Abstract
Many receptors for endocytosis recycle into and out of cells through early endosomes. We now find in dendritic cells that the DEC-205 multilectin receptor targets late endosomes or lysosomes rich in major histocompatibility complex class II (MHC II) products, whereas the homologous macrophage mannose receptor (MMR), as expected, is found in more peripheral endosomes. To analyze this finding, the cytosolic tails of DEC-205 and MMR were fused to the external domain of the CD16 Fcγ receptor and studied in stable L cell transfectants. The two cytosolic domains each mediated rapid uptake of human immunoglobulin (Ig)G followed by recycling of intact CD16 to the cell surface. However, the DEC-205 tail recycled the CD16 through MHC II–positive late endosomal/lysosomal vacuoles and also mediated a 100-fold increase in antigen presentation. The mechanism of late endosomal targeting, which occurred in the absence of human IgG, involved two functional regions: a membrane-proximal region with a coated pit sequence for uptake, and a distal region with an EDE triad for the unusual deeper targeting. Therefore, the DEC-205 cytosolic domain mediates a new pathway of receptor-mediated endocytosis that entails efficient recycling through late endosomes and a greatly enhanced efficiency of antigen presentation to CD4+ T cells.
Keywords: dendritic cell, antigen presentation, DEC-205, MHC class II, endocytosis
Introduction
Several cell surface receptors deliver ligands to the endocytic system for purposes of extensive intracellular digestion within lysosomes. Many of these receptors function exclusively in endocytosis, such as the prototype low-density lipoprotein receptor (LDLR) (Brown et al. 1983) and asialoglycoprotein receptor (Ciechanover et al. 1983). These localize to coated pits, dramatically increasing uptake via coated vesicles. After ligand release in early endosomes, the receptors recycle rapidly to the cell surface. By avoiding proteolysis during this recycling, such receptors are ideal for nutrient delivery and scavenging, e.g., cholesterol, iron, and altered glycoproteins. Other receptors, e.g., those for immune complexes (Amigorena et al. 1992a) and certain growth factors (Wilde et al. 1999), have signaling functions in addition to mediating adsorptive uptake of their ligands. The receptors typically are catabolized in lysosomes, rather than recycled, after uptake of immune complexes and growth factors (Mellman et al. 1983). This receptor downregulation can dampen signal transduction. The ligands for both recycling and nonrecycling families of endocytic receptors are digested in lysosomes down to the level of amino acids. Digestion must be complete, since amino acids and monosaccharides are then able to diffuse out of the lysosomal system. Incomplete digestion, as occurs with lysosomal enzyme deficiency, leads to vacuolar swelling (Cohn and Ehrenreich 1969; Ehrenreich and Cohn 1969).
Adsorptive endocytosis receptors, like the macrophage mannose receptor (MMR), FcR, and B cell antigen receptor (BCR), are used in the immune system to facilitate antigen capture and presentation of peptides to T cells (Bonnerot et al. 1992; Sallusto et al. 1995; Engering et al. 1997). Previously we have identified an endocytic receptor, termed DEC-205, expressed by dendritic cells (DCs). This 205-kD protein contains 10 external, contiguous, C-type lectin domains and by sequence analysis, is a homologue of the MMR. In fact, both the MMR and DEC-205 mediate adsorptive uptake, and both have cytosolic domains with requisite coated pit localization sequences (Stahl et al. 1980; Jiang et al. 1995). Therefore, we expected that both MMR and DEC-205 would recycle through early endosomal compartments and present bound antigens comparably. However, we will show that DEC-205 unexpectedly targets to late endosomes or lysosomes in developing DCs, and that DEC-205 is far superior to the MMR in presenting bound rabbit antireceptor antibodies to T cells, a classical assay for measuring the presenting function of endocytosis receptors (Chesnut and Grey 1981).
To dissect the role of the cytosolic domain in these findings, we studied a totally heterologous system: L cells stably transfected with a fusion receptor formed between the external domains of human CD16 and different cytosolic tails. We find that the DEC-205 cytosolic domain has a distinct distal region with an acidic EDE triad. The distal region and its acidic sequence are required for recycling beyond early endosomes through deeper major histocompatibility complex class II–positive (MHC II+), late endosomes and lysosomes. Such distal targeting is unique for adsorptive endocytosis receptors that have been analyzed to date, and proves to be necessary for presentation of antigenic peptides at low doses of ligand. This new pathway for receptor-mediated uptake is therefore a hybrid of the two known endocytic pathways discussed above, targeting deeper digestive compartments but recycling efficiently to produce biologically active peptides.
Materials and Methods
Cells
The MHC II (I-Ek)–expressing cell line DCEK.ICAM.Hi7 was maintained in RPMI with 7.5% Ig-depleted FCS (Atlanta Biologicals), 10 IU/ml penicillin/streptomycin, 200 μM/ml glutamine (GIBCO BRL), and 50 μM 2-mercaptoethanol (Sigma-Aldrich) (R7 medium). To maintain MHC II and intracellular adhesion molecule (ICAM)-1 expression, every other week the cells were kept in MXH medium (R7 containing 6 μg/ml mycophenolic acid, 250 μg/ml xanthine, 15 μg/ml hypoxanthine [all from Sigma-Aldrich], and 800 μg/ml G418 [GIBCO BRL]). CD16 chimeric receptor transfectants were kept in MXH medium additionally supplemented with 5 μg/ml puromycin (Calbiochem). DCs were generated from mouse bone marrow cultured in the presence of GM-CSF as described previously (Inaba et al. 1992). Day 6 DCs are primarily at an immature, antigen-capturing stage of development (Pierre et al. 1997).
Cloning and Transfection
The cDNAs coding for the intracellular domains of DEC-205 and MMR were cloned separately from the extracellular expressed domains into a TA vector (Invitrogen), and oligonucleotide directed mutagenesis using QuikChange® (Stratagene) was performed. STOP codons and a tyrosine to alanine exchange were introduced into different parts of the DEC tail (see Fig. 1 A). All mutations were verified by sequencing (DNA Technology Center, The Rockefeller University). Using standard methods, the mutated DEC tails were cloned into the BamH1-EcoR1 site of the pApuro vector (gift of Dr. Kurosaki, American Cynamid Company, Lederle Laboratories, Department of Cardiovascular Molecular Biology, Pearl River, NY) behind the sequence coding for the extracellular domain of the human FcγIII receptor (Kolanus et al. 1993). After verifying the correct insertion by sequencing, these constructs were transfected into the MHC II+ (I-Ek) fibroblast cell line DCEK.ICAM.Hi7 by calcium phosphate precipitation (Stratagene). Thereafter, cells were split into 100-mm dishes and for selection, puromycin (Calbiochem) was applied at a final concentration of 5 μg/ml. Colonies growing under selection after 2–3 wk were picked and expanded. Surface expression of CD16 was regularly monitored by FACS®, using anti-CD16 antibody (clone 3G8).
Figure 1.
