Abstract
Previous studies have identified quantitative trait loci (QTL) in the inbred High and Low Alcohol Sensitive Rat (IHAS1 and ILAS1) strains. The original development of the strains involved selection for ethanol sensitivity based on duration of the loss of the righting reflex (LORR) following a standard dose of ethanol. This paper confirms some of these QTL using a short-term selection procedure based on the difference between the blood ethanol level at loss and regain of the righting response. An F2 population of rats was developed by a reciprocal cross of IHAS1 and ILAS1 rats. Selection for 5 generations was carried out using delta-blood ethanol concentration (dBEC) as the selection trait, where dBEC = BECLR (BEC at loss of righting reflex) – BECRR (BEC at regain of righting reflex). The lines were labeled Tolerant (TOL) or Sensitive (SENS). Approximately one-third of the offspring for each generation in each line were genotyped using DNA markers that had been previously found to be linked to QTL on chromosomes 1, 2, 5, 12, and 13. By the fifth generation of selection, the lines showed a very large difference in dBEC, BECRR, and duration of LORR; BECLR showed little segregation during the selection, and latency to lose the righting reflex showed none. IHAS allele frequency increased in the SENS line for markers on chromosomes 1, 5, 12, and 13 while ILAS allele frequency increased in the TOL line. These results were in good agreement with the two previous QTL studies. On chromosome 2, the selection resulted in an accumulation of ILAS alleles in both lines. This study provides independent confirmation of the location of QTL on chromosomes 1, 5, 12, and 13 for ethanol sensitivity. It also suggests that genetic differences in duration of LORR are mediated primarily by the dBEC phenotype.
Keywords: Genetics, quantitative trait loci, ethanol tolerance, selected lines, inbred rats
Introduction
One of the best predictors of alcohol abuse and alcoholism in humans is their acute sensitivity to ethanol (Schuckit, 1992; Schuckit and Smith, 1996; Schuckit, 1994; Schuckit et al., 2000; Schuckit et al., 2001). Much research has gone into development of genetic animal models of acute ethanol responses in both mice (McClearn and Kakihana, 1981; Erwin and Deitrich, 1996; Boehm et al., 2000; Crabbe et al., 1989; Crabbe et al., 1979) and rats (Draski et al., 1992; Eriksson and Rusi, 1981). The goal of the development of animal models is two-fold. First, studies were aimed at defining the pharmacological, biochemical, and electrophysiological underpinnings of the responses of selected lines of rats and mice (Avdulov et al., 1995; Collins, 1981; Deitrich et al., 1988; Deitrich and Spuhler, 1984; Deitrich and Baker, 1995; Allan et al., 1988). Second, more recent studies were aimed at locating and identifying the specific genes that influence or control the behavioral reactions to ethanol (Bennett et al., 2002; Bennett et al., 2007; Kirstein et al., 2002). In this regard, this laboratory has developed the High and Low Alcohol Sensitive (HAS and LAS) rats from the nNIH heterogeneous population of rats by selective breeding for the duration of the loss of righting reflex (LORR) (Draski et al., 1992). The lines were selectively bred for 25 generations and subsequently inbred. Using these inbred lines, studies were undertaken to map quantitative trait loci (QTL) as a first step towards the goal of identifying genes that contribute to genetic variation for the duration of LORR response. QTL initially were mapped on chromosomes 1, 2, 5, 12, and 13; follow-up studies confirmed QTL on chromosomes 2, 5, and 13 (Radcliffe et al., 2006a; Radcliffe et al., 2004).
Acute ethanol sensitivity is often measured as the blood ethanol concentration (BEC) at the time at which a specific behavioral or other endpoint is achieved. As such, a single endpoint measurement of an acute response, such as the duration of LORR, is often taken as a measure of initial sensitivity. However, such measurements can be obscured by neuroadaptive processes that occur on both the ascending and descending limbs of alcohol distribution; e.g., acute functional tolerance (AFT) (Mellanby, 1919; Newlin and Thomson, 1990). AFT is an acute pharmacodynamic adaptation that counteracts the cellular disturbance created by the presence of ethanol. For behaviors or other neuronal responses that show AFT, true initial sensitivity is difficult to accurately assess, at least within the limits of experimental error, because neurons start adapting virtually immediately after they come into contact with ethanol; this would represent the early stages of AFT (Goldstein, 1989; Palmer et al., 1985; Radlow, 1994). Thus, ‘sensitivity’ for most acute ethanol responses is typically comprised of the combined effects of true initial sensitivity and AFT. The specific contribution of each of these is dependent on the time course of the response and AFT kinetics, and modified by genetic and environmental factors. Note that acute sensitization also is theoretically possible; indeed, acute sensitization is an important issue in the current study.
