Abstract
The class C subset of vacuolar protein sorting (Vps) proteins (Vps11, Vps18, Vps16 and Vps33) assembles into a vacuole/prevacuole-associated complex. Here we demonstrate that the class C-Vps complex contains two additional proteins, Vps39 and Vps41. The COOH-terminal 148 amino acids of Vps39 direct its association with the class C-Vps complex by binding to Vps11. A previous study has shown that a large protein complex containing Vps39 and Vps41 functions as a downstream effector of the active, GTP-bound form of Ypt7, a rab GTPase required for the fusion of vesicular intermediates with the vacuole (Price, A., D. Seals, W. Wickner, and C. Ungermann. 2000. J. Cell Biol. 148:1231–1238). Here we present data that indicate that this complex also functions to stimulate nucleotide exchange on Ypt7. We show that Vps39 directly binds the GDP-bound and nucleotide-free forms of Ypt7 and that purified Vps39 stimulates nucleotide exchange on Ypt7. We propose that the class C-Vps complex both promotes Vps39-dependent nucleotide exchange on Ypt7 and, based on the work of Price et al., acts as a Ypt7 effector that tethers transport vesicles to the vacuole. Thus, the class C-Vps complex directs multiple reactions during the docking and fusion of vesicles with the vacuole, each of which contributes to the overall specificity and efficiency of this transport process.
Keywords: Vps39/Vam6, endosome, Vps41/Vam2, rab, Vps11
Introduction
The vacuole, like the mammalian lysosome, is the primary site of hydrolase-mediated turnover of macromolecules in Saccharomyces cerevisiae (Klionsky et al. 1990). Many newly synthesized vacuolar proteins (e.g., hydrolases) require transport from the endoplasmic reticulum to the vacuole by the secretory pathway. At the trans-Golgi network, vacuolar proteins are diverted to endosomal compartments which ultimately fuse with the vacuole (Vida et al. 1993), a process regulated by >40 proteins designated vacuolar protein sorting (Vps) proteins. vps mutants have been divided into six major subgroups (classes A–F) based on distinct hydrolase missorting, vacuole morphology, and growth phenotypes (Raymond et al. 1992). Deletion of VPS39, a class B VPS gene, results in the cytoplasmic accumulation of endosomal compartments and vesicles which fail to fuse with the vacuole, fragmentation of the vacuole, and severe hydrolase missorting phenotypes (Raymond et al. 1992; Wada et al. 1992). Thus, although the precise function of Vps39 is unknown, it is likely that this protein is required for the fusion of endosomes and other types of transport intermediates with the vacuole.
Several other yeast mutants, in addition to vps39, exhibit class B vps phenotypes and many of these mutants have been found to impair endosome fusion with the vacuole. The relevant genes affected in these mutant strains have been identified through the vps and vacuole morphology (vam) genetic selections and found, in many cases, to encode homologues of proteins with well-established roles in vesicle-mediated trafficking. These proteins include Vam3, a vacuolar t-SNARE (target membrane soluble N-ethylmaleimide-sensitive fusion protein [NSF] attachment protein [SNAP] receptor), Vam7, a SNAP25 (synapse-associated protein of 25 kD)-like protein, and Ypt7/Vam4, an endosomal/vacuolar rab GTPase (Wada and Anraku 1992; Wada et al. 1992, Wada et al. 1997; Wichmann et al. 1992). Vps39/Vam6 and Vps41/Vam2 are also in the class B group, but at present the specific function of these proteins is unknown (Nakamura et al. 1997). Given the apparent functional overlap of this group of proteins, it is possible that some of these proteins physically interact. In fact, data indicate that Vps39 complexes with Vps41 (Nakamura et al. 1997; Price et al. 2000). Furthermore, a large protein complex containing Vps39 and Vps41 has been demonstrated to associate with the Ypt7 rab GTPase in its active, GTP-bound form, suggesting that this protein complex functions as a downstream effector of Ypt7 (Price et al. 2000).
Like Vps39, class C Vps proteins are also required for the fusion of hydrolase-containing endosomes with the vacuole (Banta et al. 1990; Robinson et al. 1991; Rieder and Emr 1997). Class C mutants accumulate fusion-incompetent endosomes and vesicles in the cytoplasm of the cell, lack vacuoles, and missort vacuolar hydrolases (Rieder and Emr 1997). Class C Vps proteins, Vps18, Vps11, Vps16 and Vps33, are conserved in mammalian systems, Drosophila, and Caenorhabditis elegans and have been found to assemble into a membrane-associated protein complex (the C-Vps complex) which localizes to the vacuole and prevacuolar compartments (Rieder and Emr 1997; Sevrioukov et al. 1999). Vps18 and Vps11 each contain RING domains and clathrin heavy chain repeats, protein motifs that are believed to mediate protein–protein interactions (Robinson et al. 1991; Rieder and Emr 1997; Ybe et al. 1999). Vps33 encodes a Sec1 homologue, suggesting a potential role for the C-Vps complex in regulating the assembly of SNARE complexes (Banta et al. 1990). Consistent with this notion, mutants of the vacuolar t-SNARE, VAM3, both genetically and physically interact with components of the C-Vps complex (Darsow et al. 1997; Sato et al. 2000). The C-Vps complex has also been shown to physically associate with Vam3, promoting SNARE interactions between Vam3 and Vam7 (Sato et al. 2000). These findings suggest that the C-Vps complex acts cooperatively with Vam3 to regulate the fusion of vesicular transport intermediates with the vacuole.
Protein complexes that function at distinct stages of the secretory pathway, perhaps analogously to the C-Vps complex, have been identified. The Exocyst, a complex of seven proteins, functions as a multiprotein effector of the vesicular Sec4 rab GTPase, targeting Golgi-derived secretory vesicles with the plasma membrane (TerBush et al. 1996; Guo et al. 1999). The mammalian early endosome antigen 1 (EEA1) complex catalyzes nucleotide exchange on rab5, functions as a rab5 effector, and modulates SNARE activity in promoting early and late endosome fusion (Stenmark et al. 1995; Horiuchi et al. 1997; McBride et al. 1999; Simonsen et al. 1999). An analogous complex of proteins containing Vac1, the yeast homologue of EEA1, Pep12, an endosomal t-SNARE, Vps45, a Sec1 homologue, and the Vps21 rab GTPase functions in Golgi to endosome transport (Burd et al. 1997; Peterson et al. 1999). Transport protein particle (TRAPP) is a complex of nine proteins which functions upstream of SNAREs in the docking of ER-derived vesicles with the Golgi (Sacher et al. 1998). Thus, the Exocyst, EEA1, Vac1, and TRAPP complexes are proposed to be important determinants for the specificity of vesicle fusion sites at the appropriate target membrane.
Here we present evidence indicating that the C-Vps complex regulates the fusion of transport intermediates with the vacuole by an unexpected mechanism. We find that the C-Vps complex also contains Vps39 and Vps41. Disruption of Vps39 interactions with the C-Vps complex mislocalizes Vps39 from membrane-enriched subcellular fractions to the soluble/cytosolic fraction and causes severe hydrolase missorting defects. This suggests that localization of Vps39 to the endosome/vacuole is essential for vacuolar protein transport. A protein complex containing Vps39 and Vps41 has been shown to interact with the GTP-bound form of the Ypt7 rab GTPase and to function as a downstream effector of Ypt7 (Price et al. 2000). Our findings indicate that the Vps39/Vps41-containing C-Vps complex also functions to stimulate nucleotide exchange on Ypt7. We show that Vps39 preferentially interacts with GDP-bound and nucleotide-free Ypt7, but not with other rab GTPases. Moreover, purified Vps39 stimulates GDP/GTP exchange on Ypt7 in vitro, indicating that Vps39 functions as a Ypt7 nucleotide exchange factor. Therefore, our evidence reveals a role for the C-Vps complex in stimulating nucleotide exchange on Ypt7.
Materials and Methods
Strains
For a full list of strains used in this study, please see Table .
Table 1.
Strains Used in This Study
| Strain | Genotype | Reference |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al. 1988 |
| EMY38 | SEY6210; vps39Δ::HIS3 | This study |
| vps39tsf | EMY38; pRS416.vps39-1 | This study |
| PCY2 | MATa gal4Δ gal80Δ URA3::GAL1-lacZ lys2-801 his3-Δ200 trp1-Δ63 leu2 ade2-101 | Rehling et al. 1999 |
| HF7c | MATa ura3-52 his3-Δ200 lys2-801 ade2-101 trp1-901 leu2-3/112 gal4-542 gal80-538 LYS2::GAL1-HIS3 URA3::(GAL4 17 mers)3-CYC1-lacZ | CLONTECH Laboratories, Inc. |
| WSY99 | SEY6210; ypt7Δ::HIS3 | Wurmser and Emr 1998 |
| ypt7tsf | WSY99; pRS415.ypt7-38 | This study |
| AWY2 | ypt7tsf; vps39Δ::TRP1 pRS416.vps39-1 | This study |
| vam7tsf | TKSY43; pvam7-167 | Sato et al. 1998 |
| TKSY80 | vps39tsf; vam7Δ::leu2 pvam7-167 | This study |
| vam3tsf | TDY2; pVAM3-6.416 | Darsow et al. 1997 |
| TKSY20 | vps39tsf; vam3Δ::LEU2 pVAM3-6.416 | This study |
| TKSY74 | ypt7tsf; vam7Δ::leu2 pvam7-167 | This study |
| SEY6211e | MATa leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps11-Δ2::LEU2 | Rieder and Emr 1997 |
Plasmids and DNA Methods
Standard methods using restriction and modifying enzymes (Roche) were used in carrying out DNA ligations, bacterial minipreps, and bacterial and yeast transformations (Rieder and Emr 1997). DNA fragments were purified using QIAquick Gel Extraction (QIAGEN). Protein A fusions of VPS39 and vps39 mutants were constructed by removing the VPS39 stop codon and engineering in its place an SphI site. An SphI–KpnI fragment encoding doubly iterated protein A was then ligated with VPS39 resulting in a COOH-terminal protein A–Vps39 fusion whose expression is driven by the endogenous VPS39 promoter. ypt7 point mutants and vps39 truncation mutants (for two-hybrid assays) as well as GST-Vps21 were produced by PCR using primers engineered to contain convenient restriction sites. The cloning of multicopy VPS41 (pWS26), VPS11, VPS18, VPS16, and VPS33 as well as protein A–Vps16, pMP139, and pMP141 have been described (Cowles et al. 1997; Rieder and Emr 1997; Sato et al. 2000). vps39-1 and ypt7-38 were generated through random PCR mutagenesis (amino acids 1–1,049 of Vps39 or 74–208 of Ypt7) using Taq polymerase (PerkinElmer) and were isolated using a colorimetric plate assay for secreted carboxypeptidase Y (CPY) (Burd et al. 1997).