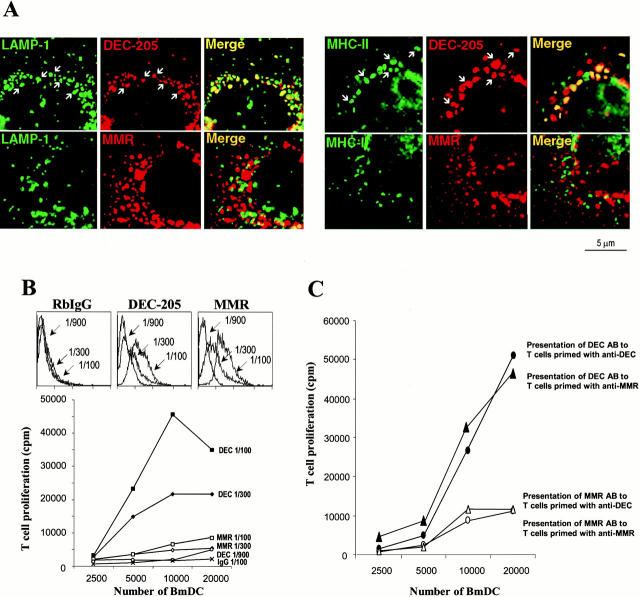
Distinct intracellular localization and function of DEC-205 and MMR in DCs. (A) Localization. Immature day 6 bone marrow DCs (BmDC) (Inaba et al. 1992) were seeded on coverslips, fixed, and double stained with rabbit anti–DEC-205 and MMR (red), and the late endosomal/lysosomal components, LAMP-1 or MHC II (green). Examples of individual granules that contain red and green label are marked by arrows, and colocalization is further shown in yellow in the merged images. (B) Surface binding and presentation of anti–DEC-205 and MMR antibodies. Day 6, immature marrow-derived DCs were incubated with different dilutions (as indicated) of anti–DEC-205, anti-MMR, or preimmune rabbit serum on ice for 1 h. Unbound antibody was washed away. The cells were incubated with FITC-labeled anti-Ig, to assess amounts of surface bound antibody by FACS® (top), or cocultured in graded doses with rabbit IgG–primed T cells, to measure presentation of peptides from rabbit antibodies (bottom). (C) DCs were incubated with 1:100 dilution of anti–DEC-205 or anti-MMR rabbit serum on ice for 1 h. Unbound antibody was washed away, and presentation of peptides from rabbit antibodies was assayed by coculturing the DCs with graded doses of T cells primed with anti–DEC-205 or anti-MMR antiserum, respectively.
Flow Cytometric Analysis of Surface Markers
Cells were harvested with trypsin (Boehringer), then incubated with cold PBS/7% normal goat serum (vol/vol) followed by incubation for 45 min on ice with the following hybridoma supernatants: anti–MHC II (14-4-4S; American Type Culture Collection), anti–ICAM-1 (YN-1; American Type Culture Collection), and anti-CD16 (3G8). B7 expression was assayed with anti–B7-1 antibody (1G10; BD PharMingen). After washing with cold PBS/1% FCS (vol/vol), FITC-labeled mouse anti–rat Ig or goat anti–mouse Ig (Jackson ImmunoResearch Laboratories) secondary reagents at a final dilution of 1 μg/ml in PBS/1% FCS (vol/vol) were added. After 45 min on ice, cells were washed and resuspended in PBS containing 1 μM propidium iodide (Sigma-Aldrich) to gate out dead cells during analysis for fluorescence using a FACScan™ (Becton Dickinson).
Surface Binding and Internalization of Human IgG
Human IgG (HuIgG; Jackson ImmunoResearch Laboratories) was diluted to 1 mg/ml in PBS. Aggregates were formed by incubation at 65°C for 30 min. Thereafter, aliquots were stored at 4°C until use, but no longer than 1 wk. In some antigen-presenting assays, HuIgG was aggregated by incubation with goat anti-HuIgG antibodies for 4 h at room temperature. To assay surface binding of HuIgG, cells were harvested and washed with cold PBS/1% FCS (vol/vol) and incubated for 45 min on ice with monomeric or aggregated HuIgG (aggHuIgG) at 0.1–100 μg/ml. After washes with cold PBS/1% FCS (vol/vol), FITC-labeled goat anti–human IgG antibodies (Jackson ImmunoResearch Laboratories) at a final concentration of 1 μg/ml in PBS/1% FCS (vol/vol) were added. After 45 min on ice, cells were washed, resuspended in PBS, and analyzed on a FACScan™. To measure internalized HuIgG, the DCEK.ICAM.Hi7 cells were seeded into 96-well plates (20,000 cells/well), cultured overnight, and transferred on ice followed by addition of HuIgG at a final concentration of 50 μg/ml. After 45 min, unbound HuIgG was removed by washing with medium, and aliquots of cells were harvested and fixed with 4% paraformaldehyde (PFA). The remaining cells were further incubated in R7 at 37°C after which the cells were harvested, fixed, and stained with FITC-labeled goat anti–human IgG antibodies as described above. To detect internalized HuIgG, cells were permeabilized before adding FITC-labeled goat anti–human antibodies, using a 10-min incubation with PBS/0.1% (vol/wt) saponin (Sigma-Aldrich). In time course experiments, the amount of internalized HuIgG was calculated by subtracting the mean fluorescence in fixed cells (surface-bound HuIgG) from that recorded with fixed and permeabilized cells (internalized and surface-bound HuIgG).
Receptor Recycling
Cells were cultured for 2 h in 10 μg/ml cycloheximide (CHX; Calbiochem), sufficient to block protein biosynthesis as assessed by [35S]methionine labeling (not shown). Surface CD16 then was saturated by incubating the cells in presence of CXH with aggHuIgG (100 μg/ml) at 37°C for 10 min, followed by chilling the cells for 1 h on ice. Thereafter, cells were cultivated in R7 supplemented with CXH, and at different times, recycled CD16 receptors were detected by incubation with 125I-HuIgG at 10 μg/ml on ice. Bound HuIgG was measured by γ counting (Wallac).
Confocal Immunofluorescence Microscopy of L Cells and DCs
L cells were seeded into LabTek tissue culture chambers (Nunc) and incubated overnight. Cells were washed twice with warm RPMI medium, fixed with 4% paraformaldehyde/PBS (wt/vol) for 20 min at room temperature, and permeabilized by incubation with permeabilization buffer (RPMI containing 10% normal goat serum [GIBCO BRL], 0.05% saponin [Sigma-Aldrich], 10 mM glycine [Sigma-Aldrich]) for 15 min at room temperature. Alternatively, DCs were grown from bone marrow precursors using GM-CSF as described (Inaba et al. 1992). At day 6, when the cultures contain numerous aggregates of immature DCs with abundant MHC II compartments (MIICs) (Pierre et al. 1997), the cells were dislodged and applied to alcian blue–coated, glass slides for fixation as above. After two washes in permeabilization buffer, cells were incubated for 45 min at room temperature with the following antibodies: anti–MHC II (M5/114; American Type Culture Collection), anti–lysosome-associated membrane protein 1 (LAMP-1) (clone ID-4B; gift of Dr. Ira Mellman, Yale University School of Medicine, New Haven, CT), anti-transferrin receptor (TfR) (C2F2; American Type Culture Collection), anti-CD16 (clone 3G8), and either monoclonal or polyclonal antibody to DEC-205 (Swiggard et al. 1995) and MMR (prepared in rabbits by immunization with cloned MMR external domains). In double labeling experiments, FITC-conjugated MHC II and LAMP-1 antibodies (BD PharMingen) were used. For unconjugated antibodies, we stained cells for 45 min in permeabilization buffer with appropriate FITC- or Texas red–labeled secondary reagents, absorbed against mouse or rat proteins and applied at a final concentration of 1 μg/ml. For detection of endocytosed HuIgG, FITC-labeled goat anti-HuIgG was applied at 1 μg/ml in permeabilization buffer. Slides were mounted in aquamount (Polysciences) and examined by confocal laser scan microscopy (ZEISS). ZEISS software was used to take and to overlay pictures. Composite figures were made using Photoshop® (Adobe Systems).
