Summary
Background
Dietary restriction (DR) is the most effective environmental intervention to extend lifespan in a wide range of species. However, the molecular mechanisms underlying the benefits of DR on longevity are still poorly characterized. AMP-activated protein kinase (AMPK) is activated by a decrease in energy levels, raising the possibility that AMPK might mediate lifespan extension by DR.
Results
Using a novel DR assay that we developed and validated in C. elegans, we find that AMPK is required for this DR method to extend lifespan and delay age-dependent decline. We find that AMPK exerts its effects in part via the FOXO transcription factor DAF-16. FOXO/DAF-16 is necessary for the beneficial effects of this DR method on lifespan. Expression of an active version of AMPK in worms increases stress resistance and extends longevity in a FOXO/DAF-16-dependent manner. Lastly, we find that AMPK activates FOXO/DAF-16-dependent transcription and phosphorylates FOXO/DAF-16 at previously unidentified sites, suggesting a possible direct mechanism of regulation of FOXO/DAF-16 by AMPK.
Conclusions
Our study shows that an energy-sensing AMPK-FOXO pathway mediates the lifespan extension induced by a novel method of dietary restriction in C. elegans.
Introduction
Dietary restriction (DR) – restriction in food intake without malnutrition – increases both the mean and maximal lifespan in a wide range of species [1]. The longevity effects of DR are conserved in worms, flies, mice, and possibly primates, underscoring the importance of this environmental intervention on lifespan. In humans, DR decreases the incidence of age-dependent diseases, including atherosclerosis and cancer [2, 3]. While numerous hypotheses have been proposed to explain the benefits of DR on longevity [4], the exact mechanisms by which DR increases lifespan are still unclear. DR decreases available nutrients [5], raising the possibility that nutrient sensing pathways may mediate DR’s beneficial effects on aging and age-dependent diseases.
The energy sensing AMP-activated protein kinase (AMPK) is an attractive candidate to mediate DR’s ability to prolong lifespan. AMPK is activated in response to decreased energy or glucose levels, which are consequences of DR [6], and in specific cases, by DR itself [7]. AMPK is a heterotrimeric protein kinase composed of a catalytic subunit (α) and two regulatory subunits (β and γ) that sense low cellular energy levels by monitoring changes in the AMP:ATP ratio [8]. AMP binding to the γ subunit induces a conformational change that allows the catalytic α subunit to be phosphorylated and activated by the AMPK-activating protein kinases LKB1 and CAMKKβ [9–13]. AMP also contributes to AMPK activation by reducing the dephosphorylation of the catalytic α subunit [14]. AMPK coordinates the adaptation of cellular metabolism and growth to decreased energy levels [6]. One of the two α catalytic subunits of AMPK (AMPKα2 or AAK-2) has recently been found to increase worm longevity [15]. However, AAK-2 is not necessary for longevity extension in response to a mutation in the eat-2 gene [16], a mutation that is thought to genetically mimic DR but might have pleiotropic effects. Therefore, whether AMPK mediates lifespan extension by DR is still unknown and the molecular mechanisms by which AMPK controls lifespan remain unclear.
FOXO transcription factors are good candidates to mediate AMPK’s ability to extend lifespan. These transcriptional regulators play an important conserved role in stress resistance by upregulating a series of target genes, including genes involved in detoxification of reactive oxygen species (ROS) [17–21]. FOXO factors are also activated in response to decreased activity of the insulin signaling pathway, an alternate nutrient sensing pathway in cells [22, 23]. In C. elegans, the FOXO transcription factor DAF-16 mediates the longevity extension due to a mutation in the insulin receptor daf-2 [17, 18]. Although the lifespan extension due to the eat-2 mutation and to several DR regimens has been shown to be independent of FOXO/DAF-16 [24–29], FOXO may still mediate lifespan extension under other DR experimental conditions.
Based on these observations, we hypothesized that AMPK and FOXO may be important mediators of the beneficial effects of DR on longevity. Using a novel method of DR in worms, we show that AMPK and FOXO/DAF-16 are necessary to mediate a large part of the lifespan extension in response to DR. In addition, we show that constitutively active AMPK increases worm stress resistance and longevity in a FOXO/DAF-16 dependent manner. Lastly, we find that FOXO/DAF-16 is a direct in vitro substrate of AMPK and that AMPK increases FOXO-dependent transcription. Together, these results place the AMPK-FOXO module as a central pathway in the regulation of lifespan by DR.
Results
Development of a new DR method, sDR, in the nematode C. elegans
We developed a new DR method in worms by transferring adult worms every two days onto freshly seeded plates with restrictive amounts of feeding bacteria (from 5×1011 to 5×108 bacteria/ml) at day 4 of adulthood (Figure 1A). We verified that worms ingested fewer bacteria under these conditions by using bacteria expressing the red fluorescent reporter dsRed (Figure 1B). This restriction in bacterial intake significantly increased worm lifespan (Figure 1C and Table S1A) and reduced worm mortality rate (Figure 1D), indicating that this regimen delays aging. Our regimen also increased resistance to the oxidative stress agent Paraquat (Figure 1E and Table S1B) and delayed the age-dependent decline in worm locomotor activity (Figure 1F), consistent with the reported ability of DR to delay aging and age-related phenotypes in a variety of organisms [1]. The increase in lifespan was not simply a result of reduced pathogenicity or toxicity of the bacteria because: i) an increase in bacterial concentration to 5×1012 bacteria/ml did not shorten worm lifespan (Figure 1C); ii) dilution of UV-killed bacteria also extended worm lifespan, albeit to a lesser extent (Figure 1G, Table S1C). Taken together, these results indicate that the food-restriction regimen that we have developed in C. elegans increases lifespan in a manner that is similar to DR in other species. In the rest of the manuscript, ad libitum will be 5×1011 bacteria/ml and dietary restriction will be 5×108 bacteria/ml. We will refer to this method as sDR for ‘solid DR’, as, contrary to other methods, the diluted bacteria are placed on solid plates.
Figure 1. sDR: a novel dietary restriction method in C. elegans.
