrestin both uncouples the receptor from the downstream heterotrimeric G protein and triggers its endocytosis (Koenig and Edwardson 1997). The internalized receptor, resident within an endosomal compartment, is dephosphorylated. As a result, the β2-adrenergic receptor recycled back to the plasma membrane is not only stripped of its ligand, but is also restored to its initial level of responsiveness.
In the yeast Saccharomyces cerevisiae, a number of plasma membrane proteins have been found to undergo a ubiquitin-dependent endocytosis that delivers the internalized protein to the vacuole (the yeast lysosome) for degradation (Bonifacino and Weissman 1998; Hicke 1999). Although receptor recycling such as that described above for mammalian cells has yet to be observed in yeast, there is evidence for trafficking pathways that link endosomal compartments back to the cell surface. For instance, a variety of mutants that block endosomal transport to the vacuole lead to an accumulation of the transported substrates at the plasma membrane (Davis et al. 1993; Piper et al. 1995; Luo and Chang 1997; Li et al. 1999). More compelling evidence is provided by a recent study of bulk endocytic membrane flow in yeast that finds the inward endocytic membrane flux to be largely balanced by a recycling return to the plasma membrane (Wiederkehr et al. 2000).
The a-factor receptor (Ste3p), one of the two G protein–coupled receptors directing sexual conjugation in yeast, is subject to two distinct modes of endocytosis: a ligand-independent, constitutive uptake mode as well as a ligand-dependent mode. To date, the Ste3p constitutive uptake mode has been the more intensively studied of the two (Davis et al. 1993; Roth and Davis 1996, Roth and Davis 2000; Roth et al. 1998). Constitutive endocytosis is rapid with receptor being delivered to the vacuole for degradation after a short surface residency. Consequently, Ste3p is a short-lived protein with a t 1/2 of 15 min in cells growing at 30°C. Constitutive endocytosis is ubiquitin dependent, with the ubiquitin added to the surface-localized receptor serving to trigger uptake (Roth and Davis 1996, Roth and Davis 2000; Roth et al. 1998). Furthermore, the signal for endocytosis is a ubiquitination signal, a 58–residue-long PEST-like sequence (Roth et al. 1998) with three embedded lysine residues serving as the ubiquitin acceptor sites (Roth and Davis 2000).
To date, the ligand-dependent uptake of Ste3p has been followed only under conditions where rapid constitutive endocytosis is impaired (Davis et al. 1993; Givan and Sprague 1997; Roth and Davis 2000). The ligand-dependent mode is distinguished from the constitutive mode both by its use of different receptor signals and by a differential involvement of the cellular proteins that catalyze uptake. Mutant receptors lacking a functional PEST-like signal though defective for constitutive endocytosis, remain competent for ligand-dependent uptake (Davis et al. 1993; Roth and Davis 2000). This uptake uses the tripeptide sequence NPF that maps to a distinct portion of the receptor COOH-terminal cytoplasmic tail domain (CTD) (Tan et al. 1996). Furthermore, an overlapping but distinct set of cellular proteins catalyzes the two uptake processes. Ste3p constitutive uptake, but not ligand-dependent uptake requires two functions: the ankyrin-repeat protein Akr1p and the redundant Yck1p–Yck2p type I casein kinase pair (Givan and Sprague 1997; Panek et al. 1997; Feng and Davis 2000). These two functions act early in the constitutive uptake pathway, at a step before receptor ubiquitination, acting apparently for the phosphoryl activation of the PEST-like endocytosis signal (Feng and Davis 2000). Other functions, for instance, the actin-associated proteins End3p and End4p are used commonly by both uptake pathways (Feng and Davis 2000; Chen, L., and G. Davis, unpublished results).
The present work that concentrates on Ste3p ligand-dependent uptake finds that the two Ste3p uptake modes also differ in terms of the fate of the internalized receptor. Rather than the vacuolar degradation associated with constitutive endocytosis, ligand-dependent uptake links instead to recycling: receptor internalized via this pathway to endosomal compartments recycles back to the cell surface.
Materials and Methods
Strains
The strains used in this work are listed in Table . New strains, all isogenic to NDY341 (Roth and Davis 1996), were constructed via well-established yeast gene replacement techniques. Details will be furnished upon request.
Table 1.