Antigen Presentation to T Cells
To obtain HuIgG- or RbIgG-specific T cells, 6–8-wk-old B10.BR mice (The Jackson Laboratory) were primed to HuIgG or RbIgG by subcutaneous injection of 50 μg HuIgG or RbIgG emulsified in complete Freund's adjuvant (Difco). In some experiments, mice were primed to RbIgG using anti–DEC-205 Rb antiserum or anti-MMR Rb antiserum. 8 d later, draining lymph nodes were removed and single cell suspensions were prepared by teasing with forceps and forcing the nodes through a nylon mesh. T cells were purified by passage over nylon wool columns and incubating the eluted cells with antibodies directed against MHC II (M5/114; American Type Culture Collection) and CD45 (B220; American Type Culture Collection) on ice for 30 min. In some experiments, anti-CD8 antibodies (TIB 211; American Type Culture Collection) were added to obtain purified CD4+ T cells. Cells were washed and incubated with goat anti–rat Dynabeads (Dynal) at a ratio of 4 beads to 1 target cell for an additional 30 min at 4°C to remove non-T cells. For antigen presentation assays with DCEK.ICAM.Hi7 cells, transfected and untransfected DCEK.ICAM.Hi7 cells were irradiated with 5,000 rads and seeded into 96-well plates (15,000 cells/well). HuIgG was added to the cells in graded doses. After overnight culture, unbound HuIgG was removed by washing the plates with warm R7 medium. Thereafter, 250,000 T cells/well in 200 μl R7 were added in triplicate, and the plates were incubated for 3–4 d. For antigen presentation assays with DCs, developing bone marrow DCs were harvested after 6 d of culture in GM-CSF, placed on ice, and incubated with graded doses of rabbit anti–DEC-205, anti-MMR, and preimmune serum for 1 h. Unbound antibodies were washed away and aliquots of the DCs were seeded in triplicates into 96-well plates. Then 200,000 T cells/well were added and cultured for 3–4 d. To assay T cell proliferation, [3H]thymidine (1 μCi/well; Amersham Pharmacia Biotech) was added for the last 12 h before harvesting. Incorporation of radioactivity was determined by scintillation counting. Data are shown are means of triplicates where the standard deviation was <10% of the mean cpm.
Results
Unique Localization and Function of DEC-205 in DCs
DEC-205 belongs to the group VI family of lectins (Drickamer and Taylor 1993) that contain 8–10 extracellular, contiguous C-type lectin domains and include the MMR. Both DEC-205 and MMR can be expressed on DCs (Jiang et al. 1995; Engering et al. 1997). Rabbit polyclonal antibodies to mouse DEC-205 (Swiggard et al. 1995) and the mouse MMR (our unpublished data) were used to localize these receptors in developing mouse DCs, generated from bone marrow progenitors with GM-CSF (Inaba et al. 1992). By day 6, the cultures were predominantly immature DCs with endocytic activity and abundant, intracellular, late endosomes or lysosomes (Pierre et al. 1997). These compartments were rich in MHC II products and are called MIICs.
By confocal laser scan microscopy, both the MMR and DEC-205 were abundant in intracellular granules (Fig. 1 A, red). As expected for a recycling endocytic receptor, very little of the MMR was found in late endosomes or lysosomes that were labeled for LAMP-1 or for MHC II (Fig. 1 A, green and merge images). In marked contrast, the bulk of the intracellular DEC-205 was localized in perinuclear MIICs, as demonstrated by colocalization with LAMP-1 and with MHC II (Fig. 1 A).
To detect a functional consequence for the distinct targeting of the MMR and DEC-205, we used the rabbit polyclonal antibodies as surrogate antigens for T cells primed to rabbit Ig, as Chesnut and Grey 1981 first did to show presentation via the BCR. When antibodies were added to immature DCs in the cold, the cells bound comparable amounts of anti-MMR and DEC-205 Ig (Fig. 1 B, top). When the same cells were added to cultures of primed T cells, the anti–DEC-205 was presented with much higher efficiency (Fig. 1 B, bottom). To rule out that the quantitative difference is due to different allotypic determinants within the MMR and DEC-205 antibodies, additional experiments were performed using T cells from mice primed to RbIgG with either anti–DEC-205 or anti-MMR antiserum (Fig. 1 C). Here again the anti–DEC-205 was presented with much higher efficiency regardless of whether the T cells were derived from animals primed with either DEC-205 or MMR antibodies. Thus, DEC-205 is normally found in MIICs, whereas MMR is predominantly found in early endosomes; when rabbit antibodies are bound to these two receptors, DEC-205 is much more efficient at presenting peptides to rabbit Ig–primed, CD4+ T cells.
Expression of Chimeric DEC-205/CD16 Receptors in Transfected DCEK.ICAM.Hi7 Cells
The targeting of endocytic receptors is determined by amino acid sequences within their intracellular domains (for review see Bonifacino and Dell'Angelica 1999). A reexamination of the cytosolic domains (“tails”) of three homologous lectins—the MMR, DEC-205, and the phospholipase A2 receptor (PLA2R)—showed the tails of the MMR and PLA2R to be very similar to each other but different from DEC-205 (gray shading in Fig. 2 A). All three tails contained a membrane-proximal, putative coated pit localization sequence (Fig. 2 A, underlined) for uptake (Collawn et al. 1990), but the distal region of DEC-205 was distinct and included a sequence of three acidic amino acids (Fig. 2 A, EDE). Interestingly, acidic sequences in other receptors are implicated in intracellular sorting (Matter et al. 1993; Voorhees et al. 1995; Wan et al. 1998; Piguet et al. 1999; Simmen et al. 1999).
Figure 2.