Vacuolar Protein Sorting
CPY, alkaline phosphatase (ALP), and aminopeptidase I (API) maturation assays were carried out in whole cells (see Fig. 2 A and Fig. 5A and Fig. B) or spheroplasts (see Fig. 5 A) by pulse-labeling with 10 μCi of Express [35S] protein labeling mix (Dupont) per OD600 unit of cells, chasing for 0–120 min, and processing cells for immunoprecipitation with antisera specific to CPY, ALP, or API (Klionsky et al. 1992) before SDS-PAGE, as described (Rieder and Emr 1997). For temperature shift experiments, cells were shifted from 26°C to 38°C 10 min before labeling.
Figure 2.
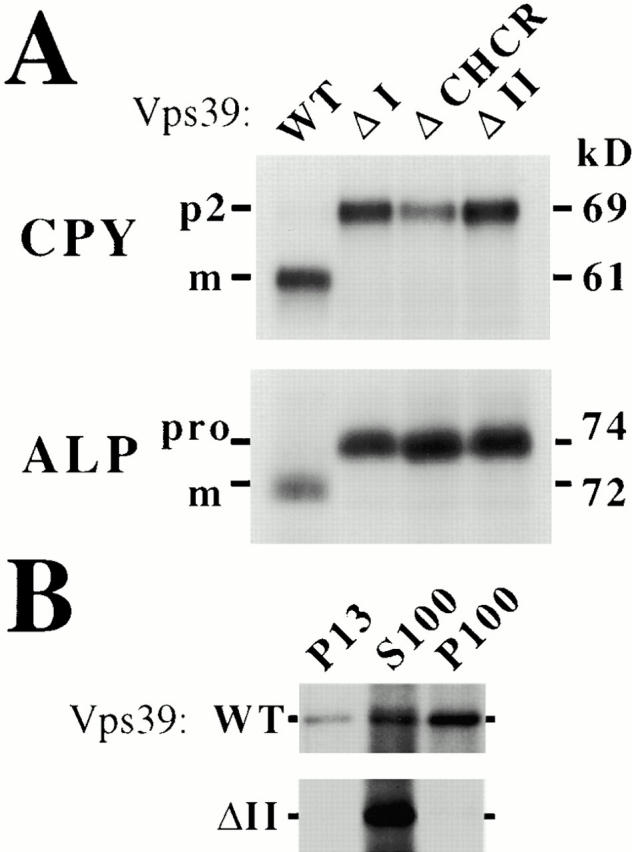
Domain I and domain II are essential for Vps39 function. vps39Δ cells expressing the indicated single-copy protein A–VPS39 construct were labeled for 10 min with [35S]methionine and chased with excess unlabeled methionine for 30 min at 30°C. (A) Labeled lysates of these cells were immunoprecipitated for CPY or ALP and the resulting immunoprecipitates were analyzed by SDS-PAGE and fluorography. m, Mature; pro, precursor ALP. (B) Subcellular Vps39 localization was determined by osmotically lysing [35S]methionine-labeled spheroplasts and sequentially centrifuging the lysates at 13,000 g and 100,000 g to yield the low-speed pellet (P13), high-speed pellet (P100), and supernatent (S100). Vps39 was immunoprecipitated from P13, S100, and P100 fractions and analyzed by SDS-PAGE and fluorography. WT, Wild-type.
Figure 5.
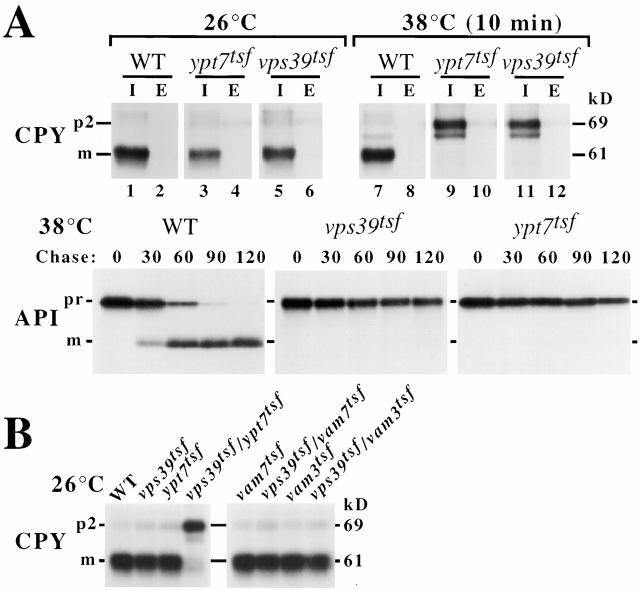
ypt7 functionally interacts with vps39. Wild-type (WT), vps39tsf, or ypt7tsf strains were grown overnight at 26°C and pulse-labeled with [35S]methionine for 10 min at 26°C or 38°C. (A) After a 30-min chase, labeled wild-type, vps39tsf, or ypt7tsf spheroplasts were separated into intracellular (I) and extracellular (E) fractions. CPY immunoprecipitates of I and E were resolved by SDS-PAGE and fluorography, revealing CPY maturation defects in the vps39tsf and ypt7tsf at 38°C. API immunoprecipitates derived from labeled wild-type, vps39tsf, and ypt7tsf cells were analyzed by SDS-PAGE and fluorography, revealing defects in API maturation in the ypt7tsf, but not wild-type cells at 38°C. (B) CPY immunoprecipitates derived from the indicated strains were analyzed by SDS-PAGE and fluorography, revealing a strong genetic interaction with vps39tsf/ypt7tsf but not other double mutants at 26°C. m, Mature; p2, precursor CPY; pr, precursor API.
Subcellular Fractionation
21 OD600 units of spheroplasts were pulse-labeled with 420 μCi of Express [35S] (20 min), chased for 1 h, and immunoprecipitated for Vps39, as described (Rieder and Emr 1997). Antiserum specific to amino acids 315–916 of Vps39 was raised by standard methods (Darsow et al. 1997).
Purification of Protein A Fusions
14 OD600 units of spheroplasts expressing protein A fusions were pulse-labeled with 420 μCi of Express [35S] for 20 min and, as above, chased for 1 h. Spheroplasts were Dounce homogonized in 1.5 ml of PBS plus protease inhibitors, centrifuged at 325 g for 5 min and 100,000 g for 30 min to clear lysates of unbroken cells and separate P total/S100 fractions, respectively. The P total was resuspended and incubated in 0.5 ml of PBS plus 250 mM NaCl and 0.5% IGEPAL CA-630 (NP-40; Sigma-Aldrich) for 5 min, after which 0.5 ml of PBS containing 0.5% NP-40 was added. The P total extract was then centrifuged at 13,000 g, and both the P total and S100 plus 0.5% NP-40 were incubated with 10 μl of IgG-coupled sepharose (Amersham Pharmacia Biotech) for 60 min. The IgG-sepharose was washed three times with 1 ml of PBS plus 0.5% NP-40 and directly resolved by SDS-PAGE or denatured for 10 min at 95°C in 50 μl of 50 mM Tris, pH 7.5, 1 mM EDTA, 1% SDS (see Fig. 3) and immunoprecipitated with polyclonal antiserum specific for Vps11, Vps18, Vps16, Vps33, or Vps39 (Rieder and Emr 1997). Standard methods (Darsow et al. 1997) were used to raise antisera specific to Vps41. For Western blot analysis with monoclonal anti-HA antibody (Roche) or polyclonal antiserum (Fig. 4 A and 7 A), unlabeled spheroplasts were treated as described (Babst et al. 1997). In Fig. 4 B, the S100 fraction derived from 4 OD600 units of spheroplasts was assayed as described above.
Figure 3.
