Abstract
Although sensory problems, including unusual tactile sensitivity, are heavily associated with autism, there is a dearth of rigorous psychophysical research. We compared tactile sensation in adults with autism to controls on the palm and forearm, the latter innervated by low-threshold unmyelinated afferents subserving a social/affiliative submodality of somatosensation. At both sites, the groups displayed similar thresholds for detecting light touch and innocuous sensations of warmth and cool, and provided similar hedonic ratings of the pleasantness of textures. In contrast, increased sensitivity to vibration was seen in the autism group on the forearm, along with increased sensitivity to thermal pain at both sites. These findings suggest normal perception along with certain areas of enhanced perception in autism, consistent with previous studies.
Keywords: Autism, Sensory, Tactile, Psychophysics, CT afferents
Introduction
Autism is a pervasive developmental disorder with an onset in early childhood and severe, often lifelong effects on communication, socialization, and tendencies toward restricted interests or repetitive behaviors (APA, 2000). Often associated with these symptoms are sensory-perceptual anomalies, which occur in approximately 70% of cases (Baranek, David, Poe, Stone, & Watson, 2006) and are associated with difficulties in adaptive behavior noted by parents of children with autism (Rogers, Hepburn, & Wehner, 2003). These symptoms are often the target of clinical interventions in an effort to reduce their occurrence, or provide environmental adaptations to improve overall participation (see Baranek, 2002 for a review).
Sensory abnormalities in autism occur in multiple forms and across various modalities (Kern et al., 2006). Some individuals with autism respond in a hypersensitive manner to sensory stimuli, such as being able to hear a distant, approaching noise (e.g., a siren) long before others are able to hear it, or being unable to tolerate a well-meaning hug or pat on the back. Some persons with autism may also respond in a hyposensitive manner, such as failing to orient when someone calls their name, or engaging in self-injurious behavior without appearing to feel pain. Both hypo-sensitivity and hypersensitivity are noted in the same individuals depending on the situation and the sensory modalities involved (Baranek et al., 2006; Dunn, Myles, & Orr, 2002; Iarocci & McDonald, 2006; Rogers & Ozonoff, 2005). Altered sensory thresholds and/or modulation difficulties are hypothesized to result in these unusual sensory features, and many individuals engage in behaviors in an attempt to counteract their effects. For example, individuals may respond to hypersensitivity by avoiding situations in which overstimulation is likely to occur; individuals with hyposensitivity may engage in “seeking” behavior to increase the sensory experience (Dunn, 2001).
Individual autobiographical accounts from verbal, high-functioning people with autism emphasize their unusual sensory experiences (Grandin, 2000; Jones, Quigney, & Huws, 2003), often describing overwhelming sensory input as an impetus for social withdrawal. Unusually acute tactile sensitivity or the inability to modulate tactile input is hypothesized to impede social behavior that involves interpersonal touch (Grandin, 1992) and aversion to social touch is among several atypical behaviors seen in infants later diagnosed with autism (Baranek, 1999). In spite of these ecologically valid reports, experimental studies of tactile perception in autism are scarce.
To date, two studies have employed rigorous psychophysical approaches to study tactile perception in autism, yielding mixed results. O’Riordan and Passetti (2006) studied high-functioning children with autism compared with typically developing children, and found no group differences in the ability to discriminate the roughness of different grades of sandpaper or to detect synthetic fibers pressed on the skin of the child’s arm. The authors concluded that there is no evidence for enhanced perception in the tactile modality, contrary to emerging findings in auditory and visual perception (Mottron, Peretz, & Menard, 2000; Mottron, Burack, Iarocci, Bellewill, & Enns, 2003; Mottron & Burack, 2001).
In contrast, a recent experiment by Blakemore, Tavassoli, Calo, Thomas, Catmur, Frith and Haggard (2006) demonstrated tactile hypersensitivity at the fingertip with superior detection of high-frequency (200 Hz), although not low-frequency (30 Hz), skin vibration in adults with Asperger’s syndrome (a disorder on the autism spectrum) compared to a general community sample of adults.
Among somatosensory submodalities (primarily touch, temperature, and pain) that may contribute to tactile hypersensitivity in autism, a class of unmyelinated tactile mechanoreceptors that has been recently identified in humans presents an intriguing hypothesis. These receptors, known as CT-afferents, are unmyelinated C fibers that respond to light (low force), slowly moving, stroking stimuli (Vallbo, Olausson, & Wessberg, 1999; Olausson et al., 2002). In humans, these low-threshold unmyelinated afferents are distributed primarily in the hairy skin and in the face, but not in the hairless, glabrous skin of the palm that is highly innervated with myelinated tactile afferents, known to be important for sensory discrimination (Kakuda, 1992). It is hypothesized that, with their distribution in hairy skin and their response preference for pleasant, stroking touch, this class of unmyelinated afferents constitutes an affiliative, social touch system (Olausson et al., 2002; Vallbo et al., 1999; Wessberg, Olausson, Fernstrom, & Vallbo, 2003). Such a system is a prime candidate for the tactile hypersensitivity associated with autism.
As described above, the few existing experimental studies of tactile sensitivity in autism have used a limited range of tactile stimuli. The goal of the present study was to investigate tactile sensitivity in adults with autism using a variety of stimuli, in order to probe different submodaltities of somatosensation. We administered a battery of five classes of somatosensory stimuli to two somatic sites in our participants. In Experiment 1, we tested participants’ ability to detect constant pressure using thin nylon, “von Frey” filaments. Since another possibility for hypersensitivity in autism is that sensory neurons fail to adapt normally, we measured adaptation to the detection of vibrotactile stimuli in Experiment 2, using a paradigm known to produce adaptation in non-autistic participants (Hollins, Sigurdsson, Fillingim, & Goble, 1996). In Experiment 3, we obtained magnitude estimates for the perceived hedonic value (pleasantness/unpleasantness) of textured surfaces; in Experiment 4, we measured thresholds for thermal sensations of cool and warmth, and in Experiment 5, we obtained thermal pain thresholds. Each experiment was performed on the dorsal forearm and the palm, chosen because the forearm is innervated by CT afferents in addition to myelinated mechanoreceptors, while the palm is devoid of CT afferent innervation (Johansson & Vallbo, 1979; Kakuda, 1992).
Specifically, our hypotheses were as follows: individuals with autism would display (a) normal thresholds for contact detection on the forearm as well as the thenar palm (O’Riordan & Passetti, 2006), (b) normal detection of low-frequency vibration on the palm (Blakemore et al., 2006), but possibly altered detection at the forearm, (c) abnormal adaptation to suprathreshold vibrotactile stimulation, (d) altered hedonic perception of textures, particularly on the forearm where CT afferents contribute to perception, (e) altered thermal sensation thresholds at either site, (f) altered thermal pain thresholds at either site.