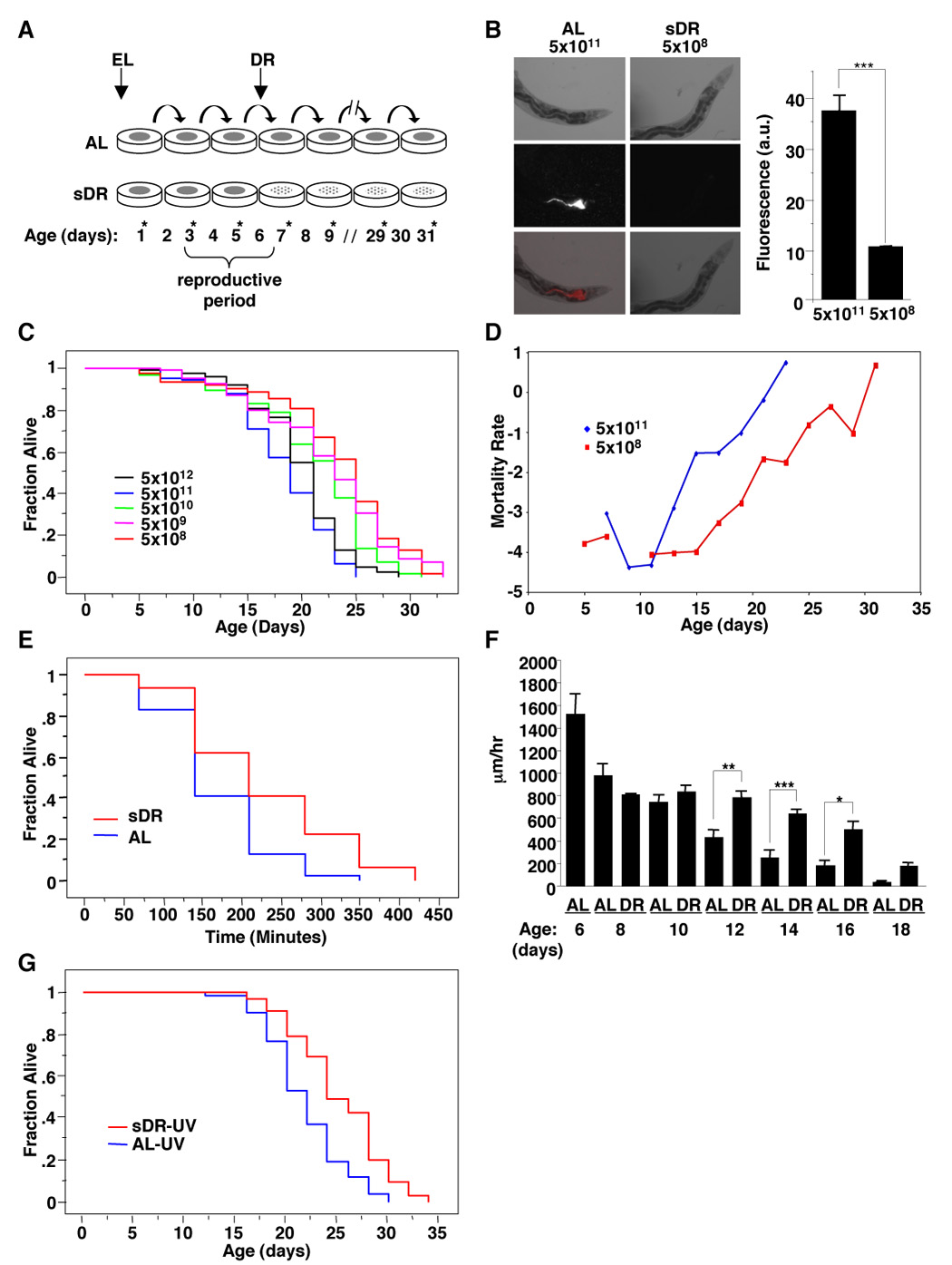
(A) Description of our dietary restriction method (sDR) in C. elegans. Populations of worms obtained by synchronized egg laying (EL) were scored and switched to freshly seeded plates every other day (*). sDR was initiated at day 4 of adulthood. (B) Representative pictures of worms fed different concentrations of bacteria expressing DsRed, a red fluorescent protein. Right panel: quantification of DsRed fluorescence of wild type (N2) worms incubated with different bacterial dilutions. Results represent the average and SEM of 3 independent experiments performed with 30 worms per condition at day 7. ***: p<0.001 in one way ANOVA. (C) Serial dilution of OP50-1 bacteria increases worm lifespan (5×108 bacteria/ml= 28.9% increase over 5×1011 bacteria/ml). Mean and standard errors for this experiment done in triplicate are presented in Table S1A. (D) Gompertz analysis shows that sDR decreases the worm mortality rate. (E) sDR treated worms displayed a 36% increase in survival compared with AL-fed worms at 200 mM Paraquat (p<0.0001). The mean and SEM for duplicate experiments done in sextuplicate is presented in Table S1B. (F) sDR delays the age-dependent decline in worm locomotor activity. Quantification of the length of worm tracks after one hour on fresh ad libitum lawns of OP50-1 bacteria was performed with Image J. Each point represents the average movement of at least 20 worms. *: p<0.05; **: p<0.01; ***: p<0.001 in one way ANOVA. (G) sDR induced by dilution of UV-killed bacteria results in a 15.9% increase in lifespan of N2 worms (statistically different from ad libitum fed worms with a p=0.0004). The mean and SEM of two independent experiments conducted in triplicate is presented in Table S1C.
sDR increases the AMP:ATP ratio and is dependent on AMPK
Since AMPK is activated by low energy levels, we asked if sDR’s ability to extend lifespan was mediated by AMPK in C. elegans. We first examined whether the sDR regimen affected energy levels in worms by measuring the AMP:ATP ratio in worm extracts using reverse phase high performance liquid chromatography (HPLC). We found that restricting the amount of seeded bacteria from 5×1011 bacteria/ml (ad libitum) to 5×108 bacteria/ml (sDR) at day 4 of adulthood significantly increased the AMP:ATP ratio in worms (Figure 2A). These results indicate that sDR leads to decreased energy levels in worms, as expected for a diet that restricts available nutrients.
Figure 2. AMPK is necessary for sDR’s beneficial effects on lifespan and age-dependent decline.
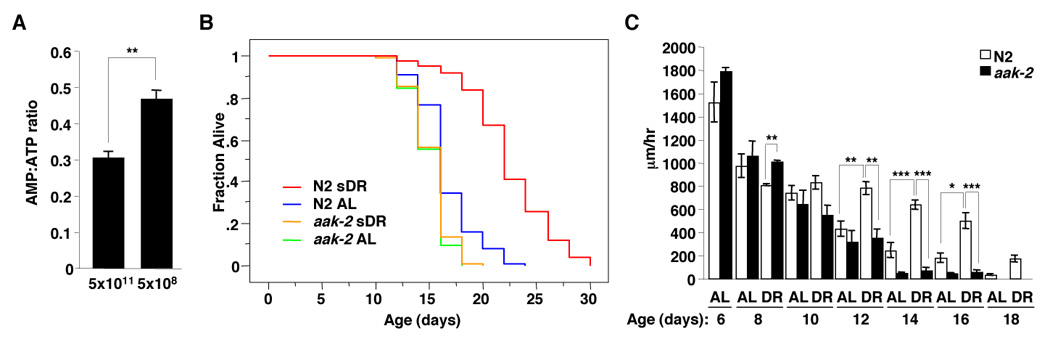
(A) The AMP:ATP ratio, measured by reverse phase HPLC, increases when worms are dietarily restricted at day 7. The results presented are the mean and SEM of two independent experiments of 5 samples each. **, p=0.0079 by Mann Whitney test. (B) AMPK mediates sDR’s ability to extend lifespan. aak-2 mutant worms displayed a mild decrease in lifespan compared to WT (N2) worms in ad libitum (AL) conditions (5×1011 bacteria/ml) (10.5%; p<0.0001). Under sDR conditions (5×108 bacteria/ml), aak-2 mutant worms had a 48% shorter lifespan compared to WT (N2) worms (p<0.0001). Comparison of five independent experiments performed in triplicate is presented in Table S2. (C) sDR delays the age dependent decline in locomotor activity in an aak-2 dependent manner. The experiment was performed as described in the legend of Figure 1F. *: p<0.05; **: p<0.01; ***: p<0.001 in one way ANOVA. Note that N2 worms are also represented in Figure 1F.
To determine if AMPK mediated the ability of sDR to extend lifespan in worms, we used a worm strain carrying a deletion in the gene encoding one of the catalytic subunits of AMPK (AMPK α2 or aak-2). The lifespan extension due to sDR was suppressed in the aak-2 mutant (Figure 2B and Table S2), indicating that AMPK is necessary for the lifespan extension due to sDR. In addition, aak-2 was also necessary for sDR to prevent the age-dependent decline in worm locomotor activity (Figure 2C). Together, these data show that AMPK is necessary for sDR to extend lifespan and to slow age-dependent decline.