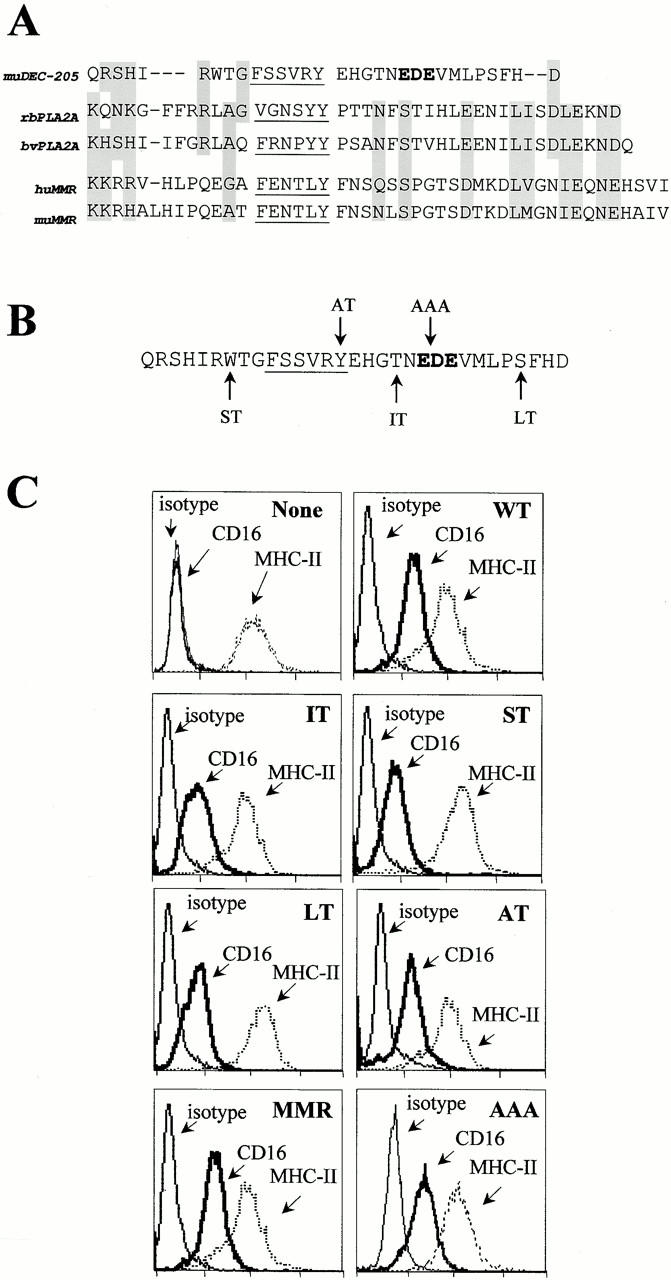
Cytosolic domains used to study the targeting of endocytosis receptors. (A) Sequences of the cytoplasmic domain of three multilectin receptors for adsorptive endocytosis: DEC-205, PLA2R, and MMR. Underlining indicates a consensus sequence for endocytosis via coated pits; boldface indicates a cluster of acidic residues (EDE), postulated to guide DEC-205 beyond early endosomes; gray shading shows similar amino acids in the tails. (B) Mutations were introduced into the DEC tail (arrows), leading to truncations at the sites denoted (ST, short tail; IT, intermediate tail; LT, long tail), or to amino acid substitutions: Y to A in alternate tail (AT), and EDE to AAA in the AAA tail. (C) Surface expression of CD16 and MHC II in untransfected DCEK.ICAM.Hi7 cells (none) or cells transfected with CD16 fusion receptors (WT, LT, IT, ST, AT, AAA, and MMR). Staining was with anti-CD16 (clone 3G8), anti–I-Ek (clone HB32), or isotype control anti–I-Ak (clone 10.216), followed by FITC goat anti–mouse F(ab′)2 antibodies.
To determine which components of the cytoplasmic tail were required for efficient targeting and presentation via late endosomes or lysosomes (Fig. 1), we constructed chimeric receptors containing the external IgG binding domain of the HuFcγIII receptor or CD16 and different DEC-205 cytosolic tails (Fig. 2 B). In addition to the wild-type (WT) DEC-205 tail, we made truncations to remove the terminal three amino acids (long tail, LT), residues 19–31 with the putative EDE distal targeting sequence (intermediate tail, IT), and residues 6–31 with the coated pit sequence (short tail, ST). We also made mutants of the DEC-205 tail, converting tyrosine to alanine in the coated pit sequence (altered tail or AT), and EDE to alanines in the putative distal targeting sequence. We used the wild-type MMR tail for comparison.
The CD16 chimeras were transfected into a murine fibroblast-like line, DCEK.ICAM.Hi7, which expresses MHC II as well as the T cell costimulatory molecules B7-1 (CD80) and ICAM-1 (CD54) (Dubey et al. 1995). Comparable surface expression of each CD16 chimeric receptor was obtained (Fig. 2 C) in the stable transfectants, without altering expression of MHC II (Fig. 2 C) or B7-1 (not shown).
Binding, Uptake, and Recycling of HuIgG by CD16 Chimeric Receptors
To test the function of the chimeric receptors, we first measured binding of HuIgG as illustrated for WT-DEC:CD16 (Fig. 3 A). The transfectants all bound ligand. Saturation occurred at 10 μg/ml, whereas the untransfected DCEK.ICAM.Hi7 cell line (Fig. 3 A, none) did not bind HuIgG. Binding required that the human IgG be heat aggregated, as expected for functional FcγRIII (Unkeless et al. 1981). On PAGE, <10% of the HuIgG aggregated (molecular mass > 200,000 D) upon heating to 65°C for 30 min (Fig. 3 B), so that saturable binding of the expressed chimeric receptors was occurring at <1 μg/ml of aggHuIgG. When the sensitivity of bound ligand to pH was measured, aggHuIgG began to elute at pH 5, and only 40% remained at pH 4 (Fig. 3 C).
Figure 3.
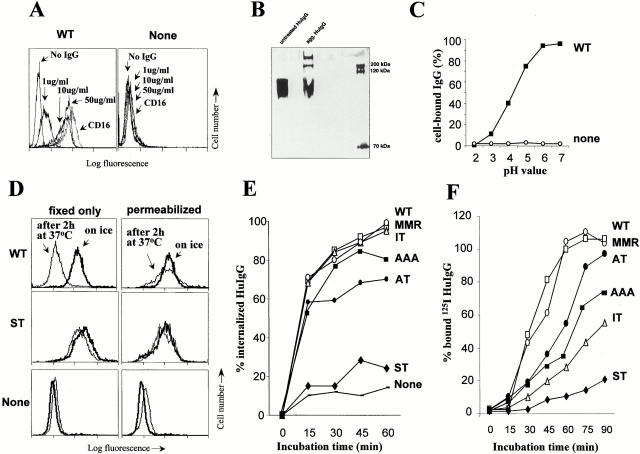
Ligand binding, uptake, and recycling via CD16 DEC-205 chimeric receptors. (A) Binding of aggHuIgG to transfected cells. WT-DEC/CD16 transfected cells (WT) and untransfected cells (None) were incubated on ice for 1 h with graded doses of aggHuIgG. After washing, surface-bound IgG was detected with FITC goat anti-HuIgG F(ab′)2 and FACS®. Cells were also stained for surface CD16 using 3G8 and FITC goat anti–mouse F(ab′)2. Binding to WT cells and all other transfectants (see Fig. 1) was similar. (B) To detect the small amount of aggHuIgG in the heated HuIgG preparations, samples were analyzed under reducing conditions by SDS-PAGE analysis. (C) Elution of HuIgG to CD16 at acidic pH. Elution of aggHuIgG to CD16 was performed at different pH's, predicting a release of ligand in mildly acidic compartments. (D) Endocytosis of aggHuIgG. Untransfected cells (none) and transfectants with different DEC:CD16 chimeric receptors (WT, ST) were incubated on ice for 1 h with aggHuIgG (10 μg/ml). Aliquots were fixed and stained for surface-bound HuIgG immediately (bold line) or incubated at 37°C for 4 h (thin line) before fixation and staining with FITC-labeled anti-HuIgG (first column, fixed only). Cells were also permeabilized before staining with FITC-labeled anti-HuIgG (second column) to reveal endocytosed HuIgG. (E) Uptake of HuIgG. Cell lines were incubated with aggHuIgG on ice, transferred to 37°C for different times, and stained with FITC-labeled anti-HuIgG F(ab′)2 without or with prior permeabilization. The mean fluorescence was determined by FACS® analysis, and the amount of internalized IgG was plotted as a percentage of total surface-bound IgG. (F) Recycling of CD16 chimeric receptors. Cells were treated for 2 h with CHX and then incubated with 100 μg/ml aggHuIgG, and transferred on ice, to saturate binding of all CD16 chimeric receptors (see Fig. 2 A). After washing, the cells were incubated at 37°C in the continued presence of CHX for different times, and recycling CD16 chimeric receptors were detected by binding of 125I-HuIgG. The amount of recycled receptors is plotted as a percentage of the amount of surface-bound 125I-HuIgG obtained with untreated cells.