Methods
Participants
Eight adults with high-functioning autism (clinical diagnoses of either Autistic Disorder or Asperger Disorder; DSM-IV-TR; APA, 2000) were recruited from a university-based autism research registry. Six of these adults had diagnoses confirmed using the Autism Diagnostic Interview-Revised (ADI-R; LeCouteur, Lord, & Rutter, 2003) and the Autism Diagnostic Observation Schedules (ADOS; Lord, Rutter, Dilavore, & Risi, 1999). The other two did not have results from these assessments available. The participants were screened for comorbid psychiatric diagnoses and peripheral injury that would affect somatosensation. All participants had an IQ of at least 70; there were seven males and one female. The mean age of the sample was 29.3 years, and ranged from 20 to 45 years.
Eight adults without autism were recruited from the community, selected to match each individual with autism on age and gender (mean age 29.0, range 21–45). Although the groups were not matched explicitly on IQ, the adults in the control group were from a general community sample with a diversity of backgrounds. Likewise, since all participants with autism were high functioning (i.e., had IQs that were within the normal range), intellectual deficits were not likely to be a confounding factor. Participant details are displayed in Table 1. All participants signed consent forms, and were paid $20/h for their time. All procedures were approved by the institutional review board for human subjects research at the university.
Table 1.
Participant information
| Autism | Age | Gender | Control | Age | Gender |
|---|---|---|---|---|---|
| A1 | 20 | M | C1 | 20 | M |
| A2 | 40 | M | C2 | 45 | M |
| A3 | 45 | F | C3 | 41 | F |
| A4 | 20 | M | C4 | 21 | M |
| A5 | 23 | M | C5 | 22 | M |
| A6 | 40 | M | C6 | 35 | M |
| A7 | 24 | M | C7 | 23 | M |
| A8 | 22 | M | C8 | 23 | M |
| Mean | 29.3 | 29.0 | |||
| SD | 10.5 | 9.7 |
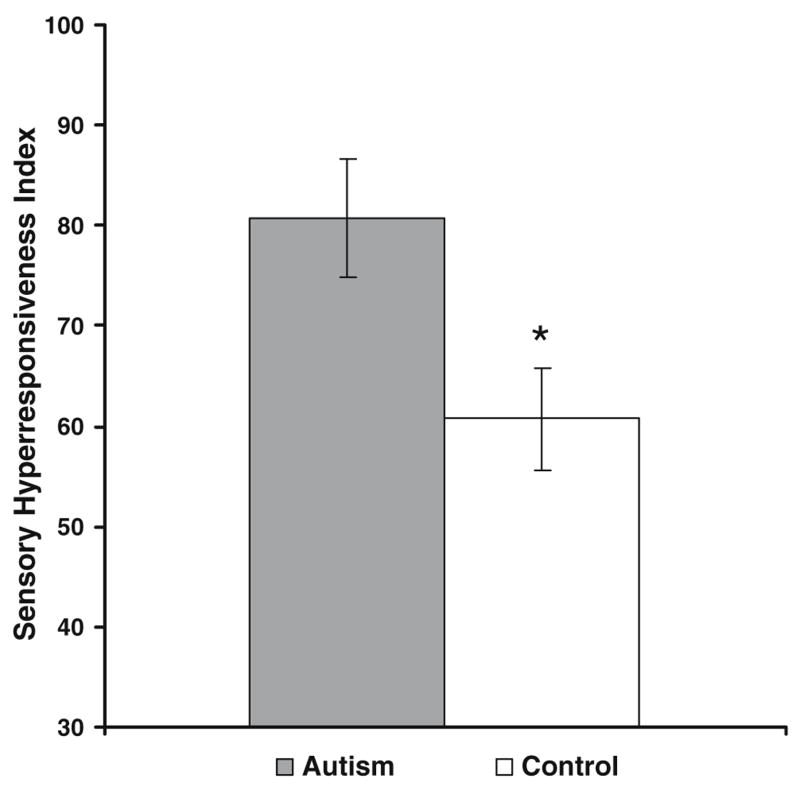
Each participant completed a brief questionnaire, the Adult Sensory Profile (Dunn, 2001) to assess whether the groups differed with respect to their experience with sensory stimuli in everyday life. The questionnaire yields scores on four subscales, two of which are theoretically linked to sensory hyperresponsiveness. The scores of these two subscales (measures of sensory sensitivity and avoidance of sensory stimuli) were combined to produce a composite, hyperresponsiveness index. When the groups were compared on this index using a general linear model, a significant difference emerged (F = 6.58; p = 0.0225), demonstrating that the autism group has a higher degree of sensory sensitivity affecting daily life than the control group (Fig. 1).
Fig. 1.
Comparison of autism and control groups on sensory hyperresponsiveness in everyday life. The sensory hyperresponsiveness index is a composite of two subscales (Sensory Sensitivity and Sensory Avoiding) of the Adult Sensory Profile (Dunn, 2001). In this and subsequent figures, error bars represent the standard error of the mean
Protocol
Participants completed two separate, identical sessions, each lasting approximately 2.5 h, with breaks offered throughout each session. Five psychophysical paradigms were used to measure different aspects of somatosensory perception in each session as described below. For each of the five paradigms, measurements were taken at two sites: (a) the hairy skin of the right dorsal forearm (innervated by CT afferents and myelinated mechanoreceptors) and (b) the glabrous skin of the right thenar palm (not innervated by CT afferents). During all four paradigms, the participants rested comfortably in a reclining dental chair, with their view of the stimulation site shielded by a dark curtain, and their right arm immobilized using an air-compressed bean bag.
Contact Detection Thresholds
A set of 20 calibrated nylon “von Frey” filaments, which delivered forces from 0.005 to 448 g, was used to determine the threshold force for detecting touch. Stimulus trials were administered using the method of limits, with four alternating blocks of two descending and two ascending trials administered after a practice descending block. Each trial consisted of a filament being manually lowered normal to the surface of the skin until contact was made with the skin, and continued force applied smoothly until the filament buckled once. The contact area was delineated with an adhesive-backed plastic ring that revealed an area of skin of about 1 cm in diameter. On the forearm, this ring also served the purpose of smoothing hair out of the way of the filament; great care was taken by the experimenter not to disturb arm hair during testing. Before each presentation, a verbal prompt was given: “pay attention now,” and after each presentation the subject reported whether or not he/she detected the applied filament. The descending blocks began with a filament that delivered a force of 8.53 g, and after each positive response, the next (thinner) filament in the set was administered in the same manner, until two consecutive negative responses were obtained. The ascending blocks began with a filament which delivered a force of 0.005 g, and after each negative response, the next (thicker) filament was administered, until two consecutive positive responses were obtained. A catch trial for which no filament was administered was included in each block to limit false positive responses and subjects were notified of false positive responses before continuing. According to an established procedure (Essick, 1992), the subject’s data would be discarded if more than one in four catch trials resulted in false alarms, however no subject’s rate of false positives reached this criterion ratio, thus all subjects’ data were retained. Thresholds were calculated by averaging the values for the trial before and after the first of the two negative (or positive) responses that ended the block, across the four blocks.
Vibrotactile Detection and Adaptation
Thirty-three hertz sinusoidal vibrations were delivered using a piezoelectric bender element controlled by a PC running Labview (National Instruments, version 7). The bender element was attached to a plastic probe that was in contact with the skin and surrounded by a plastic ring to prevent spread of the stimulus beyond the intended test site. The probe device was secured to the skin using Velcro straps.