Yeast Strains
| Strain | Genotype | Reference or Source |
|---|---|---|
| EG123 | MAT a ura3 leu2 trp1 his4 | (Siliciano and Tatchell 1984) |
| SM1229 | isogenic to EG123, except mfa1::LEU2 mfa2::URA3 | (Michaelis and Herskowitz 1988) |
| NDY341 | MATα GAL1-STE3 ura3 leu2 his4 bar1-1 | (Roth and Davis 1996) |
| NDY343 | MATα ste3Δ::LEU2 | (Roth et al. 1998); A |
| NDY349 | MATα GAL1-STE3Δ365 | This work; A |
| NDY358 | MATα GAL1-STE3Δ365 pep4Δ | This work; A |
| NDY836 | MATα GAL1-STE3(3K→R) pep4Δ | (Roth and Davis 2000); A |
| NDY841 | MATα GAL1-STE3(3K→R) | (Roth and Davis 2000); A |
| NDY1027 | MATα GAL1-STE3Δ365 pep4Δ prb1Δ::LEU2 | This work; A |
| NDY1181 | MATα GAL1-STE3Δ365(3xHA) | This work; A |
| NDY1206 | MATα GAL1-STE3 ste4Δ | This work; A |
Cell Culture, Pheromone Treatment, Protease Shaving, Protein Extract Preparation, and Western Analysis
A 90-min period of receptor expression from GAL1-driven receptor constructs was initiated from log-phase cultures growing in YPR medium (YP medium [1% yeast extract, 2% peptone] with 2% raffinose) with the addition of galactose to 2%, and was terminated with the addition of glucose to 3%.Pheromone was added 30 min after the addition of glucose or together with the glucose in the experiments that used wild-type Ste3p (see Fig. 5). Pheromone addition involved adding 0.5 vol of the a-factor–containing culture supernatant from EG123 cells carrying pKK16 (2μ STE6, MFA2; Kuchler et al. 1989) or, for the mock-treated control, an equivalent supernatant from isogenic mfa1::LEU2 mfa2::URA3 SM1229 cells (Michaelis and Herskowitz 1988). Both supernatants were prepared as previously described (Davis et al. 1993). For the initial timepoint, an aliquot containing 5 × 107 cells was removed from the cultures just before the pheromone addition step. For the subsequent, postpheromone timepoints, culture aliquot volumes were 1.5× the volume removed for the initial timepoint. After pheromone-induced turnover of Ste3Δ365p (see Fig. 1 C), extracts were prepared from the culture aliquots as previously described (Davis et al. 1993). To assess the fraction of the receptor population residing at the plasma membrane, culture aliquots were treated with Pronase (Calbiochem-Novabiochem) in the presence of the energy poisons 10 mM sodium azide and 10 mM potassium fluoride as described previously (Davis et al. 1993). SDS-PAGE and Western blotting with affinity-purified rabbit polyclonal Ste3p-specific antibodies were as described previously (Davis et al. 1993).
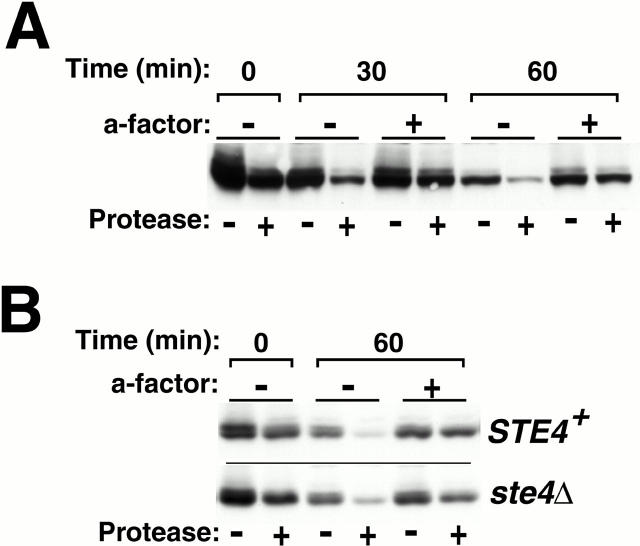
Figure 5.
a-factor produces effects on wild-type Ste3p consistent with induced recycling. After a 90-min period of galactose-induced receptor expression, cultures of GAL1-STE3 MATα cells were treated with glucose (3%) to repress further synthesis and simultaneously treated either with a-factor, or mock-treated in parallel. At the indicated times thereafter, aliquots removed from the culture at the indicated times were subjected to the intact cell protease-shaving protocol (see Materials and Methods). Finally, protein extracts were subjected to SDS-PAGE and then Western analysis with Ste3p-specific antibodies. (A) Effects of a-factor on Ste3p turnover and distribution. Cells of the wild-type GAL1-STE3 MATα strain NDY341 were treated as described above. (B) The effects of a-factor on Ste3p turnover and distribution are not secondary to induced pheromone signaling. Wild-type (NDY341) and ste4Δ (NDY1206) cells were treated as described above.
Figure 1.