To monitor endocytosis, aggHuIgG was bound to each transfectant on ice, and aliquots were transferred to 37°C for 2 h. The FACS® was used to follow uptake at the single cell level. Binding of FITC anti-HuIgG to fixed cells detected residual surface HuIgG, and to fixed permeabilized cells, detected surface and intracellular HuIgG. With WT-DEC:CD16–expressing cells, HuIgG was no longer detectable at the surface after the 2-h chase at 37°C (Fig. 3 D), but strong staining was obtained in permeabilized cells, indicating that the DEC tail mediated uptake of ligands bound to a heterologous receptor.
Similar results, i.e., endocytosis of bound HuIgG, were obtained with cells expressing the LT-, IT-, and AT-DEC:CD16 chimera (not shown). In contrast, cells transfected with the short six–amino acid tail (ST-DEC:CD16), lacking the putative coated pit localization sequence, showed no significant loss of surface IgG (Fig. 3 D), indicating that deletion of amino acid residues 6–31 prevents endocytosis of the DEC:CD16 chimeric receptor. In a more detailed time course study (Fig. 3 E), we included cells expressing AAA-DEC:CD16 and MMR:CD16. Again, the ST-DEC:CD16 chimera did not internalize, whereas half the surface Ig entered the cells via the other CD16 chimeras within 15 min. The AT-DEC:CD16 chimera, which contained a mutation of the tyrosine residue in the coated pit localization site, was capable of endocytosis although at a somewhat slower rate (Fig. 3 E). Therefore, all the chimeric CD16 receptors bound aggHuIgG, and all except the ST-DEC:CD16 mediated rapid adsorptive uptake of ligand.
To examine recycling of the DEC:CD16 chimeric receptors, we blocked protein synthesis using CHX at 10 μg/ml for 2 h. We then added a saturating dose of aggHuIgG, placed the cells on ice, washed, and transferred the L cells to 37°C. This procedure was sufficient to saturate all of the CD16 chimeric receptors (data not shown). Thereafter, at subsequent time points, we added 125I-labeled aggHuIgG to detect a reappearance of functional CD16 receptors. The intact cytosolic tails (WT, MMR) recycled within 1 h after internalization, but the truncated intermediate DEC tail (IT) and the AAA-DEC:CD16 chimera recycled less rapidly (Fig. 3 F). The 1-h recycling time could underestimate the speed of recycling via the DEC-205 tail, because the pH of the vacuolar system in L cells may be insufficiently low to quickly elute all of the bound HuIgG ligand. As expected, the ST tail did not recycle, i.e., regenerate functional CD16, because endocytosis was not occurring. To rule out replenishment of receptors from endocytic pools, rather than recycling, we repeated the experiments in cells exposed for 1 h at 37°C to aggHuIgG to occupy intracellular stores. Again, recycling of new HuIgG binding receptors took place (data not shown). We conclude that the DEC-205 and MMR tails are each capable of mediating ligand uptake, discharge, and recycling to the cell surface, and that these activities can be carried out by all of the mutant cytosolic tails we had prepared, except for the short tail lacking the coated pit localization and uptake sequence.
Intracellular Compartments Targeted by CD16 Chimeric Receptors
At this stage of the studies, the DEC-205 and MMR tails seemed similar. Distinctive features of the DEC-205 tail became apparent upon examining the intracellular targeting of CD16 chimeric receptors and HuIgG ligand, and in tests of antigen presentation to HuIgG-primed T cells. First, we did confocal immunofluorescence microscopy to simultaneously identify MHC II and LAMP-1 or the early endosomal marker TfR. In all transfectants (WT shown here), MHC II largely colocalized with LAMP-1, a marker for late endosomes/lysosomes in the perinuclear region (Fig. 4, top), and not with TfR in the periphery (Fig. 4, bottom).
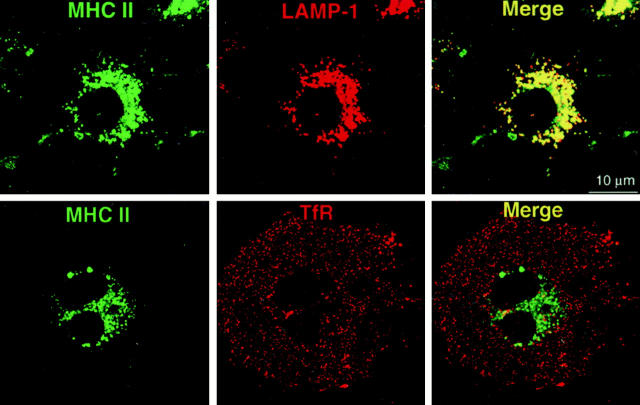
Figure 4.
Presence of MHC II+ lysosomal compartments in CD16 transfectants (WT-DEC:CD16 shown here). Stably transfected DCEK.ICAM.Hi7 cells on tissue culture chamber slides were fixed and double labeled for MHC II and lysosomal (LAMP-1) proteins (top) or TfR (bottom). Primary antibodies were visualized by FITC- and Texas red–labeled secondary antibodies. By confocal laser scan microscopy, MHC II colocalizes with LAMP-1 in all cells (yellow) but not with the TfR.
In either the absence or presence of IgG ligand, WT-DEC:CD16 chimeric receptors efficiently localized to late endosomal/lysosomal compartments, as shown by colocalization with LAMP-1 and MHC II in the perinuclear region (left columns of Fig. 5A and Fig. B, respectively). In contrast, the MMR:CD16 did not colocalize with LAMP-1 and was found primarily in the peripheral cytoplasm (Fig. 5 A). Receptor targeting to late endosomes was mediated by sequences found in the distal portion of the cytoplasmic tail of DEC-205, since DEC-IT:CD16 chimeras, which lacked amino acids 18–31 in the cytoplasmic tail of DEC-205, failed to accumulate in MHC II+ lysosomes and were found in vesicles throughout the cytoplasm (Fig. 5A and Fig. B). The ST-DEC:CD16 chimera failed to mediate endocytosis completely, and was distributed along the cell membrane (Fig. 5 A).
Figure 5.