The FOXO transcription factor DAF-16 mediates sDR-induced extension of lifespan
While FOXO transcription factors are attractive candidates to mediate the actions of environmental interventions controlling stress resistance and longevity, several alternate methods of DR in worms have been shown to be independent of FOXO/DAF-16 [24–29]. To test if DAF-16 played a role in lifespan extension in response to sDR, we assessed the lifespan of wild type or daf-16 mutant worms in ad libitum or food restriction conditions. We found that the ability of sDR to extend worm lifespan was diminished in daf-16 mutants (Figure 3A and Table S3A). These results indicate that DAF-16, like AMPK, mediates a portion of DR’s ability to extend lifespan. The ability of DR to delay the age-dependent decline in locomotor activity was also diminished in daf-16 mutant worms (Figure 3B), indicating that DAF-16 is necessary for the ability of DR to prevent the age-dependent decline in fitness.
Figure 3. FOXO/DAF-16 is necessary for sDR’s beneficial effects on lifespan and age-dependent decline.
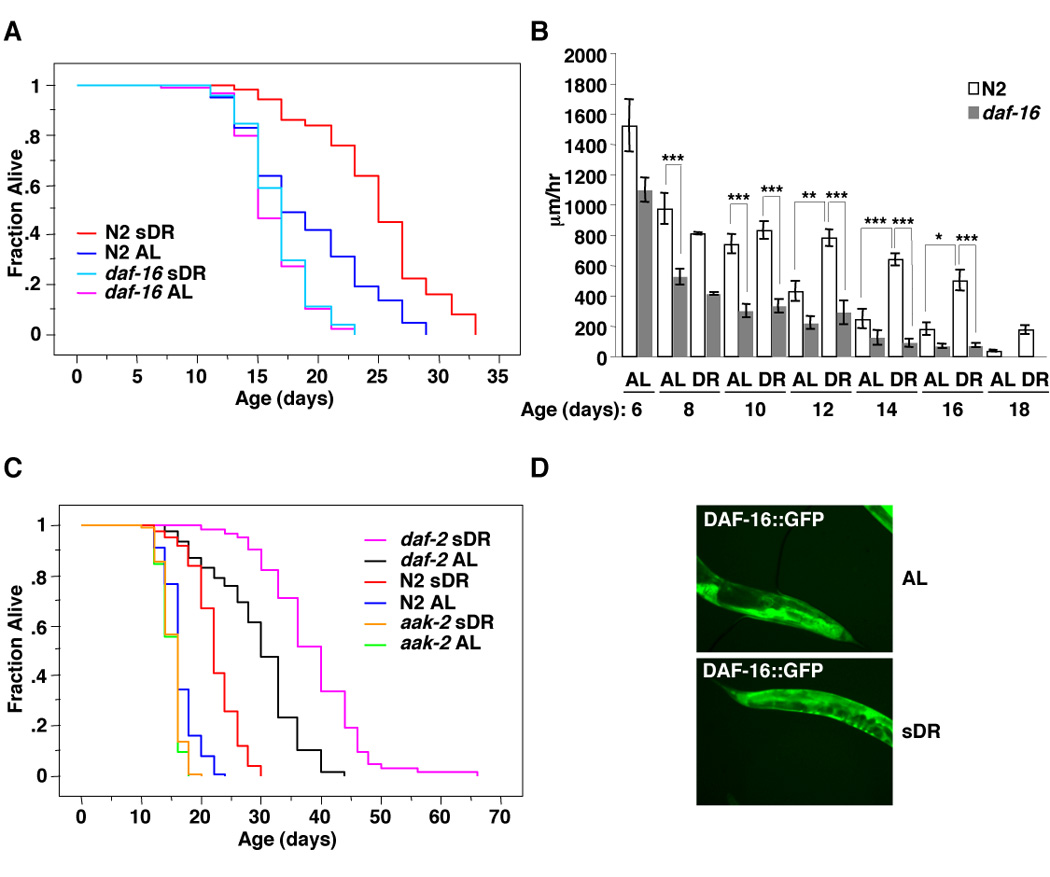
(A) DAF-16 is necessary for sDR to extend lifespan. sDR leads to a 30.6% increase (p<0.0001) in longevity in wild type (N2) worms but does not significantly affect the lifespan of daf-16 mutant worms. The mean and SEM of three independent experiments conducted in triplicates of 30 worms each is presented in Table S3A. (B) sDR delays the age dependent decline in locomotor activity in a daf-16 dependent manner. The experiment was performed as described in the legend of Figure 1F. *: p<0.05; **: p<0.01; ***: p<0.001 in one way ANOVA. Note that N2 worms are also represented in Figures 1F. (C) sDR is not dependent on the insulin receptor daf-2. sDR induced a 35.2% increase (p<0.0001) in wild type (N2) worms and a 31.0% increase (p<0.0001) in daf-2 mutant worms whereas sDR did not increase the lifespan of aak-2 mutant worms. The mean and SEM of two independent experiments conducted in triplicates of 30 worms each is presented in Table S3B. (D) sDR does not cause DAF-16 nuclear localization. DAF16:GFP expressing worms were placed on ad libitum or sDR diet for 6 hours at day 7 of life.
FOXO’s role in sDR-induced increase in lifespan may be dependent on the insulin signaling pathway, a well-known regulator of FOXO activity and a sensor of food levels [17, 18]. To test this possibility, we asked if the insulin receptor daf-2, also mediated the beneficial effects of sDR. In contrast to aak-2 and daf-16, the long lifespan of daf-2 insulin receptor mutants was further extended by sDR (Figure 3C and Table S3B), suggesting that sDR may regulate DAF-16 in a manner that is not dependent on the insulin receptor pathway. However, because the daf-2 mutation is not a null mutation it is still possible that part of the sDR-induced increase in lifespan might depend on the insulin receptor pathway.
Several known regulators of FOXO/DAF-16, including mutation in the insulin receptor or complete starvation, trigger the nuclear localization of FOXO/DAF-16 [30]. We therefore examined whether sDR triggers nuclear localization of DAF-16. Unlike starvation, sDR did not stimulate DAF-16 translocation to the nucleus (Figure 3D). Taken together, these results indicate that sDR regulates DAF-16 in a way that is distinct from starvation or mutation in the insulin receptor. These observations suggest that FOXO/DAF-16 may be regulated by AMPK in response to sDR.
FOXO/DAF-16 mediates the ability of AMPK to induce stress resistance and longevity in worms
AMPK function in worms was previously suggested to be independent of FOXO/DAF-16 [15], but the importance of FOXO as a mediator of AMPK action on lifespan has not been directly addressed. To test the possibility that FOXO/DAF-16 might mediate AMPK’s role in controlling lifespan, we generated transgenic worms expressing a constitutively active (CA) mutant of worm AMPK γ2 (R81Q). The corresponding mutation in the AMP binding pocket of AMPK γ2 in humans causes the constitutive activation of AMPK [31]. To determine if CA AMPK γ2 was sufficient to promote organismal resistance to oxidative stress, a phenotype tightly associated with increase in lifespan, we performed oxidative stress resistance assays, using the ROS generator Paraquat. Three independent CA AMPK γ2 transgenic worm lines (CA 1–3) were more resistant than control worm lines (EV 1–3) to increasing concentrations of Paraquat (Figure 4A), or to increasing times of treatment with Paraquat (Figure 4B and Table S4A). The stress protective effects of CA AMPK γ2 were reverted in worms in which daf-16 was knocked-down by RNAi (Figures 4C, 4D and Table S4A), indicating that the ability of this constitutive form of AMPK to induce stress resistance is DAF-16 dependent.
Figure 4. FOXO/DAF-16 is necessary for AMPK induced increase in stress resistance and lifespan.