Sixty stimulus trials were delivered in three blocks of 20 each. The stimuli were presented in a two-alternative forced choice tracking paradigm (Zwislocki, Maire, Feldman, & Rubin, 1958), in which the trial was divided into two consecutive, 1 s stimulation intervals, separated by an interstimulus interval of 1 s. The vibration was randomly assigned to one of these two intervals, and the computer monitor displayed an interface that visually cued the onset and offset of the intervals that composed the trial. At the end of the second interval, a cue was presented that prompted the subject to report which interval held the vibration using one of two buttons. After selection of one interval by the participant, feedback was given, and the intertrial interval (ITI) began. The ITI lasted 16 s, during the last 3 s of which a warning light was displayed, indicating that a new trial was about to begin.
The stimulus delivered during the first trial began with an amplitude of 20 μm (26.02 dB re: 1 μm peak-to-peak). An incorrect response resulted in augmentation of the amplitude of the next test stimulus by 4 dB, while three correct responses, not necessarily in a row, resulted in a 4-dB decrement. An average of the last five trials in the first block of 20 established a baseline threshold. During the second block of 20 trials, a 15 s adapting stimulus (20 dB above the baseline threshold—an order of magnitude higher) was presented coincident with the onset of the ITI (Goble & Hollins, 1993). The last 20 trials (third block) were administered in the same way as the first 20 and did not include this adapting stimulus. As for the baseline (pre-adaptation) block, detection thresholds were calculated for the adaptation and post-adaptation blocks by averaging the last five trials of the block.
Hedonic Magnitude Estimation of Textured Surfaces
Three textures were used for this experiment: a coarse burlap (Hessian) fabric, plastic mesh, and a soft cosmetic brush. The burlap and mesh were mounted on the end of a foam brush with a wooden dowel in order to be applied in a similar manner to the cosmetic brush. Each texture was applied at three subjective, manually controlled force levels: light, medium, and hard. Thus the stimuli comprised nine texture-force combinations (burlap-light, cosmetic brush-medium, etc.) and three repetitions of each combination were performed at each site. These 27 trials were preceded by a practice session with one example of each combination that allowed the participants exposure to the range of textures and practice using the rating scale. Participants were not told that there were only three textures applied at three force levels, rather, they were instructed that they would feel a range of textures, and were asked to rate each one for pleasantness on a scale from −100 to +100, with −100 representing the most unpleasant feeling they could imagine, and +100 representing the most pleasant feeling they could imagine. The three repetitions of each combination were averaged for each participant so that their dataset consisted of 27 values of average pleasantness ratings.
Thermal Sensation Thresholds
Thermal stimuli were delivered using a Peltier stimulator (30 mm × 30 mm thermoconducting surface, TSA II, Medoc, Israel). The thermoconducting surface was held on the skin (the thenar palm or the dorsal forearm) using a Velcro strap, and was set to a baseline temperature of 32°C, approximating the resting temperature of the skin. Upper and lower temperature boundaries were set at 50°and 0°C, respectively, to preclude any possibility of tissue damage. Two tasks were given; one for detection of thermal energy applied relative to the baseline temperature (warm detection), and one for detection of the removal of thermal energy (cool detection). Using a modified Marstock method of limits protocol (Fruhstorfer, Lindblom, & Schmidt, 1976), the temperature was changed at a rate of 1°C/s until the participant indicated a sensation of cool or warmth. Participants were instructed to respond with a button press as soon as they detected a change from the baseline temperature; the temperature of the thermoconducting surface was captured and recorded at this point using PC-based software, and the thermode temperature returned to baseline at a rate of 3.5°C/s. For each task, five trials were administered, and the middle three threshold values were averaged and analyzed (i.e., the lowest and highest value were not used; Jääskeläinen, 2004).
Thermal Pain Thresholds
As in the thermal sensation experiment, two tasks were given, one for cold pain and one for heat pain. The experimental setup was identical, except for the task instructions and the rate of temperature change. The thermode temperature changed from baseline at a rate of 1.5°C/s, until the participant pressed a button to indicate that the temperature had reached a point that was uncomfortable or painful. At this point, the temperature was recorded, and the thermode returned to the baseline temperature at a rate of 19.3°C/s. Participants were instructed carefully to respond as soon as the stimulation reached a point of being “painfully or uncomfortably hot (or cold).” In order to alleviate any anxiety about the pain stimuli, participants were also reminded that the device was limited to temperatures that are too mild to produce skin damage, and that their response triggered the return of the thermode to its baseline temperature.
Analyses
Each experiment was analyzed with a separate repeated measures, mixed model ANOVAs, using threshold (or magnitude estimate) as the outcome variable, and group membership, site, session, and stimulus parameters as independent variables. For the vibrotactile experiment, these parameters included block (pre-adapting, adapting, or post-adapting); for the textures, these included texture type and force level.
Results
Contact Detection
The average von Frey punctate touch detection threshold across sessions and groups was significantly (69%) lower on the palm than the forearm (F = 79.06; p < 0.0001). There was also a much smaller but significant effect of session (F = 6.24; p = 0.0167), with thresholds increasing on the second day of testing. As suggested by Fig. 2, thresholds did not differ significantly between groups, and there were no interactions between independent variables.
Fig. 2.