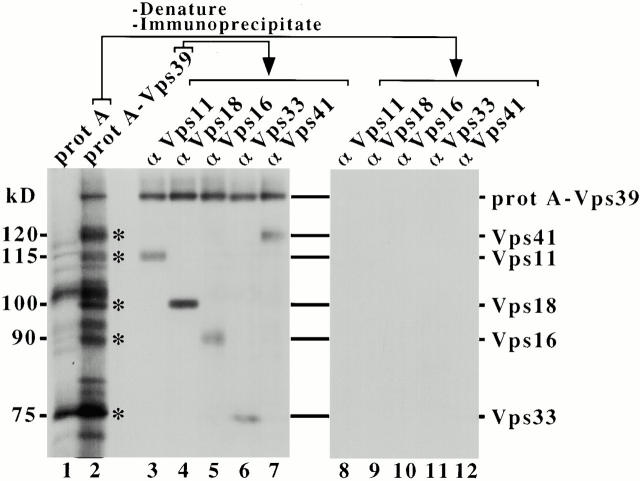
Vps39 and Vps41 are components of the C-Vps complex. Spheroplasts expressing single-copy protein (prot) A or protein A–VPS39 were labeled with [35S]methionine for 20 min and chased for 1 h at 30°C. Osmotic lysates of these strains were centrifuged at 100,000 g to generate the P total. 0.5% NP-40/250 mM NaCl extracts of the P total were incubated with IgG-sepharose to affinity-purify protein A fusions. Washed IgG-sepharose was analyzed by SDS-PAGE/fluorography. Multiple proteins coprecipitated preferentially with protein A–Vps39 (several are indicated by asterisks), but not protein A alone (lanes 1 and 2). IgG-sepharose–purified protein A fusions were also subjected to denaturation and immunoprecipitation using antibodies specific for Vps11, Vps18, Vps16, Vps33, and Vps41 (lanes 3–12).
Figure 4.
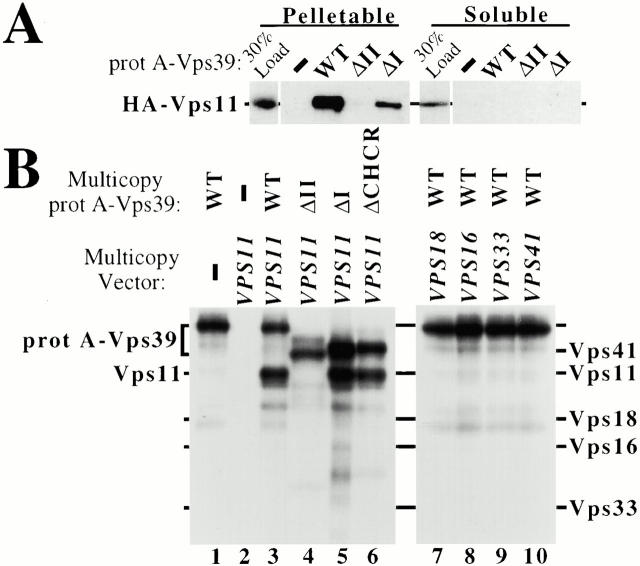
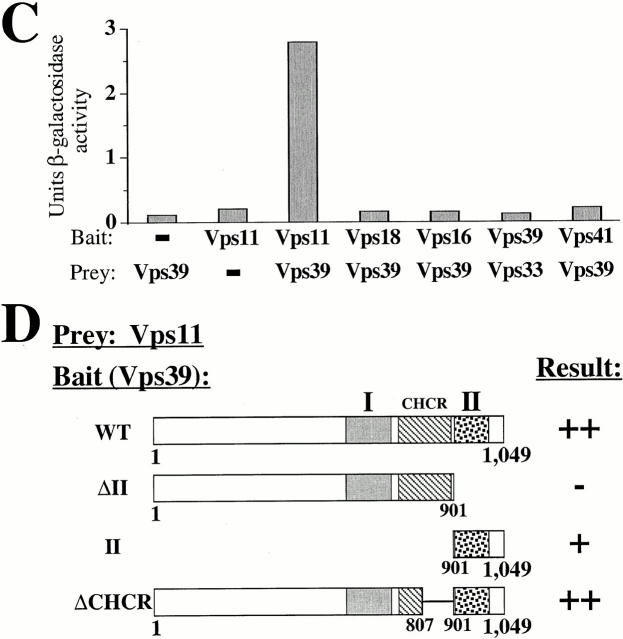
Domain II is required for Vps39 association with the C-Vps complex. (A) vps39Δ cells coexpressing HA-tagged VPS11 and protein (prot) A fusions of VPS39, vps39ΔII, or vps39ΔI from single-copy plasmids were osmotically lysed and separated by a 100,000 g spin into pelletable and soluble fractions. Extracts of each fraction were incubated with IgG-sepharose, and association of affinity-purified Vps39 with the C-Vps complex component, HA-Vps11, was assessed by Western blot assay. (B) Wild-type (WT) cells cooverexpressing the indicated multicopy protein A–VPS39 fusion with either multicopy VPS11, VPS18, VPS16, VPS33, or VPS41 were pulse-labeled with [35S]methionine, osmotically lysed, and centrifuged at 100,000 g. Protein A–Vps39 was purified from the S100 supernatent using IgG-sepharose, and association of Vps39 with the individual C-Vps complex component was determined by SDS-PAGE and fluorography. (C) Two-hybrid interactions between Vps39 and Vps11 were determined by quantifying the β-galactosidase activity resulting from expression of bait VPS11 and prey VPS39 constructs. The average of three independent transformants is presented for each component of the C-Vps complex assayed. (D) Requirement for domain II of Vps39 in Vps39/Vps11 two-hybrid interactions was assessed based on the histidine-independent growth of the indicated cotransformants (>50 transformants assayed).
Two-Hybrid Analysis
In Fig. 4 D and 6 B, interactions were assessed based on the histidine-independent growth of HF7c (CLONTECH Laboratories, Inc.). Interactions were quantified (see Fig. 4 C, 6 A, and 7 B) using the PCY2 strain as described (Rehling et al. 1999). The prenylation motif within ypt7 two-hybrid constructs was mutated (ypt7C206S, C208S).
GDP/GTP Exchange Assays
Glutathione S-transferase (GST)-Ypt7 and GST-Vps21 were purified from Escherichia coli (JM101) as described (Babst et al. 1997). Ypt7 or Vps21 eluted from glutathione-sepharose (Amersham Pharmacia Biotech) was dialyzed against buffer A (20 mM Tris, pH 7.5, 0.5 mM DTT, 150 mM NaCl, 40 μg/ml BSA, 5 mM MgCl2) for 2 h at 4°C. Spheroplasts expressing multicopy protein A–VPS39 or protein A alone were lysed by Dounce homogenization (5 OD600 units/ml buffer A, plus protease inhibitors), centrifuged at 100,000 g to generate the S100. Overexpressed Vps39 or protein A was then purified from the S100 fraction using IgG-sepharose, as described above. Purified protein A–Vps39 was quantified by silver staining. 1 μM soluble Ypt7 or Vps21 was bound to 4 μM GDP and then incubated with 6 μM [3H]GTP/6 μM GTP and IgG-sepharose–bound protein A–Vps39 or protein A derived from 15 OD600 units in 50 μl of buffer A. GDP/GTP exchange was determined by filtering Ypt7 through NitroPure nitrocellulose (Osmonics Inc.), washing the filter with 1.5 ml of buffer A, and quantifying the filter-associated [3H]GTP. Multiple experiments were performed; a representative example is presented.
rab Binding Assays
As described above, GST-Ypt7 and GST-Vps21 were purified from bacteria, eluted off glutathione–sepharose, and dialyzed against buffer A. For each assay, 50 ng of Ypt7 and Vps21 was prebound to GDP or GTPγS (Sigma-Aldrich) and incubated with ∼50 ng of protein A or protein A–Vps39 purified from 25 OD600 units of SEY6210 cells overexpressing protein A or protein A–Vps39 from a multicopy plasmid. Binding reactions were carried out in 100 μl of buffer A plus 0.5% NP-40 for 30 min at 25°C. Beads were washed three times with 1 ml of buffer A plus 0.5% NP-40, resolved by SDS-PAGE, and the presence of GST-Ypt7 or GST-Vps21 was determined by Western blotting using anti-GST antibody.
Results
Vps39 Contains Three Highly Conserved Subdomains That Are Essential for Vps39 Function
Vps39 encodes a hydrophilic, 1,049–amino acid protein that lacks a potential signal sequence or membrane-spanning region. Amino acids 733–893 of Vps39 encode a single clathrin heavy chain repeat (CHCR), an α-helical–rich protein motif that is iterated seven times in clathrin heavy chain and believed to mediate protein–protein interactions required for clathrin coat formation (Ybe et al. 1999). The single CHCR found within Vps39 is also likely to function as a protein-binding domain, although the identity of its ligand is unknown.
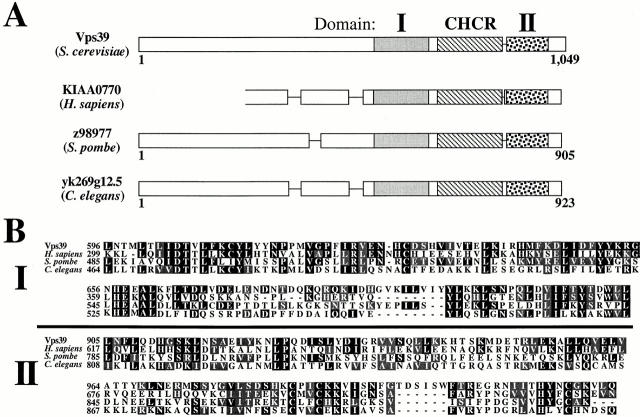
BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/) of the nonredundant protein database suggest that homologues of Vps39 exist in Homo sapiens, Schizosaccharomyces pombe, and Caenorhabditis elegans. A multiple alignment using Clustal indicated that the COOH-terminal half of Vps39 (amino acids 596–1,021) is 28, 25, and 22% identical and 45, 40, and 38% similar to the COOH termini of the H. sapiens, S. pombe, and C. elegans open reading frames (ORFs), respectively. Thus, these ORFs may reveal functionally conserved protein motifs, in addition to the CHCR, within the COOH terminus of Vps39. For instance, two regions adjacent to the CHCR that we designate domain I (amino acids 596–715) and domain II (amino acids 905–1,021) are encoded by the conserved region of Vps39 (Fig. 1A and Fig. B). We hypothesized that domain I and domain II may be essential to Vps39 function.