Ligand-dependent endocytosis of Ste3Δ365p. 30 min after a 90-min period of galactose-induced receptor expression, cultures of GAL1-STE3Δ365 MATα cells were treated with a-factor and receptor internalization (B) or turnover (C) were monitored. (A) Ste3p schematic showing positions (indicated by amino acid residue numbers) within the receptor CTD of the PEST-like signal for constitutive endocytosis (the three redundant lysyl acceptor sites for ubiquitin attachment are indicated with K) and the NPFSTD sequence required for ligand-dependent uptake. Residues removed by the Δ365 mutation are indicated above. (B) Ligand-induced internalization of Ste3Δ365p. To eliminate the vacuolar turnover of the receptor that accompanies endocytosis, the pep4Δ prb1Δ NDY1027 strain was used. At the times indicated after the addition of a-factor, aliquots were removed from culture and the intact cells were treated with proteases (+) or were mock-treated (−) in parallel (see Materials and Methods). Extracts prepared from these cells were subjected to SDS-PAGE and Western blot analysis with Ste3p-specific antibodies. (C) Ligand-induced turnover of Ste3Δ365p. Cultures of strain NDY349, isogenic to NDY1027 except for being PEP4 + and PRB1 + were treated with a-factor or were mock treated in parallel. Extracts were prepared from culture aliquots at the indicated times after a-factor addition, and were then subjected to SDS-PAGE and Western blot analysis as described for B. (D) Rates of Ste3Δ365p internalization and turnover. Receptor abundunce was quantitated for the samples of the B and C experiments (Materials and Methods) and percent internalization and percent turnover are reported at each timepoint.
Quantitative Methods
Loss of Ste3 antigen to turnover or to protease shaving was quantitated using film densitometry. Films of Western blots developed with enhanced chemiluminescent reagents (Amersham Pharmacia Biotech) were digitally scanned and the resulting TIFF files quantitated with NIH Image software. Measurements entailed comparison of the Ste3 antigen that survived turnover or protease digestion to a standard curve generated by dilution of the initial sample (i.e., receptor present at the 0-h timepoint of a turnover experiment or in the case of protease-shaving experiments, receptor present in the control sample, untreated with proteases).
Recycling Experiments
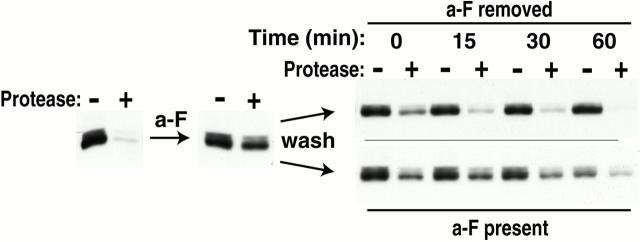
Recycling was followed with two different protocols. In the first protocol (used for Fig. 3), after a 45-min a-factor treatment (leading to the internalization of >50% of the Δ365 receptor protein), 6 × 108 cells were collected twice by centrifugation, washed with fresh 30°C YPD medium (YP medium with 2% glucose), and restored to culture. As a control for the effects of the a-factor–removing “wash” steps, half of the cells in parallel, were washed and restored to culture using fresh a-factor–containing medium (YPD plus an additional 0.5 vol of the a-factor–containing supernatant; see above). At various times after restoring the cells to culture, aliquots were removed and receptor distribution was monitored via the protease-shaving protocol (see above).
Figure 3.
Internalized Δ365 receptor returns to the cell surface after removal of a-factor ligand from the culture medium. Cells of the MATα GAL1-STE3Δ365 strain NDY349 were cultured and treated for 45 min with a-factor as described for Fig. 1. After the a-factor treatment, cells were washed and resuspended in fresh medium, either lacking or containing a-factor (see Materials and Methods). Culture aliquots were removed before the initial treatment with a-factor, after the a-factor treatment, and at the indicated times after the restoration of the cells to culture, either in the presence or absence of a-factor. To assess the surface localization of the receptor at these timepoints, intact cells were treated with extracellular proteases and extracts were prepared as described (Materials and Methods). Finally, extracts were subjected to SDS-PAGE and Western analysis with Ste3p-specific antibodies.
For the second protocol, the 45-min a-factor treatment was followed directly by protease shaving to destroy that portion of the receptor population that remained at the cell surface after the pheromone treatment. This preparative Pronase treatment was a scaled-up version of the analytical protease-shaving protocol (see above): 6 × 108 a-factor–treated cells were digested with 2,000 units of Pronase in a 9-ml vol. After this shaving, cells were collected by centrifugation and restored to culture in 25 ml of 30°C YPDS medium (YPD medium with 1 M sorbitol). Then the cell surface return of the surviving, intracellular receptor population was followed using the analytical protease-shaving protocol as described above.