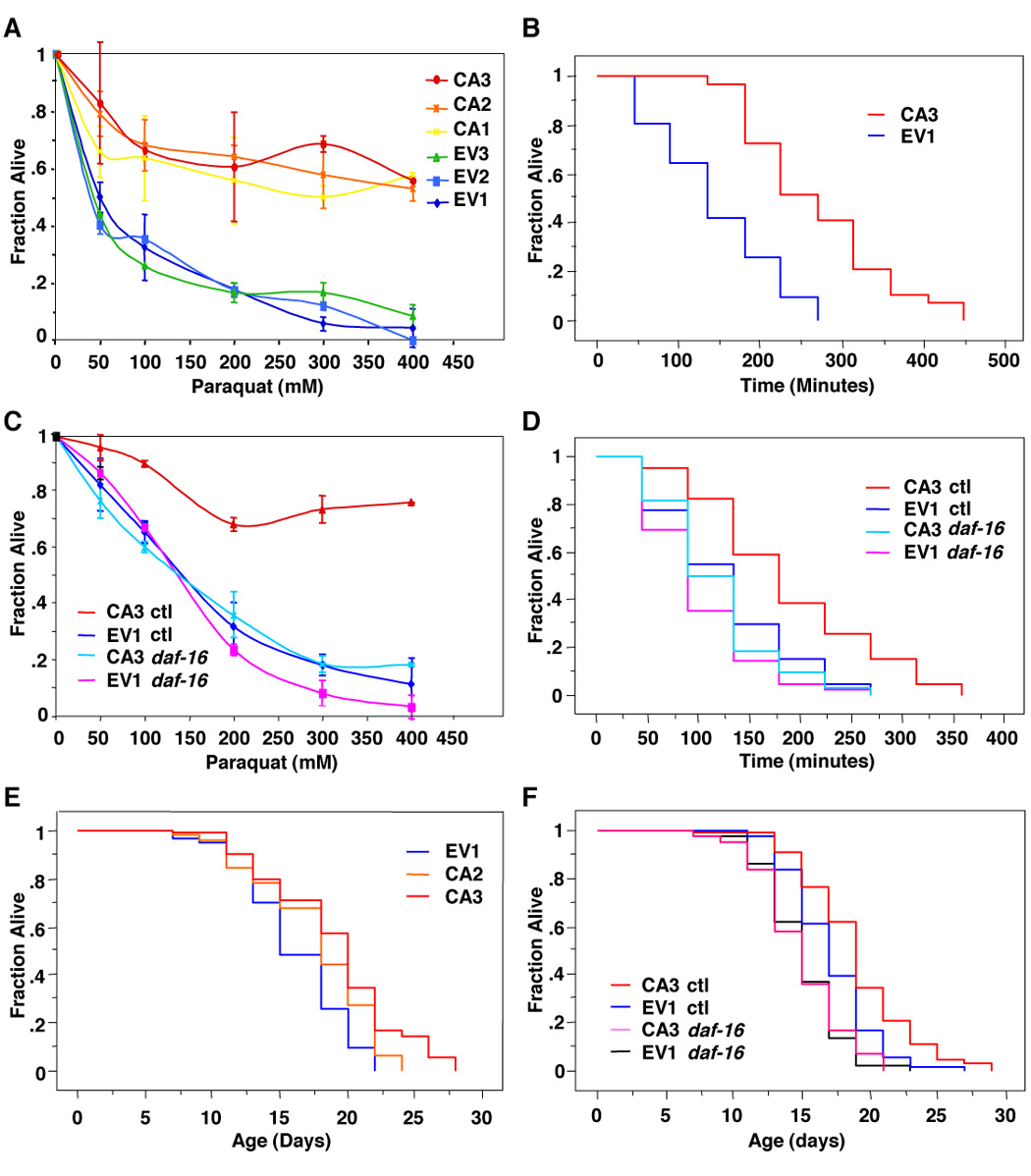
(A) Constitutively active (CA) AMPK γ2 transgenic worms display an increased resistance to increasing concentrations of the reactive oxygen species generator Paraquat compared to control transgenic worms injected with an empty vector (EV) after 7 hours of incubation. Three independent lines of EV (1–3) and CA (1–3) were analyzed. (B) CA3 displayed an 86.0% increased resistance to 300 mM Paraquat over control EV1 (p<0.0001). The mean and SEM of two independent experiments conducted in sextuplicate is presented in Table S4A. (C) Stress resistance of CA AMPK γ2 transgenic worms is dependent on the presence of DAF-16. CA3 worms were fed bacteria expressing RNAi to daf-16 or control bacteria (ctl) and submitted to increased concentrations of Paraquat for 8 hours. (D) CA3 displayed a 48.9% increased stress resistance compared to EV1 (p=0.0005) but did not significantly increase stress resistance when treated with RNAi to daf-16. The mean and SEM of two independent experiments conducted in sextuplicate is presented in Table S4A. (E) CA AMPK γ2 transgenic worms (CA2 and CA3) displayed a statistically significant increase in median lifespan (9.5% and 17.7% respectively, p<0.001) compared to EV1 control worms. A comparison experiment done in triplicate is presented in Table S4B. (F) CA AMPK γ2 lifespan extension is DAF-16 dependent. CA AMPK γ2 transgenic worms (CA3) show a statistically significant increase in median lifespan (11%; p=0.0006) compared to control transgenic worms (EV1) but did not display a significantly lifespan extension compared to EV1 when treated with RNAi to DAF-16. Comparison of two independent experiments done in triplicate is presented in Table S4B.
To examine whether DAF-16 was also required for AMPK’s ability to regulate lifespan, we examined the lifespan of CA AMPK γ2 transgenic worms in the absence or presence of daf-16. Consistent with the previous finding that AAK-2 (AMPK α2) overexpression slightly extends worm lifespan [15], two independent CA AMPK γ2 transgenic lines (CA2 and CA3) displayed a modest, but statistically significant, increase in lifespan (Figure 4E and Table S4B). The increased longevity of CA AMPK γ2 transgenic worms (CA3) was reverted when DAF-16 expression was inhibited by RNAi (Figure 4F and Table S4B), indicating that AMPK’s ability to extend lifespan requires DAF-16. Together, these results show that the worm FOXO factor DAF-16 is necessary to mediate the effects of AMPK on oxidative stress resistance and longevity.
AMPK activates FOXO/DAF-16-dependent transcription
To gain insight into the mechanism of AMPK’s action on FOXO/DAF-16 transcription factor, we examined the effect of AMPK activity on the expression of sod-3, a known DAF-16 target gene involved in both stress resistance and longevity [20, 32]. Using quantitative RT-PCR, we found that sod-3 mRNA was increased in worms expressing the constitutively active form of AMPK (CA3) compared to control worms (EV1) and that this increase was dependent on the expression of DAF-16 (Figure 5A). Consistent with this observation, sod-3 mRNA expression was also decreased in AMPK α2 (aak-2) mutant worms compared to wild-type worms (Figure 5B). These findings support the notion that AMPK enhances the ability of DAF-16 to transactivate sod-3.
Figure 5. AMPK activates FOXO/DAF-16 dependent transcription of sod-3.