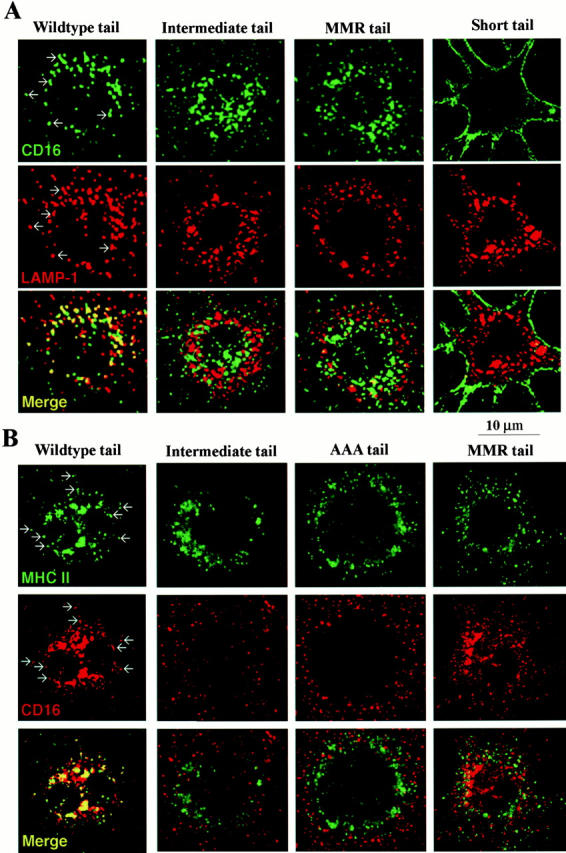
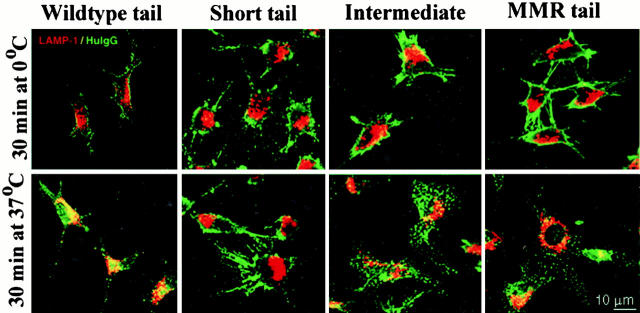
Typical distribution of CD16 chimeric receptors in DCEK.ICAM.Hi7 cells in the absence (shown here) or presence of ligand, 10 μg/ml aggHuIgG. (A) CD16 and LAMP-1 double labeling. Cells on tissue culture chamber slides were fixed and stained for CD16 (green, FITC-labeled anti–mouse Ig) and LAMP-1 (red, Texas red–labeled anti–rat Ig). Colocalization in discrete vesicles in WT transfected cells is indicated by arrows, and for all the transfectants is displayed in yellow (bottom row). (B) CD16 and MHC II double labeling. Cells grown on tissue chamber slides were fixed and stained for CD16 (red) or MHC II (green) with species-specific secondary reagents. Slides were analyzed by confocal laser scan microscopy. Examples of colocalization in single vesicles in WT transfected cells are indicated by arrows and displayed in yellow (bottom row).
To follow the targeting of bound ligand, we incubated the L cells with aggHuIgG for 30 min on ice, followed by a 30-min chase at 37°C. Surface-bound HuIgG was found on all transfectants after incubation on ice (Fig. 6 A, top), confirming the FACS® data (Fig. 3 A). After incubation at 37°C, HuIgG was mainly found in lysosomes in WT-DEC:CD16 transfectants, colocalizing with LAMP-1 in the perinuclear region (Fig. 6 A, bottom left). In contrast, HuIgG endocytosed by either IT-DEC:CD16 or MMR:CD16 transfected cells, colocalized with TfR in early endosomes (data not shown), indicating that ligands bound to these receptors failed to reach lysosomes efficiently (Fig. 6). HuIgG bound to the ST-DEC:CD16 chimera remained surface bound during the 30-min chase period. From these results, we conclude that the DEC-205 cytoplasmic domain targets CD16 chimeras and their ligands to MHC II+LAMP-1+ vacuoles, whereas the cytoplasmic domain of the MMR targets primarily to early endosomes. Sequences for targeting to late endosomes lie between positions 18 and 31 in the DEC-205 tail. The different targeting routes of MMR:CD16 and AAA-DEC:CD16 versus WT-DEC:CD16 should be reflected in a different time course for their recycling of these receptors, but in fact unoccupied receptors reappeared with a similar pace. However, the speed of recycling via early endosomal compartments is likely to have been underestimated because the pH of the early endosomes may be insufficiently low to quickly elute all of the bound HuIgG ligand.
Figure 6.
Intracellular localization of endocytosed HuIgG in CD16 transfectants. (A) Double labeling for endocytosed HuIgG and LAMP-1. Cells, grown on tissue chamber slides, were incubated with 10 μg/ml HuIgG for 1 h on ice. Unbound HuIgG was washed away and cells were either fixed immediately or further incubated in medium for 30 min at 37°C. Cells were double stained with FITC-labeled anti-HuIgG (green) and with anti–LAMP-1 antibodies (red; Texas red–labeled secondary antibodies).
Presentation of Ligands Internalized by Chimeric CD16 Receptors to CD4+ T Cells
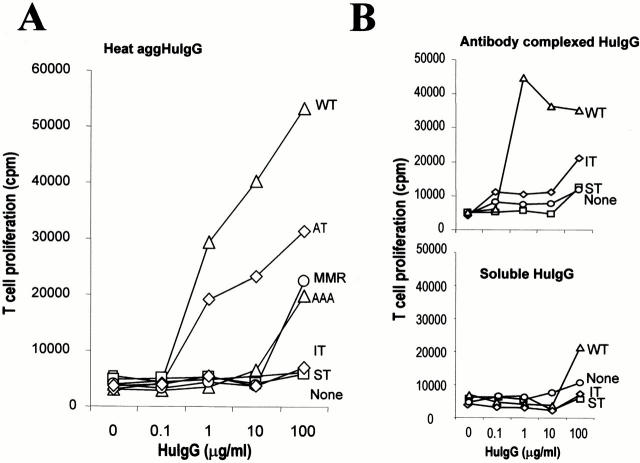
To determine whether antigen bound to CD16 chimeric receptors was processed and presented, we pulsed the transfected cells with aggHuIgG for 6 h, and assayed for presentation to primed T lymphocytes. Cells expressing WT-DEC:CD16 induced strong T cell proliferation, with saturation concentrations of 1 μg/ml aggHuIgG (Fig. 7 A). Immune complexes formed with anti-HuIgG and soluble HuIgG were also presented efficiently, whereas nonaggregated HuIgG, which did not bind to CD16 receptors (Fig. 3 A), only induced occasional T cell proliferation at high doses (100 μg/ml; Fig. 7B and Fig. C).
Figure 7.
Antigen presentation by DEC-CD16 transfected cells. (A) Presentation of aggHuIgG. Transfected and untransfected (none) DCEK.ICAM.Hi7 cell lines were incubated for 6 h with aggHuIgG, washed, and used to present antigen to 250,000 purified lymph node T cells from mice primed to HuIgG. At 72 h, the cells were pulsed with [3H]thymidine for 12 h, and incorporated radioactivity was measured. (B) Presentation of immune complexes of HuIgG. As in A, transfected and untransfected (none) DCEK.ICAM.Hi7 cell lines were incubated with either soluble or antibody aggregated HuIgG. Antigen presenting assays were then performed.