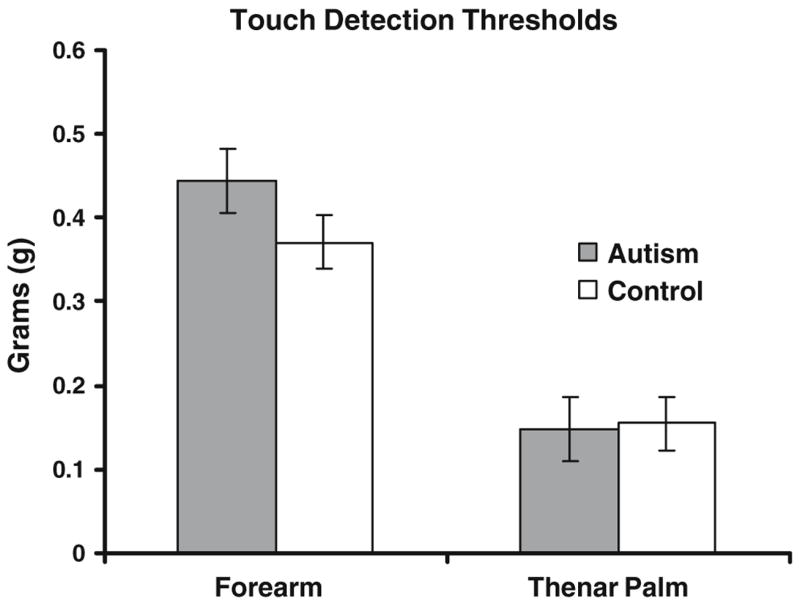
Group comparisons of von Frey contact detection thresholds, measured in grams of force delivered by von Frey filaments
Vibrotactile Detection
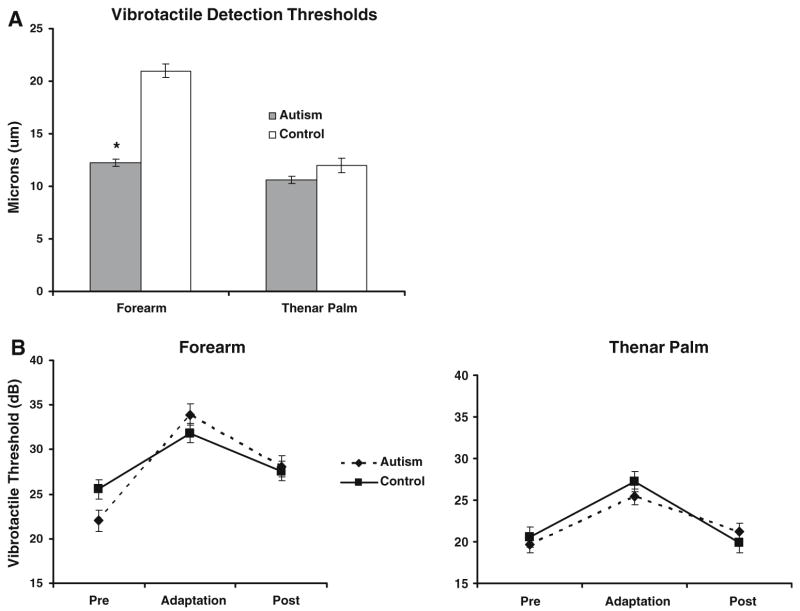
Like the previous light touch experiment, there was a main effect of site detection of a 33 Hz vibration, with (45%) lower thresholds, on average, on the palm than on the forearm (F = 52.49; p < 0.0001), and no main effect of group. However, unlike the previous experiment, there was a significant group × site interaction (F = 6.09, p = 0.0147), with the autism group having (34%) lower thresholds than controls on the forearm, but not on the palm (Fig. 3A). As in the previous experiment, there was a relatively small but significant effect of session (F = 6.11, p = 0.0145), this time with thresholds decreasing on the second day of testing.
Fig. 3.
Vibrotactile thresholds. (A) Group comparisons of baseline vibrotactile detection thresholds (in phase 1, before any adapting stimulus was presented) measured in amplitude (μm) of the sine wave stimulus. (B) Group comparisons of vibrotactile thresholds in all three phases (pre-adaptation, adaptation, and post-adaptation), measured in decibels
Vibrotactile Adaptation
Using the adaptation protocol of Hollins et al. (1996), there was a main effect of the block of 20 trials (corresponding to pre-adaptation, adaptation, or post-adaptation phases), with thresholds increasing during the adaptation phase, as expected (F = 51.41; p < 0.0001, Fig. 3B). There was an interaction between site and phase (F = 3.14; p = 0.0461), with the adaptation-related increase in threshold changing more substantially at the forearm than at the palm. The baseline (pre-adaptation) thresholds are depicted in Fig. 3A, the thresholds across all three phases in Fig. 3B.
Texture Hedonic Magnitude Estimation
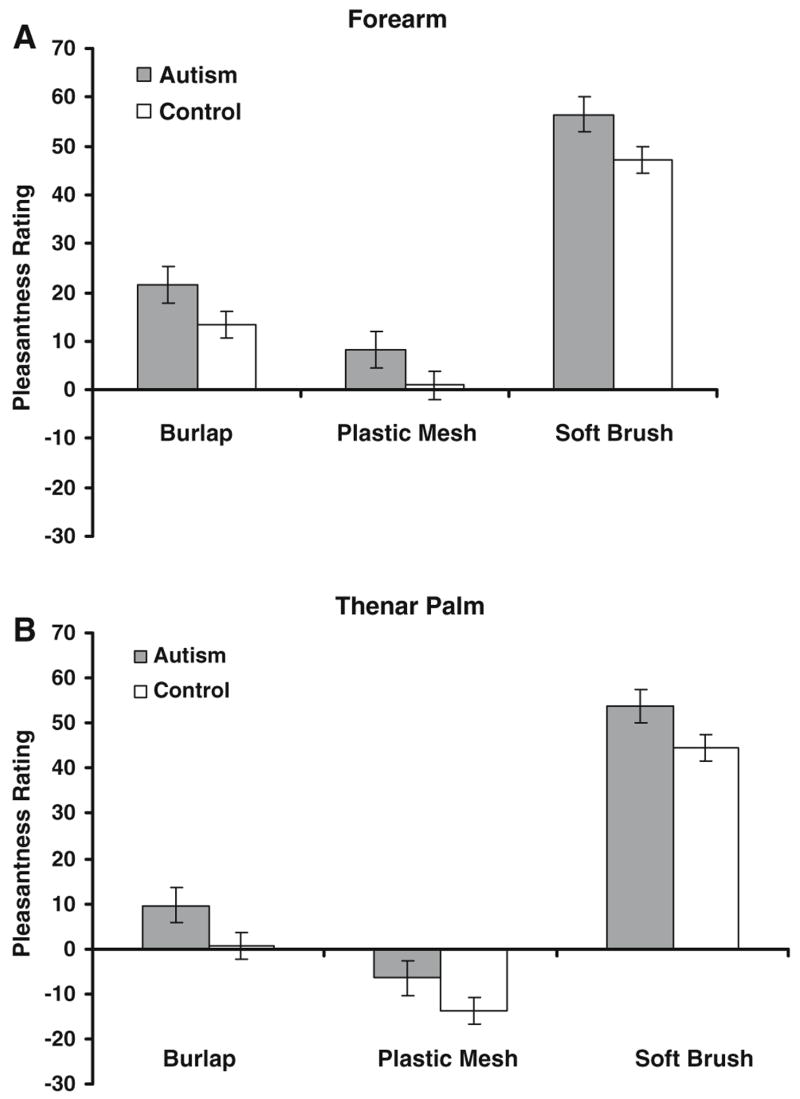
There was a strong main effect of texture (F = 246.93; p < 0.0001), with the soft cosmetic brush given the highest pleasantness ratings, the burlap given intermediate ratings, and the plastic mesh rated as least pleasant, as expected based on previous findings in neurotypical individuals (Ragin, 2002). There was a main effect of site, with textures given overall higher pleasantness ratings on the forearm as compared to the palm (F = 23.41; p < 0.0001), and a main effect of force, with lighter force generally given higher pleasantness ratings than harder force. There was again a main effect of session (F = 9.30; p = 0.0024), with pleasantness estimates being slightly lower on the second session, compared to the first. There was no main effect of group, although there was a consistent trend for the autism group to rate textures overall as more pleasant than controls (Fig. 4). There were two significant interactions: site × texture (F = 3.30; p = 0.0378), and force × texture (F = 10.05, p < 0.0001), as expected. The interaction between force and texture is an established effect (Ragin, 2002), with textures that are rated as more unpleasant increasing in unpleasantness with increasing force, while pleasant textures (i.e., the soft cosmetic brush) do not change sharply with increasing force (Fig. 5).