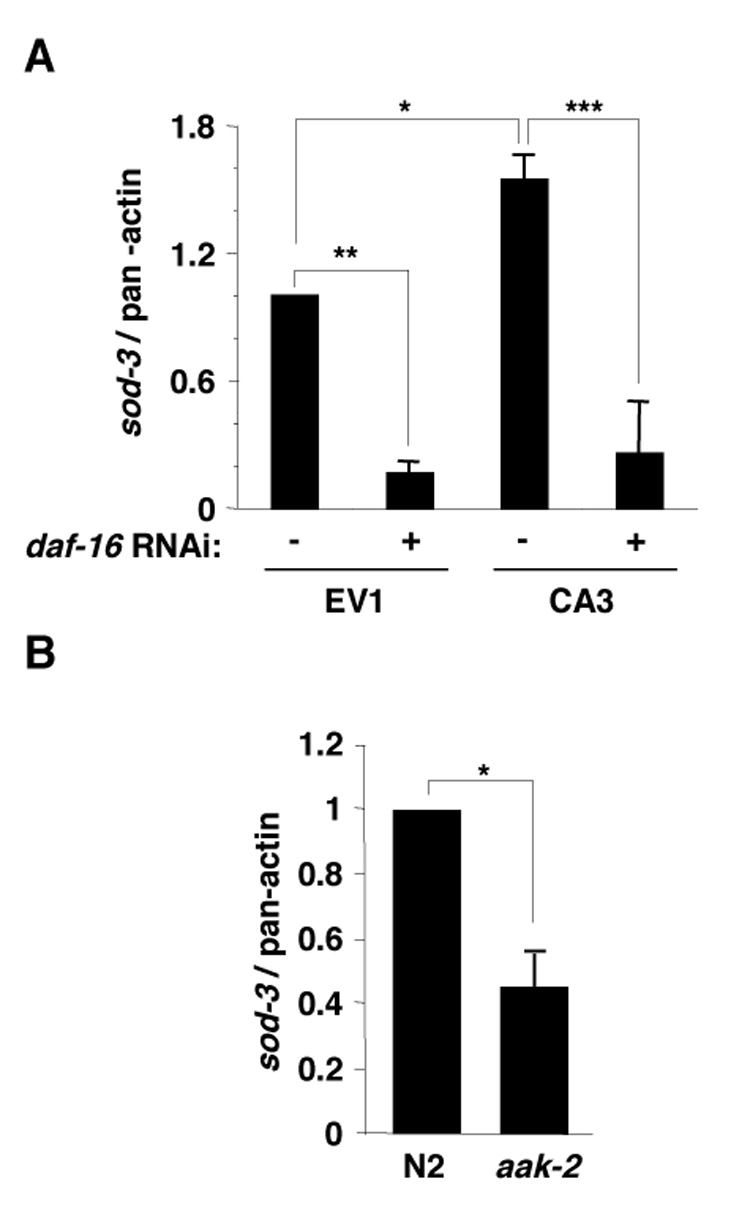
(A) sod-3 mRNA is increased in CA AMPK γ2 transgenic worms (CA3) compared with empty vector control transgenic line (EV1) and this increase is daf-16 dependent. The results presented correspond to the mean and SEM of three independent quantitative RT-PCR experiments. *: p<0.05; **: p<0.01; ***: p<0.001 in a one-way ANOVA statistical test. (B) sod-3 was decreased in aak-2 mutant worms compared with N2 wild type worms. The results presented correspond to the mean and SEM of two independent experiments: *: p<0.05 in a paired t-test.
AMPK directly phosphorylates DAF-16 in vitro
AMPK could regulate FOXO/DAF-16 activity indirectly, via the insulin receptor, or directly, via phosphorylation. Given that sDR’s beneficial effects on longevity appear to be independent of the insulin receptor, we asked if AMPK could directly phosphorylate FOXO/DAF-16. To this end, we performed in vitro kinase assays with purified AMPK and a bacterially expressed form of DAF-16 as a substrate. We found that both worm and human AMPK phosphorylated worm DAF-16 (Figure 6A). DAF-16 phosphorylation by AMPK was greatly enhanced by the presence of AMP (Figure 6A), confirming that the purified kinase responsible for DAF-16 phosphorylation is AMP-dependent. The stoichiometry of phosphorylation of DAF-16 by human and worm AMPK was higher than that of the SAMS peptide, a peptide containing a known AMPK phosphorylation site [13] (Figure 6B), suggesting that DAF-16 is a good in vitro substrate of AMPK.
Figure 6. AMPK directly phosphorylates FOXO/DAF-16 in vitro.
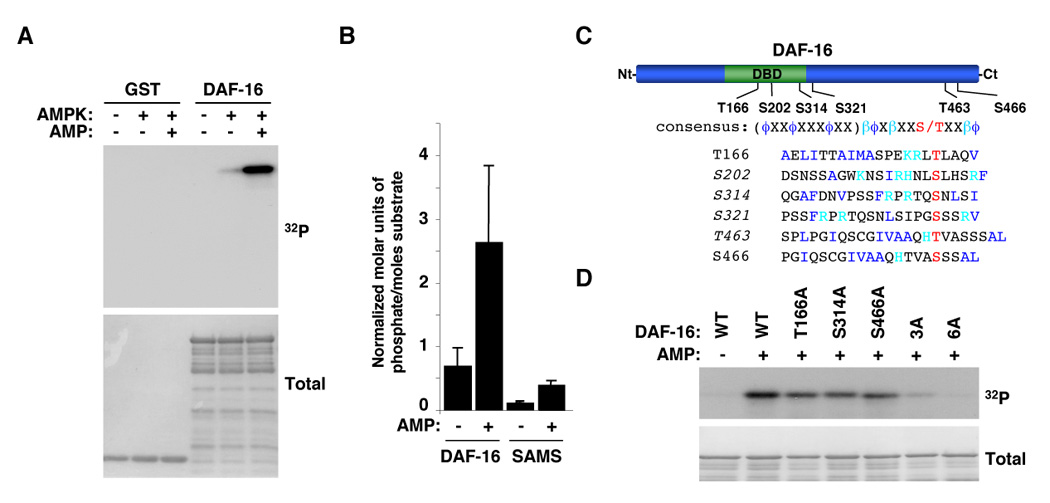
(A) AMPK phosphorylates DAF-16 in an AMP-dependent manner. Results with human AMPK are presented but both human and worm AMPK phosphorylate DAF-16 in an AMP-dependent manner. (B) Stoichiometry of phosphorylation of DAF-16 by human AMPK. The graph represents the mean and standard deviation of normalized molar units of phosphate incorporated per mole of substrate of 3 independent experiments. Similar results were obtained with the worm AMPK. (C) Schematic of DAF-16 showing the location of the residues of DAF-16 phosphorylated by AMPK in vitro. DBD: DNA binding domain. Alignment of the AMPK consensus phosphorylation motif with phosphorylation sites in DAF-16. ϕ: hydrophobic residues; β: basic residues. The sequence in parenthesis represents an amphipathic helix. The sites in italics were identified as being phosphorylated in vitro by AMPK by tandem mass spectrometry. All the sites listed here were confirmed as being phosphorylated in vitro by AMPK by site-directed mutagenesis. (D) DAF-16 mutants display a significant reduction in phosphorylation by AMPK compared to WT DAF-16 in an in vitro kinase assay. 3A: T166A/S314A/S466A mutant; 6A: T166A/S202A/S314A/S321A/T463A/S466A.