Figure 1.
Multiple protein motifs exist within Vps39. (A) A BLAST search of the nonredundant protein database revealed homologues of Vps39 from H. sapiens, S. pombe, and C. elegans. (B) A multiple alignment (Clustal) of full-length Vps39 with these ORFs indicated that the COOH-terminus of Vps39 (amino acids 596–1,021) is 28, 25, and 22% identical and 45, 40, and 38% similar to the COOH terminal regions of the H. sapiens, S. pombe, and C. elegans ORFs, respectively. The conserved region of Vps39 contains two regions adjacent to the CHCR which we designate domain I and domain II.
EMY38 (vps39Δ) cells expressing single-copy plasmids encoding wild-type VPS39, vps39ΔI (lacking amino acids 581–682), vps39ΔCHCR (lacking amino acids 809–900), or vps39ΔII (deleted for amino acids 902–1,049) were assayed for the vacuolar delivery of CPY and ALP (see Materials and Methods). Although vps39Δ cells complemented with VPS39 were able to proteolytically mature CPY and ALP, expression of vps39ΔI, vps39ΔCHCR, or vps39ΔII resulted in the accumulation of precursor forms of CPY and ALP (Fig. 2 A) and the fragmented class B mutant vacuole morphology, despite the fact that these vps39 mutants were stably expressed (data not shown). Thus, domain I, the CHCR, and domain II are important to Vps39 function.
We also tested whether deletion of any one of these subdomains is sufficient to change the subcellular fractionation of Vps39. vps39Δ cells expressing single-copy levels of either VPS39, vps39ΔI, vps39ΔCHCR, or vps39ΔII were converted to spheroplasts and pulse-labeled with [35S]methionine. Cell lysates were centrifuged at 13,000 g to yield a low-speed pellet (P13) and supernatant (S13). The S13 was centrifuged at 100,000 g to isolate the high-speed pellet (P100) and supernatant (S100). P13, S100, and P100 fractions were immunoprecipitated for Vps39. A significant portion of the total pool of wild-type Vps39 associated with the pelletable fractions (10% P13, 40% S100, 50% P100) (Fig. 2 B). Although mutation of domain I or the CHCR does not dramatically affect Vps39 localization (data not shown), deletion of domain II shifts Vps39ΔII to the soluble fraction (0% P13, >95% S100, >5% P100) (Fig. 2 B). Thus, it is likely that domain II plays an important role in localizing Vps39 to the pelletable fractions, which are enriched for cellular membranes and membrane-binding proteins, whereas domain I and the CHCR carry out a distinct aspect(s) of Vps39 function.
Vps39 Is a Component of a Protein Complex Containing Multiple Vps Proteins
One possibility raised by these results is that domain II anchors Vps39 to the pelletable cell fractions by mediating interactions with another pelletable protein(s). This possibility is especially likely since Vps39 has been isolated in a large protein complex (Nakamura et al. 1997; Price et al. 2000). Therefore, we affinity-purified a functional protein A–tagged Vps39 (protein A–Vps39) from P13 and P100 fractions and assayed for proteins which coprecipitate in a Vps39-dependent manner.
vps39Δ cells expressing single-copy protein A or protein A–VPS39 was pulse-labeled with [35S]methionine. Lysates of these strains were centrifuged at 100,000 g to generate the total cellular particulate fraction (P total) that contains the proteins and/or membranes of both the P13 and P100 fractions. Detergent/NaCl extracts of the P total were incubated with IgG-coupled sepharose. Analysis of the IgG-sepharose by SDS-PAGE and fluorography revealed multiple proteins that coprecipitated with protein A–Vps39 (indicated by asterisks), but not protein A alone, even though lysates of each strain contained ∼5.6 × 108 cpm (Fig. 3, lanes 1 and 2). The apparent molecular masses of several proteins that coprecipitated with protein A–Vps39 correspond with Vps proteins as follows: Vps41 (120 kD), Vps11 (115 kD), Vps18 (100 kD), Vps16 (90 kD), and Vps33 (75 kD). Proteins copurifying with protein A and protein A–Vps39 were therefore subjected to denaturation and immunoprecipitation using antibodies specific for these Vps proteins. These immunoprecipitates revealed that significant amounts of Vps11, Vps18, Vps16, Vps33, and Vps41 copurified with protein A–Vps39, but not protein A (Fig. 3, lanes 3–12). Protein A–Vps39 was also present in these immunoprecipitates as the protein A element renatures and associates with IgG during incubation with polyclonal antiserum. In contrast, protein A–Vps39 derived from the soluble (S100) fraction did not associate with these proteins, suggesting that this complex is enriched in the pelletable fractions (data not shown). The P total pool of a protein A–Vps16 fusion also copurified with Vps39, Vps41, Vps11, Vps18, Vps16, and Vps33 (data not shown). We conclude that a significant portion of the pelletable pool of Vps39 associates with Vps41 and class C Vps proteins, Vps11, Vps18, Vps16, and Vps33, in a heterooligomeric class C Vps protein complex (C-Vps complex). This is consistent with results which indicate that Vps39 and Vps41 are components of a large protein complex (Nakamura et al. 1997; Price et al. 2000) and with observations indicating that Vps11, Vps18, Vps16, and Vps33 form a complex (Rieder and Emr 1997). The data we present here extend these findings by demonstrating that these proteins are components of the same complex.
Domain II Mediates Vps39 Interactions with Vps11, a Component of the C-vps Complex
As described above, deletion of domain II shifted Vps39 from P13 and P100 subcellular fractions to the soluble fraction (S100) and resulted in strong CPY and ALP missorting defects (Fig. 2A and Fig. B). This raises the possibility that domain II mediates Vps39 association with the C-Vps complex to potentially anchor Vps39 in the pelletable cell fraction. Therefore, we tested the relative capacity of wild-type Vps39 and Vps39ΔII to interact with the C-Vps complex.
vps39Δ cells expressing protein A or protein A fusions of VPS39, vps39ΔII, or vps39ΔI were transformed with a single-copy plasmid encoding either hemagglutinin-tagged VPS11 (HA-VPS11, pMP139) or VPS18 (HA-VPS18, pMP141). P total and S100 fractions of these strains were incubated with IgG-coupled sepharose to purify protein A–Vps39 fusions, as described above. Western blot analysis indicated that wild-type Vps39 and Vps39ΔI derived from the membrane-associated fraction (P total), but not the soluble fraction (S100), interacted with C-Vps complex components Vps11 and Vps18 (Fig. 4 A, data not shown), suggesting that the C-Vps complex is enriched in the P total even though soluble pools of Vps11 and Vps18 exist (Rieder and Emr 1997). Vps39ΔII failed to interact with Vps11 or Vps18 (Fig. 4 A, data not shown). Thus, domain II, which appears to be an important localization determinant for Vps39, is also required for the association of Vps39 with C-Vps complex components Vps11 and Vps18. It is possible that deletion of domain II detaches Vps39 from the C-Vps complex, but otherwise leaves the C-Vps complex intact. Alternatively, inclusion of Vps39 in the C-Vps complex may be required for overall complex assembly.
It has previously been proposed that Vps39 and Vps41 interact, although it has not been determined whether this association is direct (Nakamura et al. 1997). Two approaches were undertaken to test this. First, protein A–Vps39 was affinity purified from [35S]methionine-labeled strains cooverexpressing protein A–VPS39 and either VPS41, VPS11, VPS18, VPS16, or VPS33. Multicopy vectors lead to ∼15-fold overexpression of these genes (data not shown), allowing us to study the capacity of Vps39 to directly interact with Vps41, Vps11, Vps18, Vps16, or Vps33 in the relative absence of other proteins. When only protein A–Vps39 is overproduced and purified from the soluble (S100) fraction using IgG-sepharose, as described, IgG-sepharose binds only protein A–Vps39. Cooverexpressing both protein A–VPS39 and VPS11 led to the isolation of protein A–Vps39 and another protein which comigrates with immunoprecipitated Vps11, indicating that Vps39 and Vps11 directly interact (Fig. 4 B, lanes 1 and 3). Protein A itself did not bind overproduced Vps11 (lane 2). In contrast, Vps39ΔII exhibited a marked decrease in Vps11 binding, even though Vps39ΔII was expressed and bound to IgG-sepharose, whereas Vps39ΔI and Vps39ΔCHCR each interacted with Vps11 (Fig. 4 B, lanes 4–6). The soluble (S100) pools of overexpressed Vps18, Vps16, Vps33, and Vps41 failed to interact with overexpressed protein A–Vps39 under identical conditions (lanes 7–10), despite the fact that these proteins were all readily detectable by immunoprecipitation of identically prepared soluble (S100) fractions (data not shown).
The two-hybrid system revealed that coexpression of bait VPS11 and prey VPS39 resulted in 2.8 U of β-galactosidase activity, indicative of an interaction between the Vps39 and Vps11 (Fig. 4 C). Neither bait VPS39 nor prey VPS11 constructs produced significant background (<0.2 U). Bait vps39ΔII was expressed, but failed to generate a detectable two-hybrid interaction with prey VPS11 (Fig. 4 D). Moreover, coexpression of a bait vector containing domain II alone (amino acids 901–1,049) with prey VPS11, but not empty prey vector, exhibited a two-hybrid interaction, suggesting that domain II is sufficient to bind Vps11 (Fig. 4 D). Although Vps18, Vps16, Vps33, and Vps41 were produced as Gal4 fusions (data not shown), these proteins did not exhibit two-hybrid interactions with Vps39 (Fig. 4 C). Thus, domain II mediates Vps39 association with the pelletable cell fraction, the C-Vps complex, and the C-Vps complex component, Vps11, suggesting that incorporation of Vps39 into the C-Vps complex is required for Vps39 localization to the pelletable cell fractions.