When the mutant cytosolic tails were studied, LT-DEC:CD16 chimeras were indistinguishable from WT-DEC:CD16 in inducing T cell proliferation at low antigen concentrations. AT-DEC:CD16 chimeras (tyrosine 15 substituted by alanine) showed only a slightly diminished response. In contrast, IT-DEC:CD16, which mediated endocytosis and recycling (Fig. 3E and Fig. F) but failed to target to MHC II+LAMP-1+ compartments (Fig. 5), was inefficient for antigen presentation. Cells expressing MMR:CD16 chimeras resembled those expressing IT-DEC:CD16 in that they were only able to stimulate T cell proliferation at high concentrations of HuIgG (10–100 μg/ml; Fig. 7). ST-DEC:CD16 (lacking the 25 most distal amino acids in the DEC-205 cytoplasmic domain) did not stimulate T cell proliferation above the background obtained with untransfected DCEK.ICAM.Hi7 cells. These data indicate that sequences required to target antigens for efficient presentation differ from those required for endocytosis and recycling. IT-DEC:CD16 and MMR:CD16 chimeras mediate endocytosis and recycling, but additional information found between amino acids 18 and 28 in the DEC-205 cytoplasmic domain is required for targeting to antigen processing compartments.
Importance of the Distal EDE Sequence in the Distinct Targeting of the DEC-205 Tail
Acidic amino acids are implicated in intracellular targeting, e.g., the movement of HIV-1 nef protein to lysosomes (Piguet et al. 1999), the movement of furin to the trans-Golgi network (Voorhees et al. 1995; Simmen et al. 1999), and the retrieval of the LDLR from apical to basolateral membranes of epithelial cells (Matter et al. 1993). To assess if acidic amino acids in the distal part of the DEC-205 tail were required for late endosomal targeting, we mutated the EDE residues to alanines (AAA-DEC:CD16). The AAA-DEC:CD16 chimera was fully competent for adsorptive uptake of aggHuIgG and recycling back to the surface (Fig. 3E and Fig. F), but failed to target aggHuIgG or CD16 to lysosomes (Fig. 5 B). Consistent with the absence of late endosomal targeting, presentation of antigen to IgG-primed T cells was greatly reduced (Fig. 7 A) and occurred only at antigen levels comparable to those needed for IT-DEC:CD16– and MMR:CD16-mediated presentation. Thus, the EDE in the DEC-205 tail is required for its unique lysosomal targeting and antigen presenting functions.
Discussion
A Specialized Receptor for Antigen Uptake and Presentation on DCs
High doses of protein antigens, e.g., 100–1,000 μg/ml, are typically added to antigen-presenting cells to generate the MHC II–peptide complexes that are ligands for CD4+ T cells. The efficiency of antigen binding and uptake is greatly enhanced by receptor-mediated endocytosis, as occurs with the MMR (Engering et al. 1997; Prigozy et al. 1997; Tan et al. 1997), FcR (Sallusto and Lanzavecchia 1994), and BCR (Bonnerot and Lankar 1995). The DEC-205 receptor is expressed by DCs and is a homologue to the MMR, localizing to coated pits and enhancing ligand uptake (Jiang et al. 1995). The MMR primarily recycles through peripheral, early endosomes (Engering et al. 1997; Tan et al. 1997), although some entry into late endosomes is reported (Prigozy et al. 1997). In contrast to the MMR, DEC-205 targets to deep endosomes or lysosomes in DCs rather than early endosomes, the latter being typical for other endocytosis receptors. The MMR presents mannosylated BSA very efficiently (Sallusto et al. 1995). Surprisingly, when we used polyclonal rabbit antibodies as surrogate ligands, DEC-205 was much more efficient than the MMR in presenting peptides to rabbit Ig–primed T cells. Since cytosolic domains guide the movements of adsorptive endocytosis receptors, we proceeded to study fusion receptors formed by the external domains of human CD16 and different tails, especially the MMR and DEC-205. In a totally heterologous system, i.e., transfected L cells, the DEC-205 tail mediated a unique recycling pathway through late endosomes rich in MHC II, and importantly, greatly enhanced antigen presentation relative to the MMR tail. Typically, L cells are inefficient at producing MHC II–peptide complexes from proteins, and preprocessed peptides are used to study antigen presentation (Dubey et al. 1995).
Additional work will be needed to pursue the physiological implication of these findings, i.e., that DEC-205 is used by DCs to improve antigen presentation. The following types of future experiments would be of value. First, to assess the role of the distal cytoplasmic tail in the context of a full length receptor, it would be important to mutate full length DEC-205; currently, it is not yet feasible to obtain high level expression of this large receptor. Second, the studies of CD16-DEC fusion receptors could be extended into DCs using different vectors, and such experiments are underway. Third, ligands for DEC-205 and MMR need to be identified, so that the presentation of natural ligands rather than surrogates can be tested. Interestingly, DEC-205 and not the MMR is readily detected on DCs within the T cell areas of mouse and human lymphoid tissues (Linehan et al. 1999; Guo et al. 2000).
Two Functional Regions of the DEC-205 Cytosolic Domain
The membrane-proximal region of the DEC-205 tail contains the sequence FSSVRY, which resembles the coated pit localization sequences described in many other receptors (Goldstein et al. 1979). Such sequences function in uptake of the LDLR (Chen et al. 1990; Matter et al. 1993), TfR (Collawn et al. 1990), and MMR (Ezekowitz et al. 1990). The DEC-205 tail is 31 residues in length. Deletion of residues 19–31 did not reduce endocytosis, but further deletion of residues 7–19 including the coated pit sequence ablated uptake. Mutation of the tyrosine residue to alanine did not abolish function, in contrast to decreased function of other receptors (Chen et al. 1990; Amigorena et al. 1992b; Jackman et al. 1998), but the uptake rates were lower relative to wild-type DEC-205 tail. Either the coated pit sequence of DEC-205 is not totally dependent on this tyrosine, or another sequence bypasses its need, e.g., the three acidic residues in the distal tail to be discussed next (Voorhees et al. 1995; Simmen et al. 1999).
The more intriguing region of the DEC-205 cytosolic tail was the distal region. Residues 28–31 seemed superfluous, but residues 18–27 were critical for several functions. In the absence of this “distal targeting sequence,” the DEC-205 tail did not target either receptor or ligand to late endosomes and lysosomes (Fig. 5) and did not mediate efficient antigen presentation (Fig. 7). However, the distal targeting sequence was not required for uptake (Fig. 3 E) and membrane recycling (Fig. 3 F).
A cluster of acidic amino acids (EDE) in the distal DEC-205 tail proved critical for its distinct intracellular movements (Fig. 5 and Fig. 7). Acidic clusters also mediate trans-Golgi network retrieval of the mannose-6-phosphate receptor and furin, the latter interacting with a cytosolic sorting molecule PACS-1 (Voorhees et al. 1995; Wan et al. 1998; Simmen et al. 1999). Only two acidic amino acids in the HIV-1 nef protein signal lysosomal targeting and degradation of endocytosed CD4 molecules via β-COP (Piguet et al. 1999). Acidic residues target the LDLR to a basolateral site during transcytosis (Matter et al. 1992). The LDLR-related protein contains a cluster of acidic amino acids as well. This receptor mediates uptake and degradation of sphingolipids, α2-macroglobulin, and complement component C3 into lysosomes (Hiesberger et al. 1998; Meilinger et al. 1999), but the involvement of acidic amino acids in lysosomal targeting has not been described. The experiments in this paper have compared DEC-205 with MMR, because of similarities in their external multilectin domains and their proposed function in antigen presentation. Future work will compare the cytosolic domains of DEC-205 with the LDLR family, as well as function in non–antigen-presenting cells like epithelial cells.
