Abstract
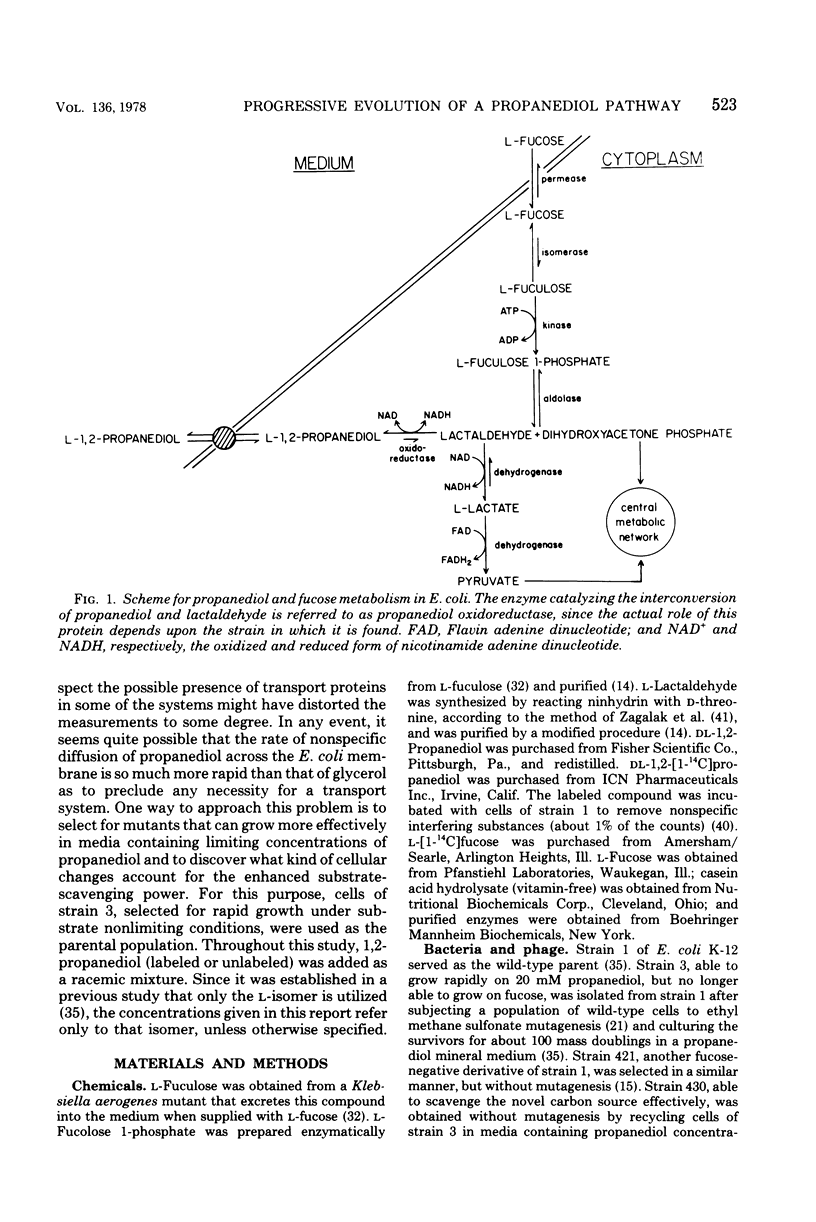
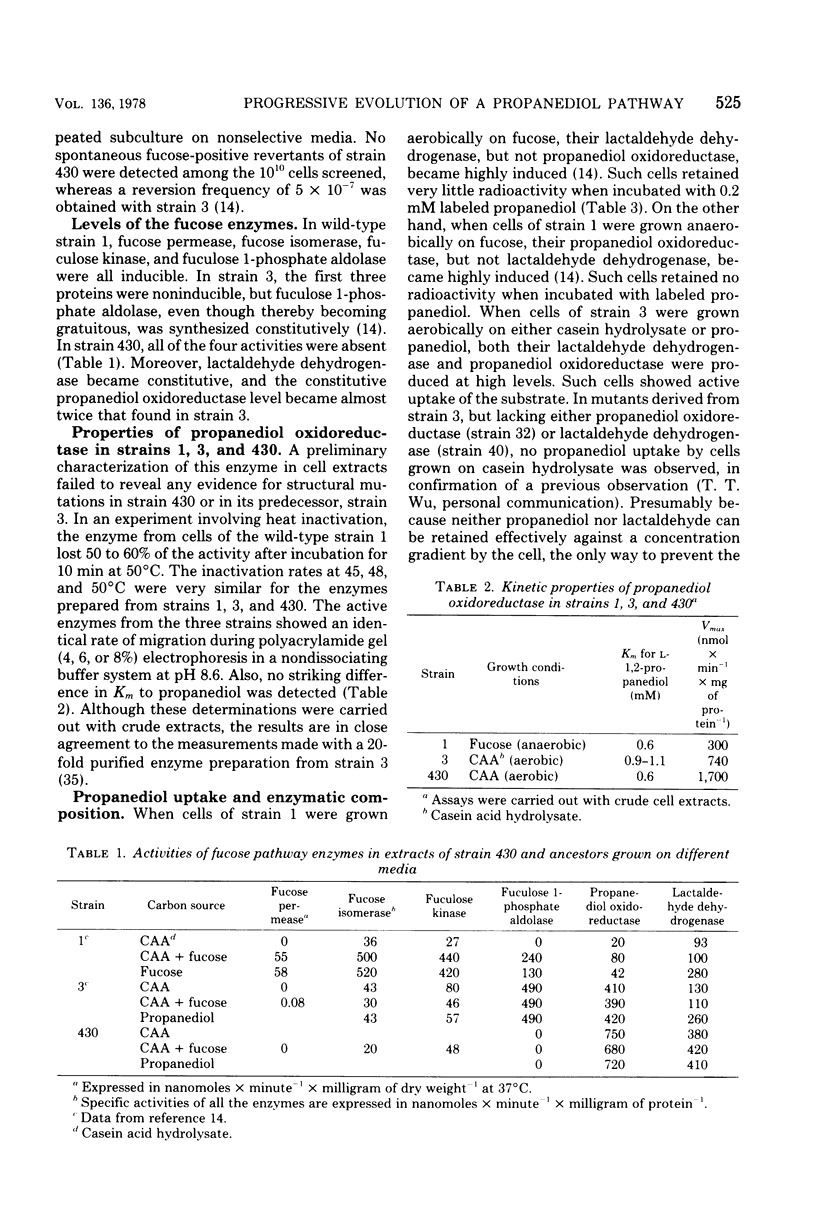
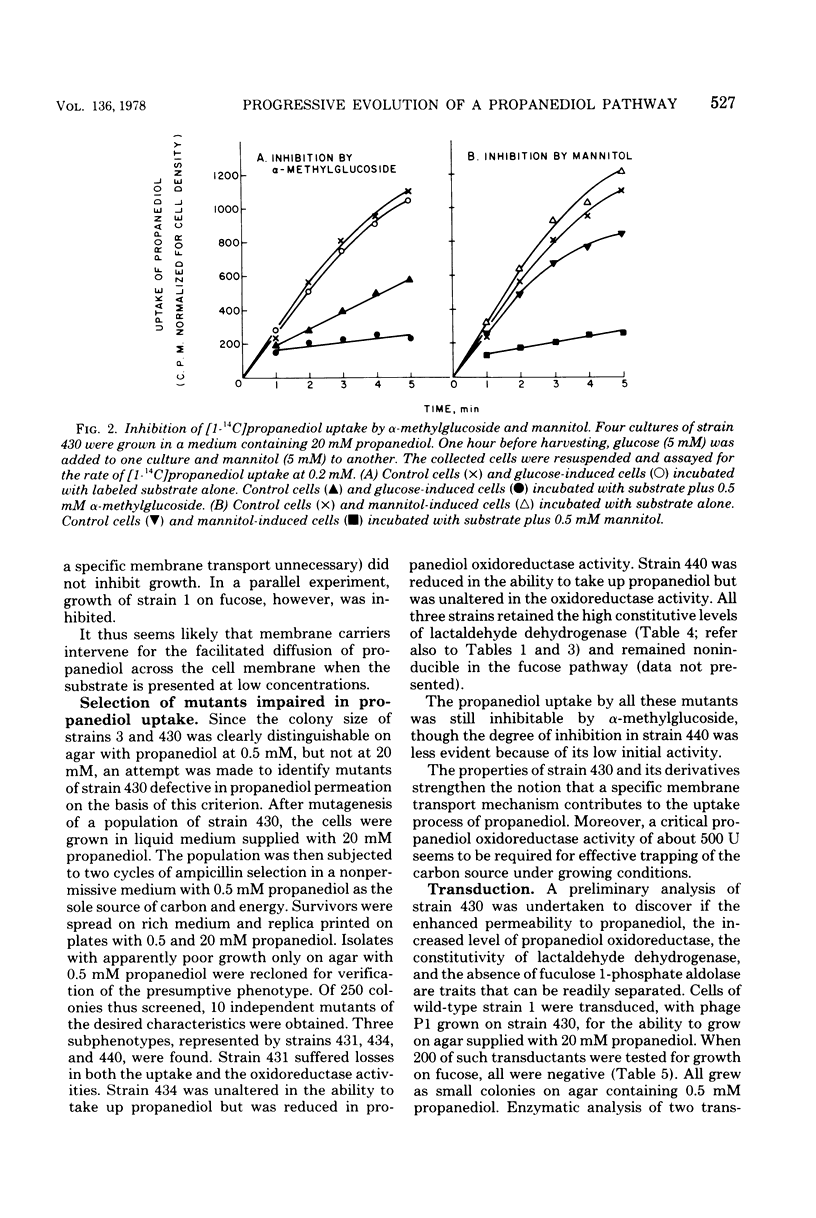
Wild-type strains of Escherichia coli are unable to use L-1,2-propanediol as a carbon and energy source. A series of mutants, able to grow on this compound at progressively faster rates, had been isolated by repeated transfers to a medium containing 20 mM L-1,2-propanediol. These strains synthesize at high constitutive levels a propanediolmicotinamide adenine dinucleotide oxidoreductase, an enzyme serving as a lactaldehyde during L-fucose fermentation by wild type cells. In this study, a mutant that can grow rapidly on the novel carbon source was subjected to further selection in a medium containing L-1,2-propanediol never exceeding 0.5 mM to obtain a derivative that has an increased power to extract the substrate from the medium. The emerging mutant exhibited four changes at the enzymatic level: (i) fuculose 1-phosphate aldolase activity is lost; (ii) the constitutive propanediol oxidoreductase activity is increased in its level; (iii) lactaldehyde dehydrogenase becomes constitutive and shows an elevated specific activity in crude extracts; and (iv) at low concentrations of propanediol, the facilitated diffusion across the cell membrane is enhanced. Changes two to four seem to act in concert in the trapping of propanediol by hastening its rate of entry and conversion to an ionized metabolite, lactate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemohammad M. M., Knowles C. J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974 May;82(1):125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- Boniface J., Koch A. L. The interaction between permeases as a tool to find their relationship on the membrane. Biochim Biophys Acta. 1967 Sep 9;135(4):756–770. doi: 10.1016/0005-2736(67)90107-1. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. On the importance of being ionized. Arch Biochem Biophys. 1958 Dec;78(2):497–509. doi: 10.1016/0003-9861(58)90374-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GOLDSTEIN D. A., SOLOMON A. K. Determination of equivalent pore radius for human red cells by osmotic pressure measurement. J Gen Physiol. 1960 Sep;44:1–17. doi: 10.1085/jgp.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Disruption of the fucose pathway as a consequence of genetic adaptation to propanediol as a carbon source in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1166–1172. doi: 10.1128/jb.126.3.1166-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Regulatory changes in the fucose system associated with the evolution of a catabolic pathway for propanediol in Escherichia coli. J Bacteriol. 1977 May;130(2):832–838. doi: 10.1128/jb.130.2.832-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- KEPES A. [Kinetic studies on galactoside permease of Escherichia coli]. Biochim Biophys Acta. 1960 May 6;40:70–84. doi: 10.1016/0006-3002(60)91316-0. [DOI] [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. The competitive inhibition of alpha-methylglucoside uptake in Escherichia coli. Biochem Biophys Res Commun. 1963 Mar 25;10:482–487. doi: 10.1016/0006-291x(63)90383-8. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Harada T. Active transport of alcohol in Corynebacterium acetophilum. J Bacteriol. 1974 Apr;118(1):149–154. doi: 10.1128/jb.118.1.149-154.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Burleigh B. D., Jr, Hartley B. S. Gene duplication in experimental enzyme evolution. Nature. 1974 Sep 20;251(5472):200–204. doi: 10.1038/251200a0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saint Martin E. J., Mortlock R. P. Natural and altered induction of the L-fucose catabolic enzymes in Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):91–97. doi: 10.1128/jb.127.1.91-97.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Solomon E., Miyal K., Lin E. C. Membrane translocation of mannitol in Escherichia coli without phosphorylation. J Bacteriol. 1973 May;114(2):723–728. doi: 10.1128/jb.114.2.723-728.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]
- Storelli C., Pesente L., Lippe C. Transporto di glicerolo e glicole propilenico attraverso la pelle isolata di Rana esculenta. Boll Soc Ital Biol Sper. 1971 Jan 15;47(1):1–3. [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]
- Wu T. T. Growth on D-arabitol of a mutant strain of Escherichia coli K12 using a novel dehydrogenase and enzymes related to L-1,2-propanediol and D-xylose metabolism. J Gen Microbiol. 1976 Jun;94(2):246–256. doi: 10.1099/00221287-94-2-246. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Lin E. C., Tanaka S. Mutants of Aerobacter aerogenes capable of utilizing xylitol as a novel carbon. J Bacteriol. 1968 Aug;96(2):447–456. doi: 10.1128/jb.96.2.447-456.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]