The rab GTPase, Ypt7, Functionally Interacts with Vps39
Although domain II is required for Vps39 association with Vps11, deletion of domain I does not appear to eliminate this interaction, suggesting that domain I has a distinct function. This notion raises the possibility that Vps39 may interact with additional Vps proteins which are essential for the docking or fusion of transport vesicles with the vacuole such as Vam7, a SNAP25 protein, Vam3, a vacuolar t-SNARE, or Ypt7, a vacuolar and prevacuolar rab GTPase (Wichmann et al. 1992; Darsow et al. 1997; Sato et al. 1998). To determine if a genetic relationship exists between vps39 and either ypt7, vam7, or vam3, temperature-sensitive vps39 and ypt7 mutants were generated.
The entire coding region of VPS39 and the region of YPT7 that encodes amino acids 74–208 were subjected to random PCR–mediated mutagenesis leading to the selection of mutants that secreted the vacuolar hydrolase, CPY, at 38°C, but not at 26°C, using a colorimetric plate assay (see Materials and Methods). 5,000 vps39 and 10,000 ypt7 candidates were screened, leading to the isolation of vps39-1 and ypt7-38. To characterize these alleles, we monitored the vacuolar delivery of CPY, ALP, and API along the three known vacuolar transport pathways (Klionsky et al. 1992; Cowles et al. 1997) in both vps39Δ cells expressing vps39-1 (vps39tsf) and WSY99 cells (ypt7Δ) expressing ypt7-38 (ypt7tsf) from single-copy plasmids.
vps39tsfand ypt7tsfcells were grown at 26°C, converted to spheroplasts, and pulse-labeled with [35S]methionine at either 26°C or 38°C. Both the intracellular (I) and extracellular (E) fractions were subjected to immunoprecipitation with an antibody specific for either CPY or ALP. Although vps39tsf and ypt7tsfcells maintained at 26°C were able to mature CPY and ALP, indicating that both hydrolases were delivered to the vacuole, the vps39tsf and ypt7tsfstrains preshifted to 38°C for 10 min before labeling accumulated intracellular pools of precursor CPY (p2CPY) and precursor ALP (proALP) (Fig. 5 A, lanes 3, 5, 9, and 11; data not shown). Only small amounts (<5%) of the cellular pool of p2CPY were secreted upon inactivation of Vps39 or Ypt7 (lanes 10 and 12). An uncharacterized form of CPY (i.e., not p1CPY) which migrates faster than p2CPY was also present (lanes 9 and 11), possibly because p2CPY accumulates in endosomes and is aberrantly processed in vps39tsf and ypt7tsfcells under these conditions. In contrast, SEY6210 (wild-type) cells exhibited the mature forms of CPY and ALP at both 26°C and 38°C (lanes 1 and 7). Similarly, after a 10-min shift to 38°C, vps39tsf and ypt7tsf cells failed to mature API, even after the 120-min chase point, while wild-type cells converted API from the 60-kD precursor form (prAPI) to the 50-kD mature form (mAPI) by 90 min (Fig. 5 A). At 26°C, wild-type, vps39tsf, and ypt7tsfcells matured API with similar kinetics (data not shown). Consistent with previous CPY and ALP sorting results using vps39 and ypt7 null mutants (Wichmann et al. 1992; Nakamura et al. 1997), these findings indicate that Vps39 and Ypt7 play important roles in the transport of newly synthesized hydrolase precursors along the CPY, ALP, and API/autophagic trafficking pathways. Thus, the sorting defects of the vps39tsf and ypt7tsfstrains are characteristic of a specific subset of vps mutants that mediate the docking and fusion of vesicular compartments with the vacuole (Darsow et al. 1997; Rieder and Emr 1997; Sato et al. 1998).
To determine if vps39 genetically interacts with ypt7, a vps39tsf/ypt7tsf double-mutant strain was generated and assayed for CPY missorting defects at 26°C. Although wild-type, vps39tsf, and ypt7tsf cells converted CPY to its mature form, strikingly, AWY2 (vps39tsf/ypt7tsf) double-mutant cells exhibited >95% p2-precursor CPY at 26°C, demonstrating that there is a strong synthetic genetic interaction between vps39tsfand ypt7tsf (Fig. 5 B). Similar results were observed when ALP maturation was assayed (data not shown). In contrast, TKSY80 (vps39tsf/vam7tsf), TKSY20 (vps39tsf/vam3tsf), TKSY74 (ypt7tsf/vam7tsf), and ypt7tsf/vam3tsf double-mutant strains each exhibited >95% mature CPY (Fig. 5 B, data not shown). This genetic evidence raises the intriguing possibility that Vps39 and the Ypt7 rab GTPase may physically associate.
Physical interactions between Vps39 and Ypt7 were tested for by two-hybrid assay. No significant background resulted from YPT7 bait plasmid or VPS39 prey plasmids; however, when YPT7 bait was cotransformed with VPS39 prey, cellular β-galactosidase activity of ∼4 U was detected (Fig. 6 A). Interactions were also detected between bait VPS39 and prey YPT7 (data not shown). Combining prey VPS39 with bait VPS21, the yeast rab5 GTPase (Horazdovsky et al. 1994; Singer-Kruger et al. 1994), or bait RAS1 resulted in <0.25 U of β-galactosidase activity. Ypt7 did not exhibit interactions with Vps11, Vps18, Vps16, Vps41, or Vam7 by the two-hybrid assay (data not shown), suggesting that the Vps39 interaction with Ypt7 is specific.
Figure 6.
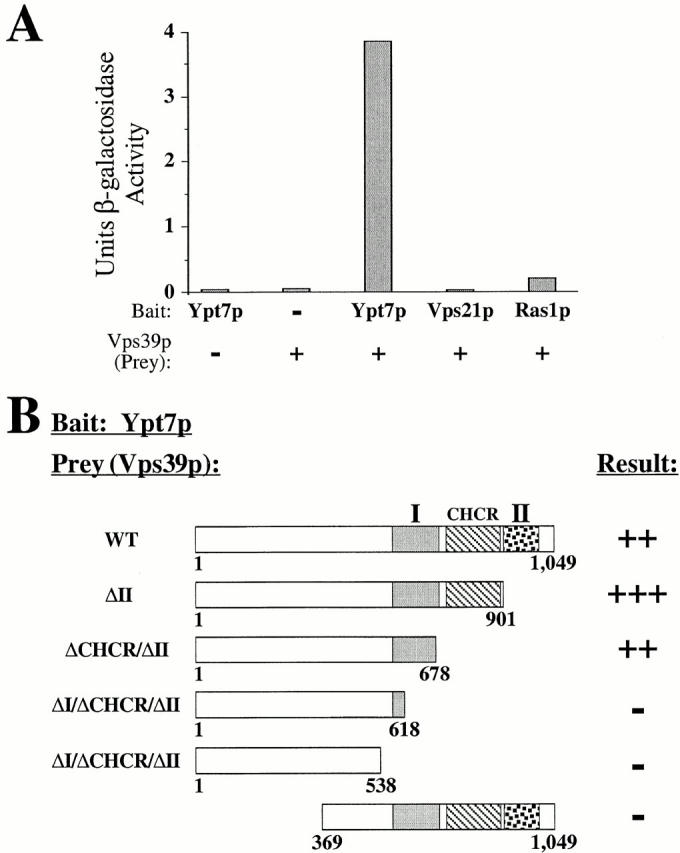
Vps39 physically associates with Ypt7. (A) Two-hybrid interactions between Vps39 and Ypt7 were assayed by quantifying the β-galactosidase activity of bait YPT7 and prey VPS39 cotransformants. The average β-galactosidase activity of three independent transformants of each strain is presented. (B) Two-hybrid interactions between Ypt7 and Vps39 require domain I. Association of the indicated Vps39 mutants with Ypt7 was assayed by cell growth under histidine-free conditions (>50 transformants tested). WT, wild-type.
Although the Ypt7–Vps39 interaction did not require the CHCR or domain II of Vps39, Ypt7 failed to interact with Vps39 lacking significant portions of domain I (Vps39ΔI/ΔCHCR/ΔII) or with Vps39 truncations lacking amino acids 1–368 (Fig. 6 B), despite the fact that these mutants were expressed (data not shown). Therefore, Ypt7 associates with Vps39 in a manner which requires domain I and additional NH2-terminal regions of Vps39, but not the CHCR or domain II. This establishes that the requirements for Vps39–Ypt7 interactions (e.g., domain I) are distinct from those of Vps39–Vps11 interactions (i.e., domain II).
Vps39 Stimulates Nucleotide Exchange on Ypt7
Our evidence indicates that association of Vps39 with the C-Vps complex component, Vps11, requires domain II of Vps39 (Fig. 4) whereas interactions between Ypt7 and Vps39 do not (Fig. 6 B). This evidence suggests that Ypt7 binds Vps39 independent of the other members of the C-Vps complex. To further test this, protein A–Vps39 was overexpressed and purified from the S100 fraction of SEY6211e (vps11Δ) cells using IgG-sepharose and assayed for nucleotide-dependent binding to Ypt7 in vitro. GST-Ypt7 and GST-Vps21 were expressed in bacteria, purified using glutathione–sepharose, and eluted off the beads. The purified GTPases were then preloaded with either GTPγS or GDP and incubated with IgG-sepharose–immobilized Vps39. Western blot analysis revealed that Vps39 preferentially bound GDP-Ypt7, but not GTPγS-Ypt7 (Fig. 7 A, lanes 1 and 2). No significant binding of Ypt7 to protein A alone occurred (lanes 3 and 4), nor did Vps21 nonspecifically associate with Vps39 or protein A (lanes 5–8). It has been found that a large protein complex containing Vps39 and Vps41 associates with the GTP-bound form of Ypt7 (Price et al. 2000). Our data show that purified Vps39 binds Ypt7 in its GDP-bound form, but not in its GTP-bound form, suggesting that a protein other than Vps39 mediates Ypt7-GTP interactions with the Vps39 and Vps41 complex.