Indirect Immunofluorescence
NDY1181 cells expressing a 3xHA epitope-tagged version of Ste3Δ365p (epitope tags fused to receptor COOH terminus) were cultured and treated with a-factor as described above. Cells were fixed, spheroplasted, and treated for indirect immunofluorescence as previously described (Davis et al. 1993). Detection of Ste3Δ365(3xHA)p used a 1:1,000 dilution of the HA.11 mAb (Berkeley Antibody Co.) followed by a Cy3-conjugated donkey anti–mouse IgG secondary antibody used at a 1:500 dilution. For detection of the vacuolar membrane protein alkaline phosphatase (ALP), an affinity-purified, rabbit polyclonal anti-ALP (provided by Steve Nothwehr, University of Missouri, St. Louis, MO) that had been pre-absorbed against pho8Δ cells (ALP is encoded by PHO8) was used at a dilution of 1:6. This was followed by a 1:500 dilution of biotinylated goat anti–rabbit IgG secondary antibody, and then fluorescein-conjugated strepavidin at 1:500. All secondary antibodies, as well as the fluorescein-conjugated strepavidin, were from Jackson ImmunoResearch Laboratories. Images were captured using a Nikon Eclipse 600 (Nikon) equipped with a Princeton Instruments Micromax CCD camera (Roper Scientific Princeton Instruments Inc.).
Results and Discussion
Kinetics of Ligand-induced Endocytosis and Turnover
To focus on the Ste3p ligand-dependent uptake mode, we have made use of a receptor mutant fully defective for the constitutive mode: Ste3Δ365p lacks the COOH-terminal 105 residues of the receptor CTD including the COOH-terminal PEST-like constitutive endocytosis signal (Fig. 1 A). Consequently, Ste3Δ365p stably accumulates at the plasma membrane. With a-factor treatment, Ste3Δ365p is internalized and delivered ultimately to the vacuole where it is degraded (Davis et al. 1993). In Fig. 1, we have examined both the rates of receptor internalization and turnover after the challenge of Ste3Δ365p-expressing cells with a-factor. After a pulse of receptor synthesis from the GAL1 promoter, a-factor is added, and receptor internalization is monitored using a protease-shaving protocol in which intact cells are treated with proteases (Fig. 1 B). Receptor localized to the cell surface is susceptible to digestion by the added extracellular proteases, while receptor that localizes to intracellular compartments (e.g., endosomes or vacuole) is protected from digestion (Davis et al. 1993; Roth and Davis 1996). The cells used for this experiment were pep4Δ prb1Δ to avoid loss of receptor protein to the normal vacuolar turnover mechanism during the experimental timecourse.
Before a-factor addition, >90% of the receptor protein was found to be susceptible to digestion by the added proteases (Fig. 1 B, 0 min timepoint), indicating that receptor initially is localized at the cell surface. In the first 40 min after a-factor addition, receptor becomes increasingly resistant to digestion, consistent with an increasing fraction of the receptor population being internalized. During this initial phase of the timecourse, we estimate a t 1/2 for internalization of 35 min (Fig. 1 D). Subsequently, the rate of receptor internalization appears to slow, reaching a plateau in which 55 to 65% of the receptors are internalized and 35 to 45% remain exposed at the cell surface.
In a parallel experiment, the rate of a-factor–induced Ste3Δ365p turnover was assessed in wild-type PEP4 + PRB1+ cells (Fig. 1 C). Cells treated with a-factor show a slow loss of receptor protein over the 2-h time course. Unlike the constitutive endocytosis of wild-type Ste3p where receptor uptake and turnover are tightly coupled (Davis et al. 1993), comparison of internalization rates with the ligand-induced turnover rate for Ste3Δ365p indicates that turnover lags substantially behind internalization (Fig. 1 D). Whereas 50% of the receptor is internalized over the initial 35 min of a-factor treatment, it takes 80 min for 50% of the receptor protein to be degraded. Thus, before its degradation, a relatively large fraction of the receptor shows a surprisingly stable accumulation inside the cell.