A Novel Pathway for an Adsorptive Endocytosis Receptor
As mentioned above (see Introduction), some receptors mediate ligand uptake and discharge in early endosomes, followed by recycling of intact receptors to the surface and further rounds of uptake. Other receptors signal cell activation and growth, and then uptake is followed by ligand and receptor digestion in lysosomes. The new pathway, illustrated by DEC-205, is a hybrid between these two. The cytosolic domain of this receptor can mediate uptake into deeper vacuoles, followed by recycling of ostensibly intact receptor. Concomitantly, there is a marked improvement in the efficiency with which peptides are salvaged and displayed as MHC–peptide complexes. It will be important now to follow the distribution and function of DEC-205 in epithelia and brain endothelium, where this receptor is also abundant. It is possible that in epithelia, as in antigen-presenting cells, DEC-205 will target to deeper proteolytic vacuoles and lead to the production of biologically active peptides.
Acknowledgments
We thank the many colleagues who gave us valuable advice during preparation of the manuscript.
These experiments were supported by a fellowship from the Deutsche Forschungsgemeinschaft to K. Mahnke (MA 1924/1-1), grants to R.M. Steinman from the Juvenile Diabetes Foundation and the National Institutes of Health (AI13013 and AI39672), to M. Nussenzweig from the Human Science Frontiers Program, and to S. Lee (National Institutes of Health Medical Scientist Training Program grant GM07739).
Footnotes
Abbreviations used in this paper: aggHuIgG, aggregated human IgG; BCR, B cell antigen receptor; CHX, cycloheximide; DC, dendritic cell; ICAM, intracellular adhesion molecule; LAMP-1, lysosome-associated membrane protein 1; LDLR, low-density lipoprotein receptor; MMR, macrophage mannose receptor; MHC II, major histocompatibility complex class II; MIIC, MHC II compartment; PLA2R, phospholipase A2 receptor; TfR, transferrin receptor.
References
- Amigorena S., Bonnerot C., Drake J.R., Choquet D., Hunziker W., Guillet J.-G., Webster P., Sautes C., Mellman I., Fridman W.H. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes Science. 256 1992. 1808 1812a [DOI] [PubMed] [Google Scholar]
- Amigorena S., Salamero J., Davoust J., Fridman W.H., Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG Nature. 358 1992. 337 341b [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., Dell'Angelica E.C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Lankar D. Role of B cell receptor Igα and Igβ subunits in MHC class II-restricted antigen presentation. Immunity. 1995;3:335–347. doi: 10.1016/1074-7613(95)90118-3. [DOI] [PubMed] [Google Scholar]
- Bonnerot C., Amigorena S., Choquet D., Pavlovich R., Choukroun V., Fridman W.H. Role of associated gamma-chain in tyrosine kinase activation via murine Fc gamma RIII. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:2747–2757. doi: 10.1002/j.1460-2075.1992.tb05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.S., Anderson R.G., Goldstein J.L. Recycling receptorsthe round-trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Chen W.-J., Goldstein J.L., Brown M.S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Chesnut R.W., Grey H.M. Studies on the capacity of B cells to serve as antigen-presenting cells. J. Immunol. 1981;126:1075–1079. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A.L., Lodish H.F. The asialoglycoprotein receptor internalizes and recycles independently of the transferrin and insulin receptors. Cell. 1983;32:267–275. doi: 10.1016/0092-8674(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Cohn Z.A., Ehrenreich B.A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J. Exp. Med. 1969;129:201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn J.F., Stangel M., Kuhn L.A., Esekogwu V., Jing S., Trowbridge I.S., Tainer J.A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Drickamer K., Taylor M.E. Biology of animal lectins. Annu. Rev. Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- Dubey C., Croft M., Swain S.L. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J. Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- Ehrenreich B.A., Cohn Z.A. The fate of peptides pinocytosed by macrophages in vitro. J. Exp. Med. 1969;129:227–245. doi: 10.1084/jem.129.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engering A.J., Cella M., Fluitsma D., Brockhaus M., Hoefsmits E.C., Lanzavecchia A., Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R.A.B., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptordemonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L., Anderson R.G.W., Brown M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Guo M., Gong S., Maric S., Misulovin Z., Pack M., Mahnke K., Nussenzweig M., Steinman R.M. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum. Immunol. 2000;61:729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Hiesberger T., Huttler S., Rohlmann A., Schneider W., Sandhoff K., Herz J. Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP) EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4617–4625. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman R.M., Stenger S., Lee A., Moody D.B., Rogers R.A., Niazi K.R., Sugita M., Modlin R.L., Peters P.J., Porcelli S.A. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8:341–351. doi: 10.1016/s1074-7613(00)80539-7. [DOI] [PubMed] [Google Scholar]
- Jiang W., Swiggard W.J., Heufler C., Peng M., Mirza A., Steinman R.M., Nussenzweig M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Linehan S.A., Martinez-Pomares L., Stahl P.D., Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organsin situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cellsthe cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J.A., Yamamoto E.M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993;74:1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Meilinger M., Gschwentner C., Burger I., Haumer M., Wahrmann M., Szollar L., Nimpf J., Huettinger M. Metabolism of activated complement component C3 is mediated by the low density lipoprotein receptor-related protein/α2-macroglobulin receptor. J. Biol. Chem. 1999;274:38091–38096. doi: 10.1074/jbc.274.53.38091. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Steinman R.M., Unkeless J.C., Cohn Z.A. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J. Cell Biol. 1983;96:887–895. doi: 10.1083/jcb.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Piguet V., Gu F., Foti M., Demaurex N., Gruenberg J., Carpentier J.L., Trono D. Nef-induced CD4 degradationa diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Prigozy T I., Sieling P.A., Clemens D., Stewart P.L., Behar S.M., Porcelli S.A., Brenner M.B., Modlin R.L., Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate antigen in the major histocompatibility class II compartment. Downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T., Nobile M., Bonifacino J.S., Hunziker W. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol. Cell. Biol. 1999;19:3136–3144. doi: 10.1128/mcb.19.4.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P.H., Sigardson E., Rodman J.S., Lee Y.S. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages. Characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Swiggard W.J., Mirza A., Nussenzweig M.C., Steinman R.M. DEC-205, a 205 kDa protein abundant on mouse dendritic cells and thymic epithelium that is detected by the monoclonal antibody NLDC-145Purification, characterization and N-terminal amino acid sequence. Cell. Immunol. 1995;165:302–311. doi: 10.1006/cimm.1995.1218. [DOI] [PubMed] [Google Scholar]
- Tan M.C., Mommaas A.M., Drijfhout J.W., Jordens R., Onderwater J.J., Verwoerd D., Mulder A.A., van der Heiden A.N., Scheidegger D., Oomen L.C. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- Unkeless J.C., Fleit H., Mellman I.S. Structural aspects and heterogeneity of immunoglobulin Fc receptors. Adv. Immunol. 1981;31:247–251. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Voorhees P., Deignan E., van Donselaar E., Humphrey J., Marks M.S., Peters P.J., Bonifacino J.S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Molloy S.S., Thomas L., Liu G., Xiang Y., Rybak S.L., Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Wilde A., Beattie E.C., Lem L., Riethof D.A., Liu S.H., Mobley W.C., Soriano P., Brodsky F.M. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]