Fig. 4.
Group comparison for hedonic texture ratings. (A) Forearm. (B) Thenar palm
Fig. 5.
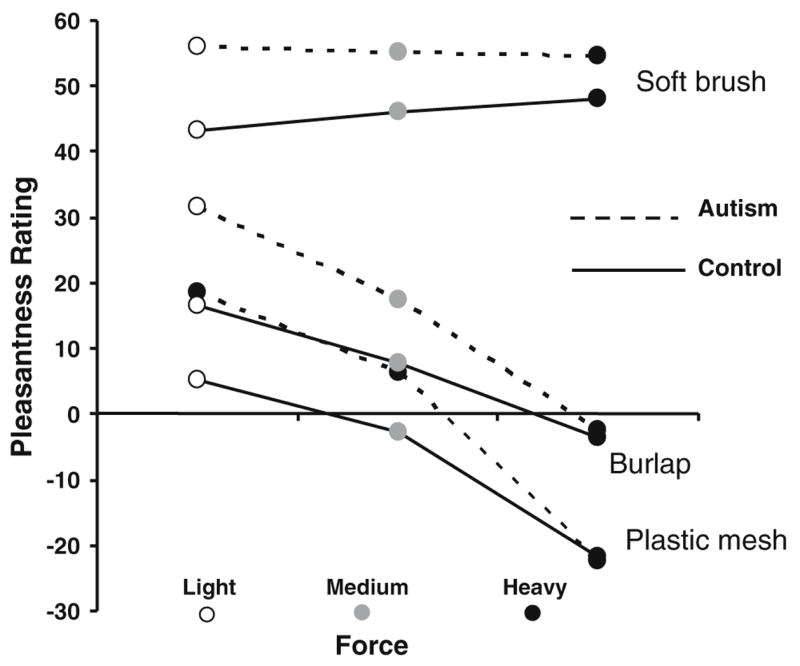
Relationship between force and hedonic ratings of textures
Thermal Sensation Detection
Separate models were run for warm and cool detection. For cool detection, there were no significant main effects and no interactions between any of the independent variables. Cool was perceived when skin temperature fell 1.51°C, on average. For warm detection, there was a highly significant effect of site (F = 109.30, p < 0.0001), with lower thresholds on the thenar palm (1.61°C, on average) than the forearm (2.91°C), and a significant interaction between site and session (F = 6.56, p = 0.0113). No significant group differences emerged for either thermal detection task; these comparisons are depicted in Fig. 6.
Fig. 6.
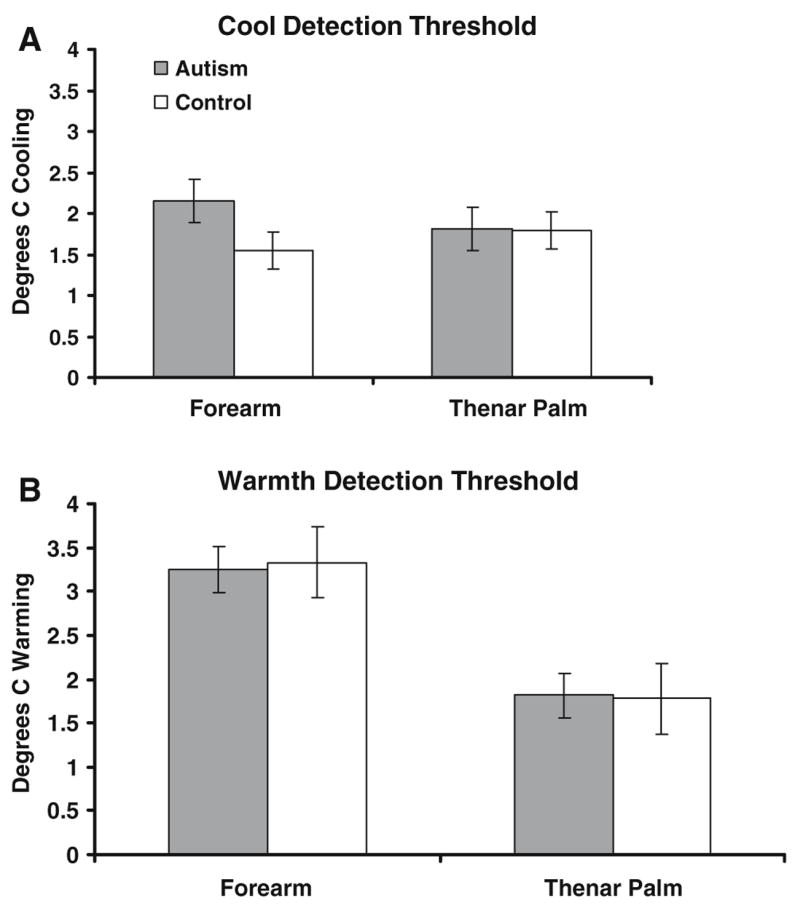
Group comparison on thermal detection thresholds. a Cool detection. b Warmth detection
Thermal Pain Perception
As in the previous experiment, separate models were run for heat and cold pain. For cold pain, there was a main effect of site (F = 5.12, p = 0.0250), and group (F = 3.84, p = 0.0518), with the autism group displaying average cold pain thresholds of 16.68°C, compared to the control group average of 9.04°C (see Fig. 7A, illustrating significantly different group averages separated by site). For heat pain, there was a significant effect of group (F = 6.79, p < 0.01), and site (F = 7.37, p = 0.0073), and a significant group × session interaction (F = 8.18, p = 0.0048). The group difference is depicted in Fig. 7B; the average threshold for the autism group was 43.66°C, while that of the control group was 46.58°C. The thresholds were lower overall on the thenar palm than on the forearm, and the interaction effect reflected that the autism group had higher thresholds (by 1.86°C, on average) on the second day of testing as compared to the first, while the thresholds for the control group remained relatively stable over the 2 days of testing (see Fig. 8).
Fig. 7.