We sought to determine the residues of FOXO/DAF-16 that were phosphorylated by AMPK. Using a combination of tandem mass spectrometry and site-directed mutagenesis, we found that AMPK phosphorylated DAF-16 in vitro at least at six residues -- T166, S202, S314, S321, T463, and S466 -- that conform to varying degrees to the consensus motif phosphorylated by AMPK (Amphipathic helix-βϕXβXXS/TXXβϕ; ϕ: hydrophobic residues; β: basic residues) [33] (Figure 6C). The concomitant mutation of T166, S314, and S466 significantly reduced the phosphorylation of DAF-16 by AMPK, indicating that these three sites are the main residues phosphorylated by AMPK in vitro (Figure 6D). The replacement of all six residues (T166, S202, S314, S321, T463, and S466) by alanine further reduced the phosphorylation of DAF-16 by AMPK, suggesting that S202, S321, and T463 are also phosphorylated by AMPK (Figure 6D). Taken together with our experiments on the effect of AMPK on sod-3, these findings suggest that AMPK could activate FOXO/DAF-16-dependent transcription via direct phosphorylation at these previously unidentified sites. However, our results do not exclude the possibility that AMPK may also regulate FOXO/DAF-16 indirectly. We have recently found that AMPK phosphorylates the human FOXO transcription factor FOXO3 at multiple sites in vitro and in cells (ELG and AB, unpublished data), raising the possibility that the direct connection between AMPK and FOXO may be conserved throughout evolution.
Discussion
sDR: a novel method of DR that extends lifespan in worms
We have developed a novel method of dietary restriction in C. elegans that we termed sDR. sDR has hallmarks of dietary restriction in many species in that it: i) reduces food intake; ii) reproducibly extends lifespan; iii) delays the age-dependent decline in physical fitness; iv) induces resistance to oxidative stress. Another well-known hallmark of dietary restriction is a reduction in fertility. sDR is initiated at day 4 of adulthood, which is past the reproductive period in worms. We found that if sDR was initiated earlier, it indeed reduced fertility, but also had deleterious effects on survival (ELG and AB, unpublished data). The fact that sDR extends lifespan at a post-reproductive age suggests that sDR extends lifespan independently of changes in fertility.
The other methods that have been used in C. elegans to induce or mimic DR include the eat-2 mutation, which reduces pharyngeal pumping [29], the dilution of feeding bacteria in liquid culture (bDR or lDR) [27, 28, 34], the total absence of bacteria in liquid culture (axenic medium) [26], a chemically defined liquid media [35], the dilution of peptone in the plates [36], and the total absence of food on plates (DD for dietary deprivation) [24, 25] (Figure 7). Compared to these other methods, sDR is relatively simple to implement because it is done in the medium commonly used for worms in the laboratory and it can be performed with any mutant strain of worm. sDR is also similar to regimens used in other species, including mammals, in which DR is achieved by a reduction of the food normally provided to the animals.
Figure 7. Proposed model for the molecular networks integrating different DR methods.
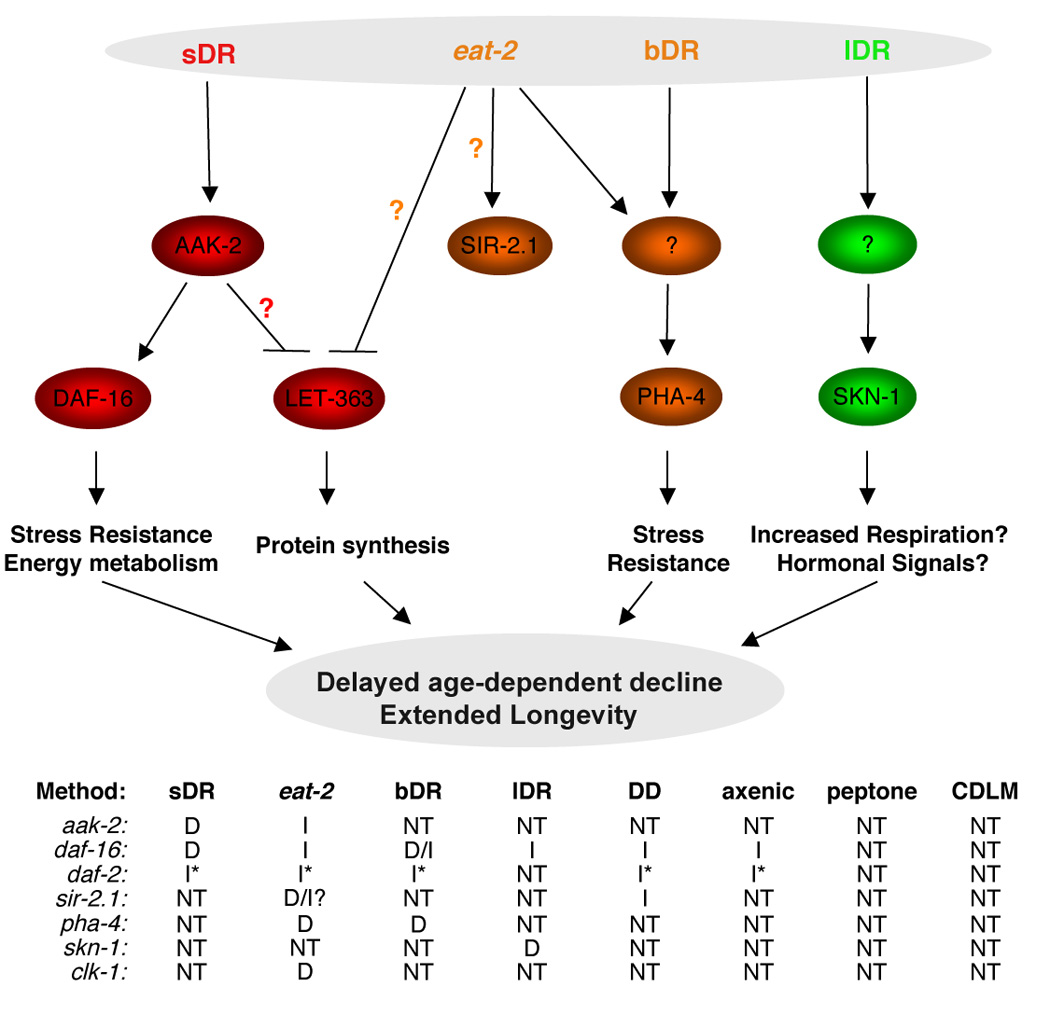
Different methods of dietary restriction may activate distinct signaling pathways or activate them to varying degrees. The table below the figure summarizes which pathways have been tested. Orange question marks indicate conflicting reports in the literature [41, 43, 50] and a red question mark indicates yet unconfirmed interactions in C. elegans. sDR: solid plate DR; bDR: liquid DR described in [28, 34]; lDR: liquid DR described in [27]; DD: dietary deprivation [24, 25]; axenic: absence of bacteria in liquid culture [26]; peptone: dilution of peptone in plates [36]; CDLM: chemically defined liquid media [35]. D: dependency; I: independency; NT: not tested. *: the daf-2 mutation is not a null mutation, which could affect the interpretation of the results. clk-1: mutant of a gene necessary for ubiquinone biosynthesis [29].
sDR reveals the importance of AMPK and FOXO in dietary restriction-induced longevity
Our findings suggest the following sequence of events: sDR activates AMPK by increasing the AMP:ATP ratio. Activated AMPK enhances FOXO/DAF-16-dependent transcription, perhaps via direct phosphorylation, leading to resistance to oxidative stress and longevity extension (Figure 7). The finding that sDR’s ability to extend lifespan is dependent on the presence of AMPK/AAK-2 and FOXO/DAF16 is in contrast to numerous studies in worms that indicate that aak-2 and daf-16 do not mediate the lifespan extension observed in eat-2 mutant [16, 29] and that daf-16 does not mediate the lifespan extension by IDR [27], axenic medium [26], DD [24, 25], or bDR, particularly when bacteria are diluted less than 10 fold from ad libitum conditions [26, 28]. However, our results are consistent with the observation that DR elicited by a chemically defined liquid media resulted in the upregulation of daf-16 and daf-16-dependent gene expression [35] and the observation that the lifespan extension by bDR when bacteria are diluted more than 10 fold from ad libitum conditions, shows a partial daf-16 dependency [26, 28].