Internalized Receptor Accumulates in an Endosomal Compartment
To identify the intracellular sites of receptor accumulation, we have used indirect immunofluorescence microscopy to follow the a-factor–induced internalization of an HA epitope-tagged Δ365 receptor (Fig. 2). Previous analysis that demonstrated the vacuole as the final destination for the a-factor–induced uptake of Ste3Δ365p, used both a pep4Δ strain background and a long period of pheromone treatment (90 min) (Davis et al. 1993). For the present analysis, the cells are PEP4 +, thus, receptor delivered to the vacuole is expected to be degraded. Furthermore, based upon the above kinetic analysis (Fig. 1 D), the 45-min a-factor treatment used for the present experiment is expected to result in substantial receptor internalization, yet minimal receptor turnover in the vacuole. Before the pheromone treatment, much of the receptor is found localized to the cell surface (Fig. 2, red signal). The a-factor treatment results both in a reduction in the surface-localized fluorescent signal and a concomitant appearance of an intracellular punctate staining. Invariably, the brightest of these punctate structures localize adjacent to the vacuole (Fig. 2, green signal), suggesting possible identity with the previously described compartment known either as the pre-vacuolar compartment or late endosome (Piper et al. 1995; Hicke et al. 1997). Additionally, less brightly stained puncta localize more peripherally, i.e., away from the vacuole. These punctate structures may correspond to the previously described “early endosome” compartment (Hicke et al. 1997). Consistent with this designation as early and late endosomes, shorter a-factor exposure times (e.g., 15 min), lead to a preferential staining of the peripheral puncta rather than the peri-vacuolar compartment (data not shown).
Figure 2.
Internalized Δ365 receptor localizes to intracellular endosomal compartments. Cells of the MATα GAL1-STE3Δ365(3xHA) strain NDY1181 were cultured and treated with a-factor as described for Fig. 1. Culture aliquots were removed for indirect immunofluorescence analysis just before the addition of a-factor and 45 min after a-factor addition. In addition, as control for the specificity of the HA.11 mAb an aliquot of a culture of the MATα ste3Δ::LEU2 strain NDY343 was processed in parallel (no HA). Cells were fixed and processed for indirect immunofluorescence analysis as described (Materials and Methods). The top row shows the fluorescent signal (red) that corresponds to HA-tagged Δ365 receptor. In addition to showing the receptor signal (red) from the same cells, the middle panel also shows the fluorescent signal (green) deriving from detection of the vacuolar membrane protein alkaline phosphatase. In the bottom row, displaying Nomarski images of the same cells, the vacuole appears as an apparent depression in the cell surface.
Recycling
The biphasic kinetics observed for Δ365 internalization (Fig. 1 D) might be explained by ongoing receptor recycling. The plateau seen for receptor internalization 40 min after a-factor addition (Fig. 1 D) could be indicative of an equilibrium in which ongoing internalization is balanced by the recycling return of receptor back to the cell surface. Drawing from examples of recycling in mammalian cells, one might anticipate that after internalization to an endosomal compartment, ligand and receptor may dissociate with unliganded receptor returning to the cell surface to repeat the endocytic cycle (rebinding ligand and re-internalizing). Such a cycle of uptake and return could be interrupted simply through removal of the uptake stimulus, i.e., the ligand. Removal of the ligand from the culture medium should block further uptake, allowing the potential recycling return of the receptor to the cell surface to be visualized. Such an experiment is shown in Fig. 3. After a 45-min a-factor treatment of Ste3Δ365p-expressing cells that resulted in ∼50% of the receptor protein being internalized (Fig. 3), cells were removed from culture and restored either to fresh medium lacking a-factor or as a control, to medium in which a-factor is maintained at its original concentration. When restored to medium containing a-factor, we find that an intracellular pool of receptor protein is maintained, with ∼50% of the receptor protein resisting digestion by added proteases (Fig. 3). As the cells used for this experiment are PEP4 +, a slow, a-factor–induced, receptor turnover also is apparent. Quite a different result is obtained when a-factor is removed from the culture medium. With withdrawal, there is a clear, time-dependent redistribution of the receptor back to the cell surface: 30 and 60 min after withdrawal, we find that 80 and 90% of the total receptor protein, respectively, is surface localized.
To focus more directly on the endosome-to-surface transport component of recycling, we have developed an alternative experimental approach where recycling is followed from a condition that has all the receptor protein initially localized endosomally (Fig. 4 A). To establish this initial condition, we use the protease-shaving technique to eliminate the portion of the receptor population that remains at the cell surface subsequent to a 45-min a-factor treatment period. Cellular energy metabolism is poisoned during the protease treatment step to block the possibility of continued membrane trafficking. After proteolysis, energy poisons are removed, the shaved cells are restored to culture, and the changing receptor localization is monitored. A clear time-dependent return of internalized receptor to the cell surface is seen (Fig. 4 A). This recycling is energy dependent as it is blocked for a control culture where the presence of the energy poisons is maintained (Fig. 4 A).
Figure 4.