Figure 7.
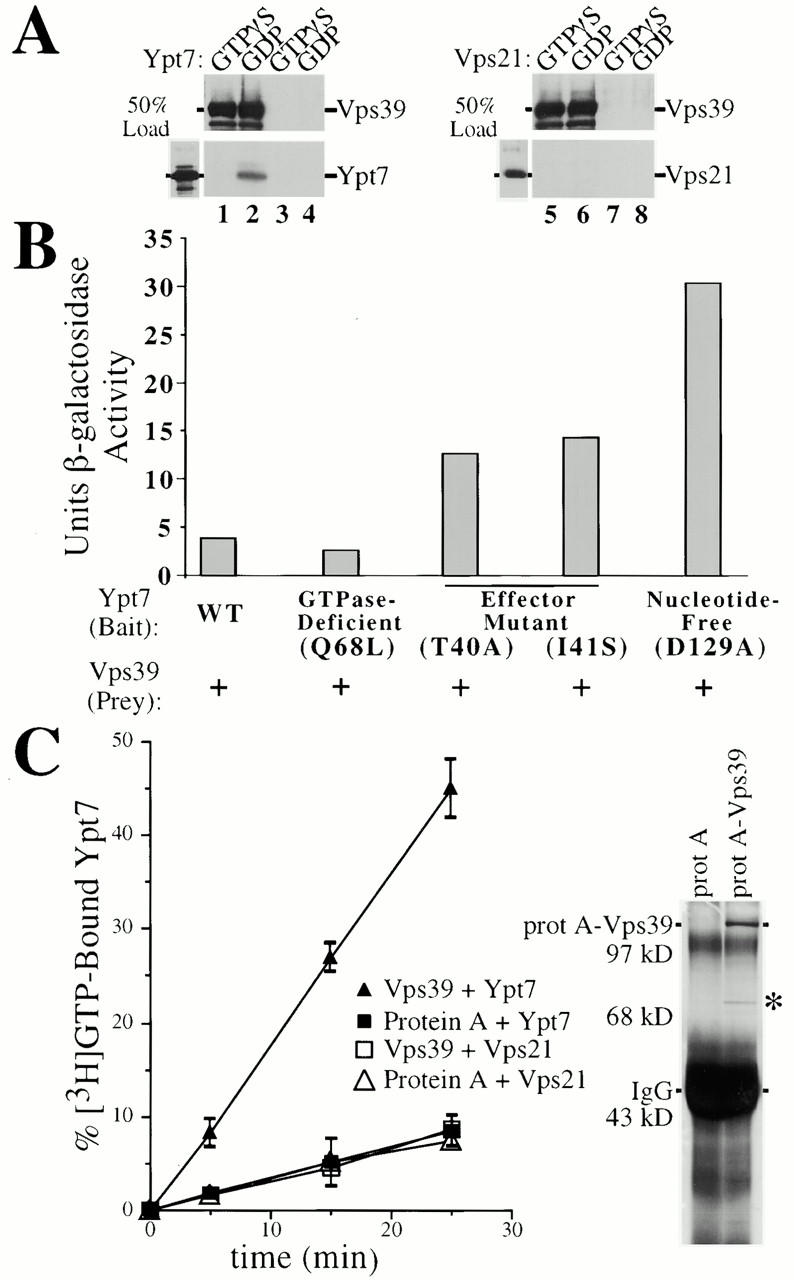
Vps39 is a Ypt7 nucleotide exchange factor. (A) Purified protein A–Vps39 interacts with GDP-bound Ypt7. Protein A or protein A–Vps39 expressed from multicopy plasmids was purified for vps11Δ cells using IgG-sepharose. 50 ng of purified Ypt7 or Vps21 bound to GDP or GTPγS was incubated with IgG-bound protein A (lanes 3, 4, 7, and 8) or protein A–Vps39 (lanes 1, 2, 5, and 6) for 30 min at 25°C, washed multiple times, and the presence of Ypt7 (lanes 1–4) or Vps21 (lanes 5–8) was ascertained by Western blot. (B) The two-hybrid assay revealed preferential interactions between nucleotide-free Ypt7 and Vps39. β-Galactosidase activity was determined in cells cotransformed with prey VPS39 and bait constructs encoding mutants of YPT7 which bias Ypt7 to a specific nucleotide binding state or impair Ypt7–effector interactions. The average β-galactosidase activity of three independent transformants of each strain is presented. (C) 1 μM purified, soluble Ypt7 or Vps21 was prebound to unlabeled GDP, incubated with [3H]GTP (12 μM), and ∼20 ng of purified protein A or protein A–Vps39 for 5-, 15- or 25-min periods. Ypt7 or Vps21 was immobilized on nitrocellulose, washed, and the [3H]GTP exchanged onto Ypt7 or Vps21 was determined. A silver-stained gel of IgG-sepharose–purified protein A and protein A–Vps39 derived from 15 OD600 units of yeast is shown. IgG heavy chain, as well as a protein which comigrates with a degradation product of protein A–Vps39 (asterisk), is indicated.
The nucleotide dependency of the interaction between Ypt7 and Vps39 was also assessed by the two-hybrid assay. Although cells coexpressing bait YPT7 and prey VPS39 vectors yielded ∼4 U of β-galactosidase activity, ypt7Q68L, a stably expressed mutant predicted to shift Ypt7 into the GTP-bound state (Der et al. 1986), exhibited a less potent interaction with Vps39 (∼3 U, Fig. 7 B). ypt7 effector domain mutants, ypt7T40A and ypt7I41S, are predicted to impair the interactions of Ypt7 with effector or GTPase activating proteins (GAPs), but not guaninine-nucleotide exchange factors (GEFs) or guanine-nucleotide dissociation inhibitors (GDIs; Becker et al. 1991). Consistent with this prediction, ypt7T40A and ypt7I41S, although stably expressed, resulted in strong CPY and ALP missorting defects as well as fragmented vacuoles, the morphology typical of class B vps mutants (data not shown). Vps39 bound both Ypt7T40A and Ypt7I41S by the two-hybrid assay (∼13 U), exhibiting stronger interactions than Vps39 and wild-type Ypt7. Perhaps, the effector mutants were unable to bind an endogenous effector/GAP, thus increasing the pool of Ypt7 available for interactions with Vps39. ypt7D129A, which is expected to remain in the nucleotide-free form in vivo due to a severalfold lower affinity for guanine-nucleotides (Weijland et al. 1994), enhanced Ypt7 binding to Vps39 approximately eightfold (31 U, Fig. 7 B). Unfortunately, Ypt7T22N, predicted to be in the GDP-bound form (Feig and Cooper 1988), was approximately sevenfold less stable than wild-type Ypt7. Nonetheless, coexpression of bait ypt7T22N and prey VPS39 yielded ∼5 U of β-galactosidase activity (data not shown), a signal greater than that resulting from wild-type Ypt7 or GTP-bound Ypt7Q68L. Since interactions between Ypt7 and Vps39 (1) are reduced when Ypt7 is in the GTP-bound form; (2) do not require a functional Ypt7 effector region; and (3) are enhanced when Ypt7 is nucleotide-free, Vps39 is likely to function as a Ypt7 exchange factor.
Therefore, we assayed the ability of Vps39 to stimulate nucleotide exchange on Ypt7. Protein A–Vps39 was overproduced in wild-type yeast from a multicopy vector and purified from the S100 fraction using IgG-sepharose. Silver staining shows that Vps39 is highly enriched during affinity purification from yeast and that the C-Vps complex is not present in these Vps39 preparations (Fig. 7 C). Ypt7 and Vps21 were bacterially produced as GST fusions, purified on glutathione-sepharose, and eluted from the beads. Ypt7 and Vps21 were then preloaded with cold GDP. Each exchange reaction contained ∼20 ng of protein A–Vps39 harvested from 15 OD600 units of cells and a relative excess of Ypt7 or Vps21 (1 μg). Protein A–Vps39 immobilized on IgG-sepharose was incubated with GDP-bound Ypt7 (or Vps21) and [3H]GTP for 5-, 15- or 25-min periods. GDP/[3H]GTP exchange was determined by binding Ypt7 to nitrocellulose and washing the filter. Vps39 stimulated 8, 27, and 45% of Ypt7 to undergo GDP for [3H]GTP exchange after 5-, 15- or 25-min reaction periods, respectively, while control IgG-sepharose bound to protein A purified from 15 OD600 units of yeast resulted in significantly less exchange (Fig. 7 C). We determined that, under these conditions, each molecule of Vps39 stimulates nucleotide exchange on approximately four Ypt7 molecules per minute, indicating that the exchange activity we observed is enzymatically driven. Ypt7 preloaded with cold GTP instead of GDP failed to undergo GTP/[3H]GTP exchange when incubated with purified Vps39 (data not shown), consistent with the nucleotide-specific interactions observed between Ypt7 and Vps39 (Fig. 7A and Fig. B). Importantly, Vps39 did not stimulate GDP/[3H]GTP exchange of Vps21, indicating that Vps39 specifically stimulates the nucleotide exchange of Ypt7 (Fig. 7 C). Given the nucleotide dependency of interactions between Vps39 and Ypt7, and the Vps39-dependent stimulation of guanine-nucleotide exchange of Ypt7, but not Vps21, we propose that Vps39 functions as a Ypt7-specific GEF.