One possible interpretation of these contradictory findings is that various methods of DR elicit distinct signaling pathways because they restrict food intake in different manners. For example, the degree of food restriction and whether food restriction is chronic or acute could influence the mechanisms underlying longevity caused by DR. eat-2 mutants, which have a germline mutation in the acetylcholine receptor that reduces their pharyngeal pumping rate, likely induce a form of DR that is chronic [37], whereas sDR, by diluting the bacteria 1000 fold at a specific adult stage, may elicit a more acute form of DR. Different DR methods may also alter proteins, carbohydrates, or lipids in different proportions, which may engage distinct mechanisms of nutrient sensing.
The differences in the mechanisms relaying sDR versus other liquid DR regimens may also be due to other parameters that differ between these two methods, including movements, energy expenditure, food sensing, oxygen levels, bacterial amounts, or osmotic conditions. Finally, some DR regimens may also extend lifespan by reducing some kind of food-related toxicity. However, sDR is unlikely to operate solely via reduced food toxicity, because an excess of bacteria is not detrimental for lifespan and because a dilution of UV-killed bacteria also extends lifespan. Dissecting the mechanisms by which different methods of DR extend lifespan and understanding the parameters that vary between the DR regimens will be important for designing efficient interventions to extend lifespan.
Molecular mechanisms mediating DR in worms
Interestingly, recent evidence indicates that the ability of eat-2 mutants and bDR to extend lifespan is mediated by a member of the Forkhead family, the FOXA/PHA-4 transcription factor [28]. IDR has also been recently shown to be sensed specifically in sensory neurons by the transcription factor SKN-1 [27]. Finally, the sir-2 gene has been shown to be important to mediate certain forms of DR in a variety of species from yeast to flies [38–41], though this observation is still subject to debate [24, 25, 42, 43]. SIR-2, an NAD-dependent protein deacetylase, may sense dietary restriction signals via alterations of the NAD:NADH ratio whereas AMPK may sense DR through changes in the AMP:ATP ratio. It will be interesting to determine whether SIR-2, PHA-4, and SKN-1 are important to mediate lifespan extension by sDR. Uncovering the genetic interactions between the SIR-2, the AMPK-FOXO, the FOXA, and the SKN-1 pathway will give important insight into the network of longevity regulation by DR (Figure 7).
AMPK increases lifespan and stress resistance via FOXO-dependent and FOXO-independent mechanisms
We have shown that AMPK regulates lifespan in a daf-16-dependent manner. However, Apfeld et al. reported that mutating one of the AMPK catalytic subunits, aak-2, in conjunction with mutating daf-16 decreased worm lifespan further than mutating each gene individually [15]. This experiment raises the possibility that DAF-16 may not be the sole contributor of AMPK’s effect on lifespan. AMPK is known to regulate the TOR pathway in mammals [44] and mTOR/LET-363 and S6 kinase mutations have been found to extend lifespan in yeast, worms, and flies [43, 45–48]. Thus, the mTOR-S6 kinase pathway may also mediate part of AMPK’s ability to extend lifespan (Figure 7). Changes in transcription (via FOXO) and translation (via TOR) may be both orchestrated by AMPK to allow the organism to adapt its longevity to low energy levels. Lastly, AMPK may also regulate the other proteins recently identified as important mediators of DR, including SIR-2, PHA-4, and SKN-1.
AMPK phosphorylation of DAF-16: a mechanism of regulation?
The direct phosphorylation of DAF-16 by AMPK may be one of the mechanisms by which DR regulates longevity in worms. DAF-16 is phosphorylated by AMPK at multiple novel sites that are located in the DNA binding domain and the C-terminal transactivation domain and these sites differ from AKT phosphorylation sites. Therefore, AMPK may regulate DAF-16 independently of its subcellular localization, consistent with our observation that sDR, which activates AMPK, does not stimulate DAF-16 nuclear translocation. Our experiments do not rule out however the possibility that AMPK regulates DAF-16 via an indirect mechanism or that AMPK and DAF-16 act in different tissues to regulate longevity in response to DR.
An AMPK-FOXO pathway in other species
Whether AMPK and FOXO play a role in DR-induced longevity in other species is not known. We have recently found that AMPK phosphorylates human FOXO3 in mammalian cells at novel regulatory sites that are distinct from the AKT sites (ELG and AB, unpublished data). Similar to our results in worms, AMPK also activates FOXO3-dependent transcription in mammalian cells (ELG and AB, unpublished data), raising the possibility that the mechanism of regulation of FOXO by AMPK may be conserved.
A number of compounds that activate AMPK, including metformin, phenformin, and resveratrol, have been proposed to act as potential “caloric restriction mimetics” in mammals [48–52]. It will be interesting to test whether these compounds affect lifespan in an AMPK-dependent manner. Understanding the mechanisms underlying the conserved extension of lifespan by DR will help harness the power of this intervention to slow aging and the onset of age-related diseases.
Experimental Procedures
Worm strains and RNA interference
N2 and daf-16 (mu86) strains were a kind gift from Dr. Man-Wah Tan. The zIs356 IV (TJ356) (daf-16:GFP strain) was a kind gift from Dr. Tom Johnson. The aak-2 (ok524) and daf-2 (e1370), strains were generously provided by Theresa Stiernagle at the Caenorhabditis Genetics Center. HT115 (DE3) bacteria transformed with vectors expressing dsRNA of the genes of interest were obtained from the Ahringer library (a gift from Dr. M.-W. Tan) and were grown at 37°C and seeded onto standard nematode growth medium (NGM) plates [49] containing ampicillin (100 µg/ml) and IPTG (0.4 mM). Adult worms were placed on these plates and removed after 4–6 hours to obtain synchronized populations of worms. Each vector was sequenced to verify the presence of the appropriate gene of interest.
Worm transgenic lines
The PRKAG2 (AMPKγ2) gene (WBGene00020633/WP:CE29350) and regulatory regions were amplified by PCR from wild type N2 worm genomic DNA and was sub-cloned into the L3691 pPD117.01 vector (a kind gift from Dr. Andy Fire). To generate a constitutively active mutant, we replaced Arg81 by Glu (R81Q mutant) [31] using site-directed mutagenesis. Purified plasmids of interest (the L3691 vector without the coding region of AMPKγ2, L3691 vector encoding WT AMPKγ2, or L3691 vector encoding the R81Q mutant of AMPKγ2) were co-injected with pha-1 encoding plasmid into pha-1 mutant worms. Worms were placed at 24°C after injection and were allowed to lay eggs to rescue the pha-1 phenotype. Individual F1 strains were selected from various injections and were clonally expanded. Incorporation of extrachromosomal arrays was verified by PCR of genomic DNA isolated from worms.
Lifespan in C. elegans
Worm lifespan assays were performed at 20°C. Worm populations were synchronized by placing young adult worms on NGM plates seeded with the E. coli strain OP50-1 (unless otherwise noted) for 4–6 hours and then removed. The hatching day was counted as day one for all lifespan measurements. Worms were changed every other day to new plates to eliminate confounding progeny, and were marked as dead or alive. Worms were scored as dead if they did not respond to repeated prods with a platinum pick. Worms were censored if they crawled off the plate or died from vulval bursting. For each lifespan assay, 90 worms were used in 3 plates (30 worms/plate). The data were plotted using the Kaplan-Meier Survival curves and statistical significance was determined by Logrank (Mantel-Cox) tests. Lifespan assays were repeated at least once and showed similar trends in relative lifespan effects. Representative Kaplan-Meier survival curves are shown in the figures. Means, SEM, and p values are shown in Tables S1–4.