Cell surface return of internalized Δ365 and 3K→R receptors. (A) Energy-dependent recycling of Ste3Δ365p. MATα GAL1-STE3Δ365 cells of strain NDY349 were cultured and treated with a-factor as described for Fig. 1. After the 45-min a-factor treatment, cells were collected and the intact cells were preparatively digested with extracellular proteases in the presence of energy poisons (10 mM sodium azide and 10 mM potassium fluoride). The shaved cells were then collected by centrifugation and restored to culture in fresh medium either lacking or containing the energy poisons. Culture aliquots were removed either before the initial treatment with a-factor, after the a-factor treatment, and at the indicated times after the restoration of the shaved cells to culture. Surface localization of the receptor at these timepoints was assessed via the intact cell proteolysis protocol (Materials and Methods). Finally, extracts were subjected to SDS-PAGE and Western analysis with Ste3p-specific antibodies. (B) Energy-dependent recycling of Ste3(3K→R)p. Cells of the MATα GAL1-STE3(3K→R) strain NDY841were cultured, treated with a-factor, and processed as described for A.
The experiments above define a recycling pathway associated with the ligand-dependent endocytosis of the Δ365 Ste3p CTD tail truncation mutant. In addition to the PEST-like signal, the 105-residue Δ365 deletion interval also could include sequences that participate in other, unforeseen aspects of receptor trafficking. For instance, it is possible that endosomal accumulation of internalized Ste3Δ365p could reflect the loss of hypothetical receptor sequences that specify endosome to vacuole transport. More subtle receptor mutations that disable constitutive endocytosis have been generated in recent studies of the Ste3p PEST-like signal (Roth et al. 1998; Roth and Davis 2000). The 3K→R mutation is a Lys-to-Arg substitution of the three lysine residues of the PEST-like signal that serve as the redundant ubiquitin acceptor sites (Fig. 1 A). The triply substituted Ste3(3K→R)p fails to be ubiquitinated, fails to undergo constitutive uptake, and consequently accumulates at the cell surface (Roth and Davis 2000). Nonetheless, Ste3(3K→R)p does remain competent for ligand-dependent endocytosis, with kinetics of uptake similar to that observed for the Δ365 truncation mutant (Roth and Davis 2000). Furthermore, comparison of internalization and turnover rates for the ligand-induced endocytosis of 3K→R reveals a turnover lag like that seen for Ste3Δ365p (Fig. 1 D): a-factor–induced internalization of Ste3(3K→R)p is relatively rapid whereas the associated turnover lags slowly behind (data not shown). To test if Ste3(3K→R)p also undergoes recycling, we have applied the protocol used for Ste3Δ365p in Fig. 4 A. Again, we see much the same result as was obtained for Δ365: internalized 3K→R receptor shows a time- and energy-dependent return to the cell surface (Fig. 4 B).
Recycling of the Wild-type Receptor
How might the ligand-induced receptor recycling mechanism impact wild-type Ste3p? In the absence of ligand, Ste3p is subject to a rapid degradative endocytosis. As we have seen above (Fig. 3 and Fig. 4), the ligand-dependent endocytosis of Δ365 and 3K→R mutant receptors links primarily to recycling. If a-factor provides the switch that converts wild-type Ste3p uptake from the degradative to the recycling mode then we should expect several changes after treatment of wild-type Ste3p-expressing cells with a-factor. First, considering the pronounced intracellular accumulation of Ste3Δ365p after a-factor treatment (Fig. 1 and Fig. 2), we might expect a ligand-induced redistribution of the wild-type receptor, with an increased proportion of the receptor population localizing intracellularly to endosomal compartments. Second, with recycling induced instead of degradation, a-factor treatment might also lead to an overall slowing of the turnover rate.
In Fig. 5 A, we have examined the effects of a-factor treatment on the localization and turnover of wild-type Ste3p. Surface localization of the receptor is again assessed using the protease-shaving protocol. Unlike the Δ365 and 3K→R receptors, the residency of wild-type Ste3p at the plasma membrane is short-lived (5 to 10 min for cells growing at 30°C). To maximize exposure of the receptor to ligand, a-factor was added together with glucose at the end of a 90-min period of galactose-induced receptor synthesis. At that time (Fig. 5 A, 0 min timepoint), ∼60% is surface localized and ∼40% is localized intracellularly. The bulk of the intracellular receptor population is newly synthesized receptor that has not yet arrived at the cell surface: consistent with this, the proportion of receptor that is surface localized increases 30 min after the glucose-mediated shut-off of new receptor synthesis (Fig. 5 A, 30 min, no a-factor timepoint).
Comparing receptor turnover and distribution in the a-factor treated cultures to that of the unstimulated control, we do indeed find effects consistent with those forecast for the induction of a recycling mode of endocytosis (Fig. 5 A). 30 and 60 min after the a-factor challenge, a much higher fraction of the receptor population is found to distribute intracellularly compared with the mock-treated control. In addition, a subtle, yet reproducible slowing in the rate of receptor turnover for the a-factor–treated cultures is also seen. A more substantial receptor stabilization may require exposure to high concentrations of a-factor as is thought to occur during the late stages of mating (Brizzio et al. 1996; Elia and Marsh 1996).