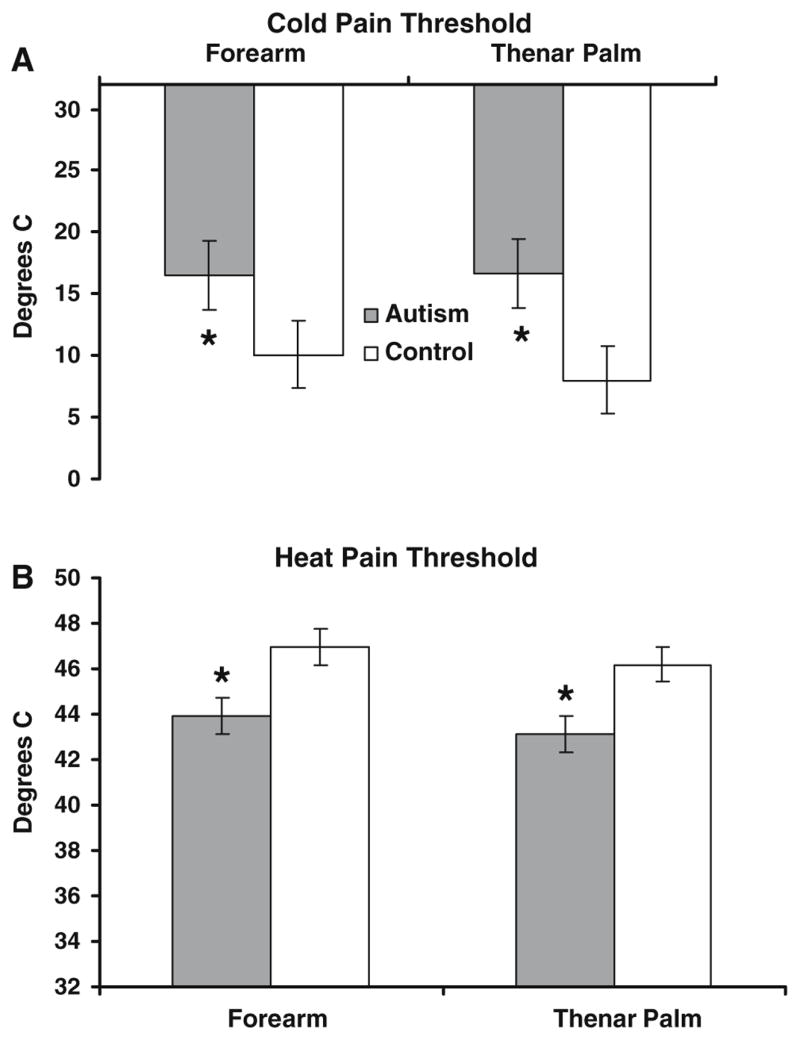
Group comparison on thermal pain thresholds. (A) Cold pain. (B) Heat pain. Asterisks denote statistically significant group differences at p = 0.05
Fig. 8.
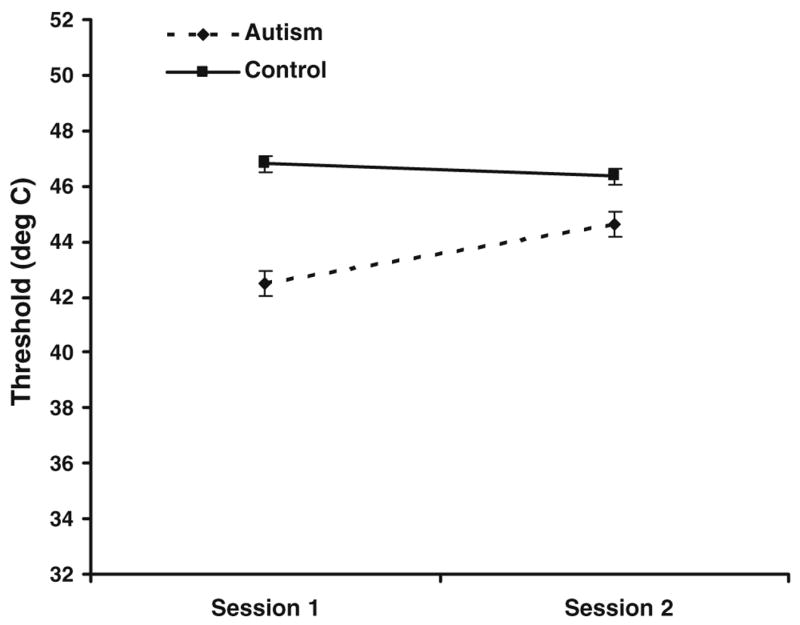
Heat pain thresholds vary by session for autism but not control group
Discussion
As predicted, thresholds on several psychophysical indices of tactile sensitivity were comparable between adults with autism and controls. Our finding that the von Frey tactile thresholds were unaltered compared with controls confirms the findings of O’Riordan and Passetti (2006), converging on the conclusion that the perceptual phenomenon of altered tactile sensitivity in autism as reported in the clinical literature, is not attributable to a difference in sensitivity to detecting light pressure against the skin as measured experimentally.
Our investigation of possible differences in neural adaptation to a suprathreshold vibrotactile stimulus also revealed no differences between our experimental group and controls. As is clear from Fig. 3B, the autism group shows an adaptation-related elevation in threshold during the second block of trials, and then returns to the baseline threshold, as do controls. While it has long been demonstrated that peripheral afferents demonstrate fatigue in response to vibration exposure (Lundstrom & Johansson, 1986), the adaptation tapped by this protocol is likely to reflect both peripheral and central neuronal activity (Bensmaia, Leung, Hsiao, & Johnson, 2005; Goble & Hollins, 1993; Whitsel et al., 2003). Thus, failure of central neurons to adapt to somatosensory stimulation does not appear to account for reports of tactile hypersensitivity in autism.
For detection of the 33 Hz vibrotactile stimulus used in Experiment 2, we replicated the result of Blakemore et al. (2006) at a similar frequency by demonstrating no group differences in thresholds on the palm at low frequencies of vibration. While we were encouraged by this consistency, it was not our explicit goal to replicate the entire Blakemore et al. study, thus we did not test the higher frequency vibration at which they noted group differences. However, at the forearm site, our group of adults with autism exhibited lower thresholds than the control group, indicating greater vibrotactile sensitivity, in spite of Experiment 1’s result that light touch sensitivity is similar in both groups regardless of testing site (see paragraph above). Rapidly adapting mechanoreceptive afferents are vigorously activated by vibration but not the nylon filaments; thus the disparity could reflect differences in this system. There is also evidence that individuals with autism process dynamic sensory information differently (Milne et al., 2002), particularly when it is higher in complexity than comparison stimuli (Bertone, Mottron, Jelenic, & Faubert, 2003), as is the case with vibrotactile as compared to light touch stimuli.
Since the hairy skin of the forearm is more often stimulated in the context of affiliative touch than the glabrous skin of the palm, which is specialized for fine haptic discrimination, this is an important finding to further characterize in autism. The forearm site was chosen in part because of its innervation by CT afferents, which is posited to constitute a social or affiliative touch system (Olausson et al., 2002; Vallbo et al., 1999; Wessberg et al., 2003). However, it is unclear whether the effect seen in the present study can be ascribed to CT afferents, since (a) myelinated mechanoreceptive afferents also innervate the forearm and underlie discriminative sensitivity, and (b) CT afferents do not respond optimally to vibrotactile stimuli, instead responding preferentially to slow, stroking stimuli such as the textures used in Experiment 3.
Contrary to our hypothesis, in Experiment 3 we did not find a significant effect of group or a significant interaction between group and site on the pleasantness ratings of textures. This argues against a role for CT afferents in tactile hypersensitivity in autism, and needs to be reconciled with the findings in Experiment 2. We did note a small, nonsignificant, but consistent trend for individuals with autism to rate each of the textures overall as more pleasant than controls (see Fig. 4). If verified by further investigation, this effect may provide insights into some of the sensory seeking behaviors that are characteristic of some individuals with autism. Idiosyncratic sensory stimuli (such as a flickering light or the texture of a certain material) may be unusually rewarding, and thus an individual with autism may pursue these sensory experiences repeatedly or fixate upon them to the neglect of other events in the environment. It would be of interest to uncover neural mechanisms underlying sensory seeking behaviors, which are often disruptive to the daily lives of individuals with autism and their families. Furthermore, utilizing sensory rewards (i.e., stimuli perceived to be particularly pleasant) may be useful for practitioners planning individualized interventions for persons with autism.