Dietary restriction in C. elegans
OP50-1 bacteria were serially diluted from 5×1012 bacteria/ml to 5×104 bacteria/ml. Bacteria were resuspended in S Medium to inhibit bacterial growth. 150 µl of these solutions were plated on each 35 mm plate on the day of transfer. 30 adult worms were placed on each plate containing various concentrations of bacteria starting at day 7 of life (day 4 of adulthood). Worms were switched to fresh plates every other day. Worms placed on bacterial concentrations ranging from 5×104 bacteria/ml to 5×107 bacteria/ml died within two days after being placed on these extreme dietary restriction diets. Dietary restriction (sDR) was considered 5×108 bacteria/ml and ad libitum (AL) was 5×1011 bacteria/ml. OP50-1 grown to saturation overnight normally resulted in a concentration of bacteria of 5×1011 bacteria/ml. The lifespan assays were conducted in the absence of FUDR. For lifespan experiments using UV-killed bacteria, OP50-1 bacteria were UV-irradiated for four minutes in a UV Stratalinker 2400 (0.6 Joules). This type of UV treatment completely prevented bacterial growth in solid and liquid cultures.
AMP:ATP ratio quantification
100 day 7 hand-picked C. elegans were placed in ad libitum or sDR conditions for 12 hours under the same conditions as for lifespan assays. Worms were then picked onto bacteria free plates, pooled, and washed twice with M9 buffer (KH2PO4, 22mM; K2HPO4, 34 mM; NaCl, 86 mM; MgSO4, 1mM). All but 20 µl of M9 buffer was removed from worm pellet. 80 µl of ice cold 8% (v/v) perchloric acid was added to worm samples. The samples were then sonicated and 1N KHCO3 was added to neutralize perchloric acid. The samples were briefly centrifuged and supernatants were filtered through a .22µm filter and then reverse phase HPLC was performed on a LC-18 250 × 4.6 mm 5µm column. Samples were separated by addition of buffer A (KH2PO4 pH 6.0, 0.1M). Nucleotides were detected at 254 nm with a Varian Pro Star detector. Peak areas were measured with Varian Star Workstation software. Nucleotide identities were confirmed by co-migration with AMP and ATP standards (Sigma).
AMPK in vitro kinase assay
Purified AMPK (Upstate Biotechnology or purified from 293T cells) was incubated with various substrates (1 µg) in the kinase reaction buffer (HEPES pH 7.0, 15 mM; DTT, 450 µM; MgCl2, 18.75 mM; β-glycero-phosphate, 6.25 mM; EGTA, 1.25 mM; ATP, 125 µM) and 12.5 µCi radiolabeled ATP, with or without 150 µM AMP, at 30°C for 15 minutes. Phosphorylation was detected by incorporation of radiolabeled γ32P ATP. Quantifications were performed using the Amersham Storm phospho-imager and the Image Quant 5.2 software. The stoichiometry of phosphorylation was measured by excising Coomassie-stained bands of substrate and by quantifying the amount of radioactivity incorporated using a scintillation counter. The molar incorporation of phosphate was calculated and was divided by the molar amount of substrate. To compare independent experiments, the average moles of phosphate/molar amount of substrate was used to normalize the data.
Liquid chromatography followed by tandem mass spectrometry
A Coomassie-stained band corresponding to GST-DAF-16 phosphorylated by AMPK was excised from an SDS-PAGE gel, divided in half, reduced with DTT, alkylated with iodoacetamide, and digested with either trypsin or chymotrypsin. Peptide mixtures were separated by microcapillary reverse-phase chromatography and online analyzed in a hybrid linear ion trap-orbitrap (LTQ-Orbitrap, Thermo Electron) mass spectrometer. Mass spectra were database searched using the SEQUEST algorithm. All peptide matches were initially filtered based on enzyme specificity, mass measurement error, Xcorr and ΔCorr’ scores and further manually validated for peptide identification and site localization.
Worm RNA extraction and reverse transcription followed by quantitative PCR
RNA was extracted by addition of 1 ml of Trizol (Invitrogen) for 100 ml of worm pellet, followed by six freeze-thaw cycles in liquid nitrogen. The RNA extraction was performed according to the Trizol protocol. The expression of sod-3 was determined by reverse transcription of one µg total RNA using the Superscript II kit (Invitrogen) followed by quantitative PCR analysis on a Bio-rad iCycler using iQ SYBR green (Bio-rad) with the following primers:
sod-3 F: CAACTTGGCTAAGGATGGTGGAG,
sod-3R: GCATTGGCAAATCTCTCGCTG,
pan-actin F: TCGGTATGGGACAGAAGGAC,
pan-actin R: CATCCCAGTTGGTGACGATA
The experiments were conducted in triplicate and the results were expressed as 2(-(Gene of interest number of cycles-actin number of cycles)). Control PCR reactions were also performed on total RNA that had not been reverse-transcribed to test for the presence of genomic DNA in the RNA preparation.
Constructs, oxidative stress resistance in worms, quantification of bacterial ingestion using DsRed, DAF-16 localization assays, worm locomotor activity, and worm and human AMPK purification were performed as described in detail in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. Andy Fire and Man-Wah Tan for helpful discussion, for helping us setting up all C. elegans assays, and for kindly providing reagents and strains. We thank Dr. James Chen for his help with the AMP:ATP ratio measurement by HPLC. We thank Dr. Kang Shen for helpful discussion and for his help in the cloning of the worm AMPK subunits. We thank Dr. Leon Avery for helpful discussion regarding eat-2 mutants. We thank Dr. Zach Serber for helping us with stoichiometry calculations. We thank Drs. Laura Attardi, Greg Barsh, Judy Lieberman, Mike Greenberg, Sue Paradis, Tom Rando, Julien Sage, Man-Wah Tan, and the members of the Brunet laboratory for helpful discussion and for critically reading the manuscript. This research was supported by an NIH grant (NIA AG026648-01), an American Institute for Cancer Research grant, and a Pfizer/AFAR Innovations in Aging Research grant to A.B.. E.L.G. was supported by a PHS grant (CA 09302) from the NCI and by an NSF graduate fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masoro EJ. Overview of caloric restriction and ageing. Mechanisms of ageing and development. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. Jama. 2004;291:1226–1230. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mechanisms of ageing and development. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mechanisms of ageing and development. 1988;46:89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 6.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Pallottini V, Montanari L, Cavallini G, Bergamini E, Gori Z, Trentalance A. Mechanisms underlying the impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in aged rat liver. Mechanisms of ageing and development. 2004;125:633–639. doi: 10.1016/j.mad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 13.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apfeld J, O'Connor G, McDonagh G, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes & development. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis R, O'Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 17.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 18.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 19.Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647. doi: 10.1126/science.1083614. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 21.Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- 22.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 23.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 24.Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, Zou S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 26.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 27.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 28.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 29.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson T, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 31.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. Faseb J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 33.Michell BJ, Stapleton D, Mitchelhill KI, House CM, Katsis F, Witters LA, Kemp BE. Isoform-specific purification and substrate specificity of the 5'-AMP-activated protein kinase. J Biol Chem. 1996;271:28445–28450. doi: 10.1074/jbc.271.45.28445. [DOI] [PubMed] [Google Scholar]
- 34.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mechanisms of ageing and development. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 35.Szewczyk NJ, Udranszky IA, Kozak E, Sunga J, Kim SK, Jacobson LA, Conley CA. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J Exp Biol. 2006;209:4129–4139. doi: 10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- 36.Hosono R, Nishimoto S, Kuno S. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp Gerontol. 1989;24:251–264. doi: 10.1016/0531-5565(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 37.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 39.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mechanisms of ageing and development. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 44.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 45.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 46.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 47.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