Pheromone treatment induces an array of responses including induced transcription, arrest of the cell cycle in G1, and a polarization of cell growth that results in a deformation of the cell body into a mating projection or “shmoo”. Therefore, we have considered the possibility that the a-factor effects on Ste3p turnover and distribution might be indirect, resulting from interference by one of these other pheromone-induced responses. To this end, we have tested the consequence of deletion of the gene encoding the Gβ subunit of the heterotrimeric αβγ G protein (i.e., ste4Δ cells) on the a-factor effects on Ste3p turnover and redistribution. Activation of the heterotrimeric G protein is the first intracellular signaling step for the pheromone signaling pathway and ste4Δ cells fail to mount a pheromone response. Interestingly, ligand-induced endocytosis for both the a- and α-factor receptors is distinguished from the other pheromone responses by G protein independence: uptake of both proceeds in ste4Δ cells (Zanolari et al. 1992; Davis et al. 1993). Apparently, the endocytic apparatus uses features other than G protein activation to recognize the liganded status of the receptor. Comparison of the a-factor effects on wild-type Ste3p distribution and turnover in STE4 + and ste4Δ cells, indicates a lack of requirement for G protein–induced signaling (Fig. 5 B). Thus, the noted effects of a-factor on Ste3p trafficking share the unique feature of ligand-dependent endocytosis: they too are G protein–independent, indicating that they likely do result from a ligand-induced switch from a degradative endocytosis to a recycling mode.
Endocytic Recycling in Yeast
Our results with Ste3p recycling fit nicely within recycling paradigms established for a variety of mammalian receptors. Ligand binding triggers uptake of surface receptor to an endosomal compartment. By analogy to the mammalian paradigm, we expect at this point, dissociation of ligand from the receptor. Ligand may travel onward to the vacuole for degradation while the receptor returns to the surface for reutilization.
Endocytic recycling also has been suggested as participating in the trafficking of two other yeast membrane proteins, the chitin synthetase Chs3p and the vesicle-associated membrane protein (VAMP)-like vesicle soluble NSF attachment protein receptor (v-SNARE) Snc1p (Chuang and Schekman 1996; Lewis et al. 2000). Interestingly, recycling does not appear to be a prominent feature of α-factor pheromone receptor (Ste2p) trafficking (Jenness and Spatrick 1986): differing from Ste3p, pheromone treatment increases Ste2p vacuole-directed transport and degradation (Schandel and Jenness 1994; Hicke and Riezman 1996).
Endocytosis and Mating
What role might the two Ste3p endocytic modes play in the mating process? Recycling allows reutilization of the receptor. Thus, a-factor, in addition to inducing the synthesis of new receptor protein through induced STE3 transcription, also conserves preexisting receptor through recycling. In keeping with this, Ste3p levels are found to be dramatically elevated in mating MATα cells (Roth, A., and N. Davis, unpublished results). Recycling may also play a role in the redistribution of surface receptor that occurs during mating. During the late stages of mating, pheromone receptors concentrate within the polarized mating projections of their respective cells (Jackson et al. 1991; Davis, N., unpublished results). In large part, this is due to the polarization of the secretory apparatus and consequent delivery of newly synthesized receptor exclusively to the mating projection. Endocytosis may play a role as well. Mating projection formation is a chemotropic response: the external pheromone gradient is detected, interpreted, and the polarized new growth of the cell body is directed along the gradient towards the highest concentrations of pheromone. The consequence is growth of the mating projection towards the producing cell. In theory, the two Ste3p endocytic mechanisms could combine to provide a simple and direct mechanism for imprinting the map of the external pheromone concentration gradient onto the cell surface distribution of receptor. Consider the consequences of placing a cell having Ste3p evenly displayed at its surface into a ligand concentration gradient. For portions of the cell exposed to low a-factor concentrations, the receptor would be largely unliganded, and consequent constitutive endocytosis would lead to receptor loss. On the other hand, in portions receiving high pheromone input, receptor would be preferentially liganded and recycling would be induced. If recycling is locally restricted, that is if internalized receptor is delivered back to surface sites relatively near to where it was internalized from, then receptor will be retained in parts of the cell surface receiving the high pheromone input, while disappearing from those areas receiving low input. Thus, these two endocytic mechanisms alone, without the need to invoke either signal transduction and/or polarized delivery of new receptor protein, are theoretically capable of generating the polarized receptor distribution characteristic of mating cells. This mechanism could play a role in setting up the initial spatial cues that direct chemotropism, or alternatively, could help to reinforce polarized cues already in place.