A limitation of Experiment 3 is in the nonsocial nature of the texture stimuli used. The perceptual and neural basis of social (interpersonal) touch is a current area of investigation; future studies should incorporate methodologies that specifically target the social context in which tactile hypersensitivity seems to occur most often in autism (Baranek, 1999; Grandin, 2000).
While no group differences were apparent in the detection of innocuous thermal stimuli (Fig. 6), there were significant group differences in thresholds for both heat and cold painful stimuli, as hypothesized (Fig. 7). In both cases, the autism group showed a greater degree of pain sensitivity, that is, their cold and heat pain thresholds were lower relative to the control group (i.e., cold pain was perceived at higher temperatures and heat pain at lower temperatures). In addition, sensitivity to heat pain also exhibited an interaction between group and session. The autism group was more sensitive to heat pain during the first session compared to the second session, while the average threshold of the control group remained stable over the two sessions (Fig. 8). Individuals with autism may have been more anxious about the unknown element of the heat pain stimulus, responding prematurely in the first session. Familiarity with the stimuli in the second session may have alleviated this anxiety in the second session, while controls may not have experienced this differential anxiety across sessions. However, it is unlikely that our results can be purely attributed to anxiety, as there was an overall group effect. In addition, there was a significant difference in cold pain sensitivity between the two groups, without an interaction between group and session, indicating that both cognitive and perceptual effects mediated hypersensitivity to thermal pain in the autism group.
In summary, this study demonstrates that high-functioning individuals with autism display normal tactile perception in light touch sensitivity, vibrotactile detection on the palm, vibrotactile adaptation, and innocuous thermal sensation, but significantly increased sensitivity to noxious thermal stimulation, and also to low-frequency vibration on the forearm. In addition, persons with autism tended to rate textures as more pleasant than controls. The group differences reflect the possibility of C-fiber involvement, but the study is limited by a relatively small sample size, and thus it is possible that negative findings partially reflect a lack of power. One cannot rule out the possibility that the positive findings are due to chance given the large number of tests performed, but we find it unlikely for two reasons: (1) two of the three significant findings were for painful stimuli which would be surprising if due to chance, and (2) the magnitude of the third significant group difference (vibrotactile detection at the forearm) was very large (with the autism group’s average threshold 34% lower than controls) to be attributable to chance.
In addition, we considered the possibility that group averages might obscure differences if the autism group had thresholds that followed a bimodal distribution (i.e., a subset within the autism group with very low thresholds corresponding to hyperresponsiveness, and another with very high thresholds corresponding to hyporesponsiveness). However, as is evident from the figures, variation was similar between the two groups, and frequency histograms (not shown) of thresholds on each task were also similar. A much larger sample size would be needed to assess distributions of sensory thresholds in autism; this kind of study should be done in the future. The altered thresholds we note for pain and low-frequency vibration have implications for understanding neural mechanisms associated with sensory-perceptual phenomena reported in the autism literature, and may be important to research further. Future studies should explore the contexts in which altered pain and pleasantness somatosensory sensitivity is exhibited in autism, and investigate the neural basis of observed hypersensitivity in autism using techniques such as neuroimaging and electrophysiological recordings.
Although clear evidence for a role of CT afferents in tactile hypersensitivity in autism was not gained, these findings add to a growing literature describing normal and sometimes enhanced perceptual abilities in autism. While the CT afferent system presents an attractive hypothesis for altered tactile sensitivity in autism, it is clear that multiple sensory modalities are affected. Of the empirical studies of vision to date, many suggest that individuals with autism exhibit enhanced perception of certain stimulus properties, specifically local, circumscribed properties of a visual stimulus (Shah & Frith, 1983, 1993; Joliffe & Baron-Cohen, 1997; Mottron et al., 2003). An analogous finding in the auditory domain (Mottron et al., 2000) reveals that individuals with autism have an advantage for discriminating local changes in a musical melody (changes from note to note), but are similar to controls in processing more global melodic changes. Our finding of enhanced discriminative abilities for vibration applied to the forearm and enhanced affective sensitivity to thermal pain are consistent with this body of literature.
Touch input from parents is ubiquitous during normal parent–infant interactions (Stack & Muir, 1990), has significant effects on social attention within the infant–parent dyad (Gusella, Muir, & Tronick, 1988; Roggman & Woodson, 1989), and shapes infants’ affective and attentional responses in formative early social interaction (Stack & Muir, 1992). An early developmental abnormality in processing this information is likely to have far-reaching socio-cognitive effects throughout the lifespan. Alternatively, sensory abnormalities may be secondary to other symptoms of autism. The tendency toward social isolation may severely restrict somatosensory input in early development, leading to altered neural organization within somatosensory cortex. The immense capacity for experience-dependent plasticity of the somatosensory cortex has been demonstrated in both human (Merzenich & Jenkins, 1993) and nonhuman primates (Elbert, Pantev, Wienbruch, Rockstroh, & Taub, 1995; Sterr et al., 1998).
Further psychophysical investigation of sensory abilities in autism is required to clarify the roles of sensation, perception and affect in autism. Another important step will be determining how to apply experimental methodology such as that employed in this study to younger people with autism, as sensory symptoms are known to change throughout development in autism (Kern et al., 2006). However mixed the experimental results may be at this point, altered sensitivity in autism is very real to those individuals who experience it, and often interferes with daily life activities. It is important to continue searching for the source of these hyper/hyposensitivities in order to inform development of efficacious environmental adaptations and other interventions to maximize coping, optimal functioning, and social participation for individuals with autism whose lives are affected by sensory sensitivities.
Acknowledgments
The authors thank the participants and their families, and the University of North Carolina Autism Research Registry. This work was partially supported by NIH grants R01-HD42168 (awarded to G.B.) and NS045685 (awarded to G.E.), funds available from F. McGlone (Unilever R&D Port Sunlight), and a grant from the Foundation of Hope for Research and Treatment in Mental Illness (awarded to K.P). Dr Pelphrey is supported by NIMH grant K01-MH01725, and a John Merck Scholar’s Award. Dr Cascio is supported by training grants T32-HD040127-04 (PI: Joseph Piven) and T32-MH019111-15 (PI: John Gilmore).
Contributor Information
Carissa Cascio, Center for Neurodevelopmental Disorders Research, University of North Carolina, Chapel Hill, NC 27599, USA.
Francis McGlone, Department of Neurological Sciences, University of Liverpool, Liverpool L69 3BXM, England.
Stephen Folger, Center for Neurosensory Disorders, University of North Carolina, Chapel Hill, NC 27599, USA; Department of Physical Therapy Education, Elon University, Elon, NC 27244, USA.