Discussion
The C-Vps complex (Vps18, Vps11, Vps16, Vps33) is conserved in mammalian cells, Drosophila, and C. elegans (Sevrioukov et al. 1999). The vacuolar and prevacuolar C-Vps complex regulates the assembly of Vam3 and/or Vam7 SNARE complexes, an event required for the fusion of hydrolase-containing transport intermediates with the vacuole (Darsow et al. 1997; Rieder and Emr 1997; Sato et al. 2000). Here, we find that the C-Vps complex also contains Vps39 and Vps41. Failure to incorporate Vps39 into the C-Vps complex mislocalizes Vps39 from membrane-enriched cell fractions to cytosolic fractions and results in severe vacuolar protein sorting defects, suggesting that Vps39 is essential to C-Vps complex function. A Vps39- and Vps41-containing protein complex has been found to associate with the GTP-bound Ypt7 rab GTPase and function as a downstream effector of Ypt7 (Price et al. 2000). Unexpectedly, our data indicate that this protein complex also functions to stimulate nucleotide exchange on Ypt7. We find that Vps39 preferentially interacts with the nucleotide-free form of Ypt7, and stimulates nucleotide exchange on Ypt7. These observations extend the functional role of the C-Vps complex as a regulator of vacuolar SNARE function and directly couple it to rab GTPase signaling.
Vps39 Stimulates Nucleotide Exchange on Ypt7
VPS39 and YPT7 encode class B VPS genes. Inactivation of the vps39tsf or the ypt7tsf resulted in the intracellular accumulation of precursor CPY and ALP and also blocked API transport. These missorting phenotypes are indicative of prevacuolar compartment (e.g., endosome) to vacuole transport defects in both the CPY and ALP sorting pathways (Darsow et al. 1997; Rieder and Emr 1997; Sato et al. 1998). Furthermore, coexpression of vps39tsf with ypt7tsf, but not other temperature-sensitive vps mutants (i.e., vam3tsf or vam7tsf), revealed that a strong genetic interaction exists between vps39 and ypt7. This suggests that Vps39 and Ypt7 function at a late stage in the vacuole transport pathway common to the CPY, ALP, and API/autophagy sorting pathways. Although the SNAREs Vam7 and Vam3 are likely to carry out the fusion of transport vesicles with the vacuole (Darsow et al. 1997; Sato et al. 1998), the endosomal/vacuolar rab GTPase Ypt7 is believed to mediate initial tethering of transport intermediates to the vacuole (Haas et al. 1995; Ungermann et al. 1998). Therefore, these findings favor the idea that Vps39, like Ypt7, is essential for the initial recognition and docking of CPY, ALP, and API/autophagy transport intermediates with the vacuole.
The observed genetic interaction between vps39 and ypt7 is indicative of a potential physical interaction between Vps39 and Ypt7. Indeed, we found that Ypt7, but not other GTPases (i.e., Vps21, Ras1) bind Vps39 (Fig. 6 A and 7 A). This interaction was reduced when Ypt7 was in its GTP-bound form and did not require a functional Ypt7 effector domain, suggesting that Vps39 does not function as a GAP or effector of Ypt7. In contrast, nucleotide-free Ypt7 exhibited an approximately eightfold increased affinity for Vps39, strongly suggesting that Vps39 functions as a Ypt7 exchange factor. Purified Vps39 protein stimulated GDP/GTP exchange on Ypt7, but not Vps21 in vitro (Fig. 7 C). Since Vps39 was isolated from yeast, it is formally possible that a Vps39 binding protein is carrying out the exchange reaction. However, analysis of the Vps39 preparations indicated that the protein A–Vps39 fusion is the only major band detected (Fig. 7 C). Domain I as well as NH2-terminal regions of Vps39 are likely to be critical to carry out Ypt7 exchange. Deletion of domain I curtailed Ypt7 binding of Vps39. Deletion of the coding region for amino acids 1–369, which corresponds with a segment of human Vps39 (KIAA0770, amino acids 5–141) that is 27% identical and 43% similar to Rom1, a Rho1 exchange factor (Bonifacino, J., personal communication), also disrupted Ypt7 binding, but did not affect interactions between Vps39 and Vps11 (see below). Vps39 is not homologous to any other known exchange factor. Thus, domain I and NH2-terminal sequences of Vps39 define a novel rab-binding motif.
Function of the C-Vps Complex
Ypt7 localizes to and functions on both donor (e.g., endosome) and acceptor (vacuole) compartments, consistent with a proposed symmetrical requirement for rabs in membrane fusion events (Haas et al. 1995; Barbieri et al. 1998). This suggests that a mechanism exists to localize Vps39 to Ypt7-containing membranes. However, the targeting of Vps39 to the membranes does not depend on interactions with Ypt7, as Vps39ΔII bound Ypt7 but failed to associate with the particulate cell fractions. Vps11, Vps18, Vps16, and Vps33 (class C Vps proteins) form a prevacuolar and vacuolar protein complex required, like Vps39, for the fusion of CPY, ALP, and API transport intermediates with the vacuole (Rieder and Emr 1997). Affinity purification of Vps39- and Vps16-associated proteins revealed that Vps39 and Vps41 are additional components of this complex (Fig. 3). This is consistent with observations indicating that Vps39 is part of a 38 S protein complex which contains Vps41 (Nakamura et al. 1997; Price et al. 2000). Association of Vps39 with the C-Vps complex occurs in the membrane-enriched pelletable fractions; the soluble/cytosolic pool of Vps39 does not appear to bind the C-Vps complex. Thus, incorporation of Vps39 into the C-Vps complex may serve to localize and/or activate Vps39 such that it triggers nucleotide exchange on Ypt7 in the context of a fully assembled C-Vps complex. Although assembly of Vps39 into the C-Vps complex could be essential for multiple aspects of C-Vps complex function, this could be the underlying basis for the strong CPY and ALP missorting defects resulting from deletion of domain II (Fig. 2 A).
Recent observations by the Wickner lab have resulted in the identification of the C-Vps complex—referred to as homotypic fusion and vacuole protein sorting (HOPS)—as a downstream effector of GTP-bound Ypt7 (Seals et al. 2000). The activated Ypt7 rab GTPase is believed to tether acceptor (i.e., vacuole) and donor (e.g., endosome) membrane compartments, facilitating v- and t-SNARE pairing and hence, membrane fusion (Ungermann et al. 1998). This suggests that the C-Vps complex not only activates Ypt7 through Vps39, but also may act as a Ypt7 effector through an as yet undefined protein partner(s). Our two-hybrid assay did not reveal individual interactions between GTP-bound Ypt7 and components of the C-Vps complex (i.e., Vps18, Vps11, Vps16, Vps39, and Vps41). Perhaps this result indicates that a fully assembled C-Vps complex is required to form a binding surface for activated Ypt7, ensuring that docking/fusion does not proceed until all components of the C-Vps and SNARE complexes are present.
Additional biochemical and genetic evidence suggests that the C-Vps complex interacts with unpaired Vam3 (vacuolar t-SNARE), possibly by preventing nonproductive binding of Vam3 with the pool of its partner v-SNARE, Vti1, present in the same membrane (vacuole) (Sato et al. 2000). By this mechanism, the C-Vps complex facilitates the formation of productive trans-SNARE interactions between endosomal Vti1 and vacuolar Vam3, allowing for the selective recognition and efficient fusion of vacuolar and prevacuolar compartments. Thus, the C-Vps complex carries out at least three functions essential for membrane fusion: (1) stimulates nucleotide exchange on Ypt7; (2) functions as a downstream effector of GTP-bound Ypt7; and (3) preserves a pool of Vam3 in an unpaired/activated state in order to promote trans-SNARE interactions. The multifunctional role of the C-Vps complex in rab-mediated tethering and SNARE-mediated fusion is depicted in Fig. 8. According to this model, Vps39 associates with the C-Vps complex, localizing Vps39 to Ypt7 on prevacuolar cargo vesicles and the vacuole. This enables Vps39 to convert inactive GDP-Ypt7 to its active GTP-bound state (Fig. 8, step 1). During the initial stages of the fusion reaction, Vam3 is maintained in an unpaired state through interactions with the C-Vps complex. After activation of Ypt7, GTP-bound Ypt7 associates with an effector protein(s) contained within the C-Vps complex (step 2) (Price et al. 2000). Since Ypt7 and the C-Vps complex localize to both prevacuolar vesicles and the vacuole (Haas et al. 1995; Rieder and Emr 1997), this interaction is likely to tether these compartments, bringing Vti1 and Vam3 into close proximity. At step 3, the C-Vps complex releases Vam3, allowing Vti1/Vam3/Vam7 trans-SNARE complexes to form and drive membrane fusion. Thus, the C-Vps complex orchestrates at least three distinct interactions that ensure the high degree of specificity and efficiency required during endosome/vacuole fusion.
Figure 8.