Acknowledgments
We thank Steve Nothwehr for affinity-purified anti-ALP antibodies and Jeff Loeb for the use of his microscope and help with the immunofluorescence microscopy. We thank Sandy Lemmon and Bob Fuller for helpful suggestions. We thank Amy Roth for her early work on the ligand-dependent Ste3p endocytosis and for helpful comments on this manuscript.
This work was supported by a grant from the National Science Foundation (MCB 99-83688).
Footnotes
Abbreviations used in this paper: ALP, alkaline phosphatase; CTD, COOH-terminal cytoplasmic tail domain.
References
- Bonifacino J.S., Weissman A.M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V., Gammie A.E., Nijbroek G., Michaelis S., Rose M.D. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J. Cell Biol. 1996;135:1727–1739. doi: 10.1083/jcb.135.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.S., Schekman R.W. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J. Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A.L., Lodish H.F. Sorting and recycling of cell surface receptors and endocytosed ligandsthe asialoglycoprotein and transferrin receptors. J. Cell Biochem. 1983;23:107–130. doi: 10.1002/jcb.240230111. [DOI] [PubMed] [Google Scholar]
- Davis N.G., Horecka J.L., Sprague G.F., Jr. Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia L., Marsh L. Role of the ABC transporter Ste6 in cell fusion during yeast conjugation. J. Cell Biol. 1996;135:741–751. doi: 10.1083/jcb.135.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Davis N.G. Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosisAkr1p is required for kinase localization to the plasma membrane. Mol. Cell. Biol. 2000;20:5350–5359. doi: 10.1128/mcb.20.14.5350-5359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan S.A., Sprague G.F., Jr. The ankyrin repeat-containing protein Akr1p is required for the endocytosis of yeast pheromone receptors. Mol. Biol. Cell. 1997;8:1317–1327. doi: 10.1091/mbc.8.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L., Brown M.S., Anderson R.G., Russell D.W., Schneider W.J. Receptor-mediated endocytosisconcepts emerging from the LDL receptor system. Annu. Rev. Cell. Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Hicke L. Gettin' down with ubiquitinturning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Hicke L., Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L., Zanolari B., Pypaert M., Rohrer J., Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol. Biol. Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C.L., Konopka J.B., Hartwell L.H. S. cerevisiae α pheromone receptors activate a novel signal transduction pathway for mating partner discrimination. Cell. 1991;67:389–402. doi: 10.1016/0092-8674(91)90190-a. [DOI] [PubMed] [Google Scholar]
- Jenness D.D., Spatrick P. Down regulation of the α-factor pheromone receptor in S. cerevisiae . Cell. 1986;46:345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- Koenig J.A., Edwardson J.M. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol. Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R.E., Thorner J. Saccharomyces cerevisiae STE6 gene producta novel pathway for protein export in eukaryotic cells. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J., Nichols B.J., Prescianotto-Baschong C., Riezman H., Pelham H.R. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kane T., Tipper C., Spatrick P., Jenness D.D. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell. Biol. 1999;19:3588–3599. doi: 10.1128/mcb.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Chang A. Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J. Cell Biol. 1997;138:731–746. doi: 10.1083/jcb.138.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol. Cell. Biol. 1988;8:1309–1318. doi: 10.1128/mcb.8.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek H.R., Stepp J.D., Engle H.M., Marks K.M., Tan P.K., Lemmon S.K., Robinson L.C. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R.C., Cooper A.A., Yang H., Stevens T.H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae . J. Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.F., Davis N.G. Ubiquitination of the yeast a-factor receptor. J. Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.F., Davis N.G. Ubiquitination of the PEST-like endocytosis signal of the yeast a-factor receptor. J. Biol. Chem. 2000;275:8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- Roth A.F., Sullivan D.M., Davis N.G. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J. Cell Biol. 1998;142:949–961. doi: 10.1083/jcb.142.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel K.A., Jenness D.D. Direct evidence for ligand-induced internalization of the yeast α-factor pheromone receptor. Mol. Cell. Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano P.G., Tatchell K. Transcription and regulatory signals at the mating type locus in yeast. Cell. 1984;37:969–978. doi: 10.1016/0092-8674(84)90431-8. [DOI] [PubMed] [Google Scholar]
- Tan P.K., Howard J.P., Payne G.S. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae . J. Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A., Avaro S., Prescianotto-Baschong C., Haguenauer-Tsapis R., Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae . J. Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B., Raths S., Singer-Kruger B., Riezman H. Yeast pheromone receptor endocytosis and hyperphosphorylation are independent of G protein-mediated signal transduction. Cell. 1992;71:755–763. doi: 10.1016/0092-8674(92)90552-n. [DOI] [PubMed] [Google Scholar]