Vinay Tannan, Center for Neurosensory Disorders, University of North Carolina, Chapel Hill, NC 27599, USA.
Grace Baranek, Center for Neurodevelopmental Disorders Research, University of North Carolina, Chapel Hill, NC 27599, USA.
Kevin A. Pelphrey, Department of Psychology and Neuroscience, Duke University, Durham, NC 27708, USA
Gregory Essick, Center for Neurosensory Disorders, University of North Carolina, Chapel Hill, NC 27599, USA.
References
- American Psychiatric Association. DSM-IV-TR diagnostic and statistical manual of mental disorders. 4. Washington, DC, USA: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WD, Watson LR. Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Leung YY, Hsiao SS, Johnson KO. Vibratory adaptation of cutaneous mechanoreceptive afferents. Journal of Neurophysiology. 2005;94:3023–3036. doi: 10.1152/jn.00002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: A “complex” issue. Journal of Cognitive Neuroscience. 2003;15:218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassoli T, Calo S, Thomas RM, Catmur C, Frith U, et al. Tactile sensitivity in Asperger syndrome. Brain and Cognition. 2006;61:5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Dunn W. The sensations of everyday life: Empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy. 2001;55:608–620. doi: 10.5014/ajot.55.6.608. [DOI] [PubMed] [Google Scholar]
- Dunn W, Myles BS, Orr S. Sensory processing issues associated with Asperger syndrome: A preliminary investigation. American Journal of Occupational Therapy. 2002;56:97–102. doi: 10.5014/ajot.56.1.97. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270(5234):305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Essick GK. Comprehensive clinical evaluations of perioral sensory function. Oral and Maxillofacial Surgery Clinics of North America. 1992;4:503–526. [Google Scholar]
- Fruhstorfer H, Lindblom U, Schmidt WG. Method for quantitative estimation of thermal thresholds in patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1976;39:1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble AK, Hollins M. Vibrotactile adaptation enhances amplitude discrimination. Journal of the Acoustical Society of America. 1993;93:418–424. doi: 10.1121/1.405621. [DOI] [PubMed] [Google Scholar]
- Grandin T. An inside view of autism. In: Schopler E, Mesibov GB, editors. High functioning individuals with autism. New York: Plenum; 1992. pp. 105–126. [Google Scholar]
- Grandin T. My experiences with visual thinking, sensory problems, and communication difficulties. 2000 Available online: http://www.autism.org/temple/visual/html.
- Gusella JL, Muir DW, Tronick EZ. The effect of manipulating maternal behavior during an interaction on 3- and 6-month-olds; affect and attention. Child Development. 1988;59:1111–1124. doi: 10.1111/j.1467-8624.1988.tb03264.x. [DOI] [PubMed] [Google Scholar]
- Hollins M, Sigurdsson A, Fillingim L, Goble AK. Vibrotactile threshold is elevated in temporomandibular disorders. Pain. 1996;67:89–96. doi: 10.1016/0304-3959(96)03083-7. [DOI] [PubMed] [Google Scholar]
- Iarocci G, McDonald J. Sensory integration and the perceptual experience of persons with autism. Journal of Autism and Developmental Disorders. 2006;36:77–90. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen S. Clinical neurophysiology and quantitative sensory testing in the investigation or orofacial pain and sensory function. Journal of Orofacial Pain. 2004;18:85–107. [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in the glabrous skin. Journal of Physiology (London) 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliffe T, Baron-Cohen S. Are people with autism or Asperger’s syndrome faster than normal on the embedded figures task? Journal of Child Psychology and Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Jones RP, Quigney C, Huws JC. First-hand accounts of sensory perceptual experiences in autism: A qualitative analysis. Journal of Intellectual and Developmental Disability. 2003;28:112–121. [Google Scholar]
- Kakuda N. Conduction velocity of low-threshold mechano-receptive afferent fibers in the glabrous and hairy skin of human hands measured with microneurography and spike-triggered averaging. Neuroscience Research. 1992;15:179–188. doi: 10.1016/0168-0102(92)90003-u. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, et al. The pattern of sensory processing abnormalities in autism. Autism. 2006;10:480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, Rutter M. Autism diagnostic interview-revised (ADI-R) Los Angeles: Western Psychological Corporation; 2003. [Google Scholar]
- Lord C, Rutter M, Dilavore P, Risi S. The autism diagnostic observation schedule (ADOS) Los Angeles: Western Psychological Corporation; 1999. [Google Scholar]
- Lundstrom R, Johansson RS. Acute impairment of the sensitivity of skin mechanoreceptive units caused by vibration exposure of the hand. Ergonomics. 1986;29(5):687–698. doi: 10.1080/00140138608968303. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. Journal of Hand Therapy. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman T, Yimiya N, Zelazo PR, editors. The development of autism: Perspectives from theory and research. New Jersey: Lawrence Erlbaum; 2001. [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Bellewill S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: Evidence from multiple paradigms. Journal of Child Psychology and Psychiatry. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Mottron L, Peretz I, Menard E. Local and global processing of music in high-functioning persons with autism: Beyond central coherence? Journal of Child Psychology and Psychiatry. 2000;41(8):1057–1065. [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Wallin BG, Starck G, Ekholm S, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Passetti F. Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders. 2006;36:665–675. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- Ragin YK. Masters thesis at the University of North Carolina, Chapel Hill. 2002. Development and testing of a psychophysical protocol for studies on the affective components of touch. [Google Scholar]
- Rogers SJ, Hepburn S, Wehner W. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33:633–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Roggman LA, Woodson R. Touch and gaze in parent-infant play. Poster presented at the Society for Research in Child Development Conference; Kansas City. 1989. [Google Scholar]
- Shah A, Frith U. An islet of ability in autism: A research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design test? Journal of Child Psychology and Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Stack DM, Muir DW. Tactile stimulation as a component of social interchange: New interpretations for the still-face effect. British Journal of Developmental Psychology. 1990;8:131–145. [Google Scholar]
- Stack DM, Muir DW. Adult tactile stimulation during face-to-face interactions modulates five-month-olds’ affect and attention. Child Development. 1992;63:1509–1525. [PubMed] [Google Scholar]
- Sterr A, Muller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. Journal of Neuroscience. 1998;18(11):4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. Journal of Neurophysiology. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Olausson H, Fernstrom KW, Vallbo AB. Receptive field properties of unmyelinated tactile afferents in the human skin. Journal of Neurophysiology. 2003;89:1567–1575. doi: 10.1152/jn.00256.2002. [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Kelly EF, Quibrera M, Tommerdahl M, Li Y, Favorov O, et al. Time-dependence of SI RA neuron response to cutaneous flutter stimulation. Somatosensory and Motor Research. 2003;20:45–69. doi: 10.1080/0899022031000083834. [DOI] [PubMed] [Google Scholar]
- Zwislocki J, Maire F, Feldman AS, Rubin H. On the effect of practice and motivation on the threshold of audibility. Journal of the Acoustical Society of America. 1958;30:254–262. [Google Scholar]