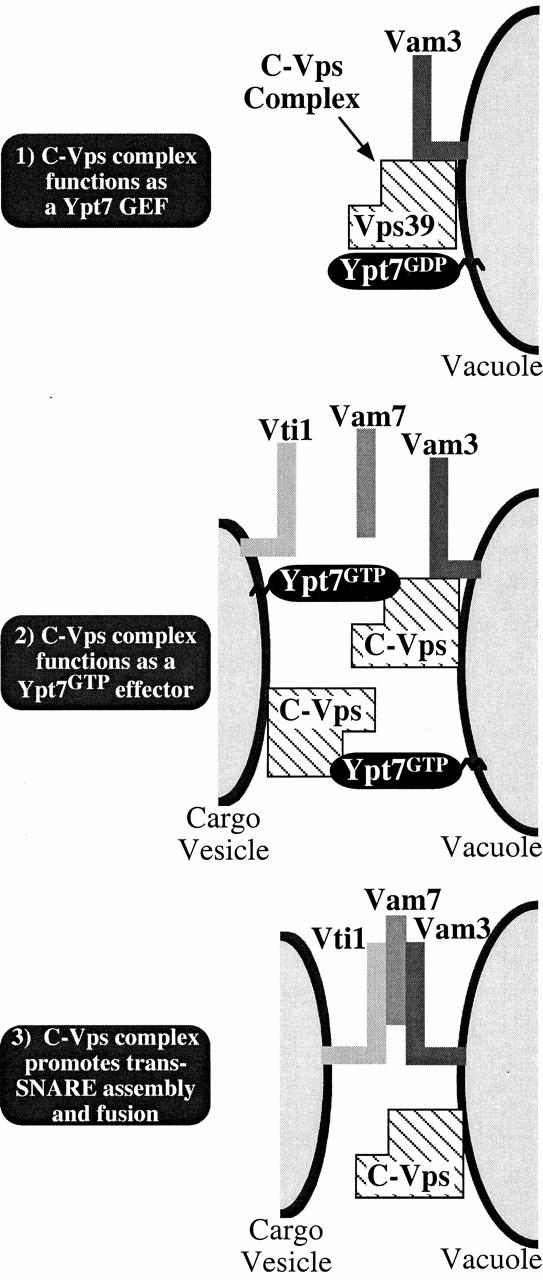
Proposed functional roles of the C-Vps complex (Vps39, Vps41, Vps11, Vps18, Vps16, Vps33) in the fusion of prevacuolar hydrolase-containing cargo vesicles with the vacuole. Our observations support a model in which association of Vps39 with the C-Vps complex localizes Vps39 to Ypt7 on cargo vesicles and the vacuole, enabling Vps39 to convert inactive Ypt7GDP to its active GTP-bound state (step 1). Vam3 (t-SNARE) is maintained in an unpaired state through interactions with the C-Vps complex. During step 2, Ypt7GTP associates with an effector protein(s) contained within the C-Vps complex, tethering these compartments such that Vti1 (v-SNARE) and Vam3 are brought into close proximity. At step 3, the C-Vps complex releases Vam3, allowing trans-SNARE complexes containing Vti1, Vam3, and Vam7 (SNAP25) to form and drive membrane fusion.
The proteins which constitute the C-Vps complex, although not conserved with the components of other protein complexes with recognized roles in vesicular trafficking, functionally overlap with these complexes. Similar to the C-Vps complex, the EEA1 complex, required for the fusion of early and late endosomes in mammalian systems, contains the exchange factor (Rabex5) and effectors (Rabaptin5, EEA1) of rab5 and modulates SNARE activity through NSF and EEA1 (Stenmark et al. 1995: Horiuchi et al. 1997; McBride et al. 1999; Simonsen et al. 1999). Vac1, the yeast homologue of EEA1, integrates signals from GTP-bound Vps21 and the Sec1 homologue, Vps45, in the regulation of SNARE-mediated fusion of Golgi-derived vesicles with the prevacuolar endosome (Burd et al. 1997; Peterson et al. 1999). Furthermore, the Exocyst, a protein complex required for the docking of secretory vesicles with the plasma membrane, functions as a multiprotein effector of the Sec4 rab GTPase (Guo et al. 1999). However, unlike the C-Vps complex, the Sec4 exchange factor (Sec2) is not included in the Exocyst (TerBush et al. 1996; Walch-Solimena et al. 1997). Thus, the C-Vps complex, the EEA1/Vac1 regulatory complexes, and the Exocyst each appear to function by regulating the activity of ubiquitously conserved elements of the membrane trafficking apparatus (rabs and SNAREs). It will be interesting to determine whether this emerging theme applies to other large protein complexes required for membrane fusion such as TRAPP, which plays an essential role in endoplasmic reticulum to Golgi transport (Sacher et al. 1998).
Acknowledgments
We thank William Wickner for helpful discussions; Eric Marcusson and William Snyder for strains and reagents; and Mike Peterson for contributions to protein A purifications.
A.E. Wurmser and T.K. Sato are members of the Biomedical Sciences Graduate Program and supported by the Program Project Grant, National Institutes of Health grant CA58689. S.D. Emr is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: ALP, alkaline phosphatase; API, aminopeptidase I; CHCR, clathrin heavy chain repeat; CPY, carboxypeptidase Y; EEA1, early endosome antigen 1; GAP, GTPase activating protein; GST, glutathione S-transferase; NSF, N-ethylmaleimide-sensitive fusion protein; ORF, open reading fame; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; TRAPP, transport protein particle; Vps, vacuolar protein sorting.
References
- Babst M., Sato T.K., Banta L.M., Emr S.D. Endosomal transport function in yeast requires a novel AAA–type ATPase, Vps4p. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L.M., Vida T.A., Herman P.K., Emr S.D. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol. Cell. Biol. 1990;10:4638–4649. doi: 10.1128/mcb.10.9.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M.A., Hoffenberg S., Roberts R., Mukhopadhyay A., Pomrehn A., Dickey B.F., Stahl P.D. Evidence for a symmetrical requirement for Rab5-GTP in in vitro endosome-endosome fusion. J. Biol. Chem. 1998;273:25850–25855. doi: 10.1074/jbc.273.40.25850. [DOI] [PubMed] [Google Scholar]
- Becker J., Tan T.J., Trepte H.H., Gallwitz D. Mutational analysis of the putative effector domain of the GTP-binding Ypt1 protein in yeast suggests specific regulation by a novel GAP activity. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:785–792. doi: 10.1002/j.1460-2075.1991.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G., Peterson M., Cowles C.R., Emr S.D. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles C.R., Snyder W.B., Burd C.G., Emr S.D. Novel Golgi to vacuole delivery pathway in yeastidentification of a sorting determinant and required transport component. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T., Rieder S.E., Emr S.D. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C.J., Finkel T., Cooper G.M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986;44:167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Feig L.A., Cooper G.M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol. Cell. Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Scheglmann D., Lazar T., Gallwitz D., Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky B.F., Busch G.R., Emr S.D. VPS21 encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Lippe R., McBride H.M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Klionsky D.J., Herman P.K., Emr S.D. The fungal vacuolecomposition, function, and biogenesis. Microbiol. Rev. 1990;54:266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Cueva R., Yaver D.S. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J. Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Hirata A., Ohsumi Y., Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae . J. Biol. Chem. 1997;272:11344–11349. doi: 10.1074/jbc.272.17.11344. [DOI] [PubMed] [Google Scholar]
- Peterson M.R., Burd C.G., Emr S.D. Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol. 1999;9:159–162. doi: 10.1016/s0960-9822(99)80071-2. [DOI] [PubMed] [Google Scholar]
- Price A., Seals D., Wickner W., Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C.K., Howald-Stevenson I., Vater C.A., Stevens T.H. Morphological classification of the yeast vacuolar protein sorting mutantsevidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P., Darsow T., Katzmann D.J., Emr S.D. Formation of AP-3 transport intermediates requires Vps41 function. Nat. Cell Biol. 1999;1:346–353. doi: 10.1038/14037. [DOI] [PubMed] [Google Scholar]
- Rieder S.E., Emr S.D. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.S., Klionsky D.J., Banta L.M., Emr S.D. Protein sorting in Saccharomyces cerevisiaeisolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.S., Graham T.R., Emr S.D. A putative zinc finger protein, Saccharomyces cerevisiae Vps18p, affects late Golgi functions required for vacuolar protein sorting and efficient alpha-factor prohormone maturation. Mol. Cell. Biol. 1991;11:5813–5824. doi: 10.1128/mcb.11.12.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Jiang Y., Barrowman J., Scarpa A., Burston J., Zhang L., Schieltz D., Yates J.R., III, Abeliovich H., Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.K., Darsow T., Emr S.D. Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol. Cell. Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.K., Rehling P., Peterson M.R., Emr S.D. Class C Vps protein complex regulates SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 2000;6:661–667. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen G., Margolis N., Wickner W.T., Price A. A Ypt/Rab effector complex containing the sec1 homolog vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukov E.A., He J.P., Moghrabi N., Sunio A., Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila . Mol. Cell. 1999;4:479–486. doi: 10.1016/s1097-2765(00)80199-9. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Gaullier J.M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B., Stenmark H., Dusterhoft A., Philippsen P., Yoo J.S., Gallwitz D., Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato K., Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Vida T.A., Huyer G., Emr S.D. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J. Cell Biol. 1993;121:1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. II. VAM7, a gene for regulating morphogenic assembly of the vacuoles. J. Biol. Chem. 1992;267:18671–18675. [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Wada Y., Nakamura N., Ohsumi Y., Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae . J. Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R.N., Novick P.J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijland A., Parlato G., Parmeggiani A. Elongation factor Tu D138N, a mutant with modified substrate specificity, as a tool to study energy consumption in protein biosynthesis. Biochemistry. 1994;33:10711–10717. doi: 10.1021/bi00201a019. [DOI] [PubMed] [Google Scholar]
- Wichmann H., Hengst L., Gallwitz D. Endocytosis in yeastevidence for the involvement of a small GTP-binding protein (Ypt7p) Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Emr S.D. Phosphoinositide signaling and turnoverPtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe J.A., Brodsky F.M., Hofmann K., Lin K., Liu S.H., Chen L., Earnest T.N., Fletterick R.J., Hwang P.K. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature. 1999;399:371–375. doi: 10.1038/20708. [DOI] [PubMed] [Google Scholar]