The fact that acute neuroadaptive processes such as AFT do occur has important implications for the study of acute ethanol responses in both humans and model organisms. For example, two experimental groups might show a similar response if it is measured soon after ethanol administration, while the response could diverge later on as a result of differential acquisition of AFT (see Newlin and Thomson, 1990). As mentioned above, QTL were mapped for the duration of LORR in crosses derived from the IHAS and ILAS selected rat lines, yet it is not known for which component of ‘acute sensitivity’ the QTL represent: initial sensitivity, AFT, or some combination (Radcliffe et al., 2006a; Radcliffe et al., 2004). AFT is known to occur for the LORR response, at least in mice (Ponomarev and Crabbe, 2002), and it was speculated that a substantial portion of the genotype-dependent difference in duration of LORR in mouse lines selected for a differential LORR response was related to acquisition of AFT rather than initial sensitivity (Radcliffe et al 2006b). Thus, the current experiment was undertaken to test the hypothesis that at least some of the duration of LORR QTL in the IHAS and ILAS lines would also control AFT. A bi-directional short-term selection was carried out using the development of acute tolerance or acute sensitization following a standard hypnotic dose of ethanol as the selection trait. The founding population was an F2 derived from an intercross of IHAS1 X ILAS1 rats. At each generation of selection, rats were genotyped at the previously mapped QTL to test for significant cosegregation of IHAS and ILAS alleles with the selection trait. In this way we hoped to dissect the relationship between alleles controlling initial sensitivity and those controlling the acute neuroadaptation which develops during the LORR response.
Materials and Methods
Animals
All rats were bred at the Center for Laboratory Animal Care at the University of Colorado at Denver and Health Sciences Center (UCDHSC; Denver, CO) and were 51 to 88 days of age at the time of testing (mean: 65.0 ± 0.2). The founding population was an F2 intercross created from reciprocal matings of IHAS1 X ILAS1 F1 rats. This was the same type of cross used in earlier QTL mapping studies (Radcliffe et al., 2004; Radcliffe et al., 2006a), but from a completely independent group of F2 rats. The animals were maintained on a 12-h light/dark cycle in an environment of constant temperature and humidity (22°C, 40% humidity) and given access to normal rodent chow (Harlan Teklad 22/5) and water ad libitum. The procedures described in this report have been established to ensure the absolute highest level of humane care and use of the rats, and have been reviewed and approved by the UCDHSC IACUC.
Acute Sensitivity and Tolerance
The F2 rats were tested for hypnotic sensitivity to ethanol using the same procedure that was used to select the IHAS and ILAS with a slight modification (Draski et al., 1992). Following alcohol administration (3.5 g/kg; 15% w/v ethanol in normal saline; ip), rats were placed on their back in a V-shaped trough and the time at which they could no longer right themselves was recorded. A 40 μL retro-orbital blood sample was drawn at this time for determination of the blood ethanol concentration at loss of righting (BECLR). The BECLR sample was not obtained either in the original selection of the HAS and LAS nor in the subsequent QTL studies using those lines. Duration of LORR was the elapsed time from the loss of the righting reflex until the time at which they could right themselves at least 3 times within a 1 minute span. A second 40 μL retro-orbital blood sample was drawn for determination of the blood ethanol concentration at regain of righting (BECRR). Delta BEC (dBEC) was the quantitative difference between BECRR and BECLR (i.e., dBEC = BECLR – BECRR) and was used as the sole selection trait (see below). Positive or negative dBEC indicated acute tolerance or sensitization, respectively. Values for duration of LORR are expressed in minutes and BEC measures as mg ethanol per dl blood (mg%). Blood ethanol concentrations were determined using a reliable spectrophotometry-based enzyme assay with comparison against a linear standard curve (Lundquist, 1959). Values shown in figures are mean ± SEM.
Selection for dBEC
Tolerant (TOL) and sensitive (SENS) lines were created using standard two-way selection procedures. Selection criterion was based on the dBEC score as described above. A total of 14 mating pairs, 7 for each line, consisting of the rats that showed the highest (TOL) or lowest (SENS) dBEC were selected from the founding F2 population (n = 128; 61 males and 67 females). Seven breeding pairs/line were chosen as a balance between maximal intensity of selection and avoidance of over- or under-producing the desired number of offspring in each generation which was approximately 60 to 70 per line (numbers are shown in table 1). The 7 males and 7 females with the highest or lowest dBEC scores from within the offspring of the high line or the low line, respectively, were selected as breeders for the next generation and this process was repeated for a total of 5 generations. Breeders were selected based exclusively on dBEC scores and were not chosen until all subjects from a given generation had been phenotyped. Several additional males and females were selected as backup breeders in case one of the primary pairs was unable to produce, but these animals were never needed. Each group of 7 mating pairs were representative of no fewer than five of the families from the preceding generation and brother–sister matings were entirely avoided. Up to 5 litters from a single mating were utilized.
Table 1.
One sample t statistic for test of dBEC vs. zero in the TOL and SENS selected lines.
| Generation | Line | n | ta |
|---|---|---|---|
| F2 | -- | 128 | −4.64 ** |
| S1 | TOL | 67 | −4.38 ** |
| SENS | 61 | −6.79 ** | |
| S2 | TOL | 55 | −1.04 |
| SENS | 43 | −5.72 ** | |
| S3 | TOL | 62 | 6.01 ** |
| SENS | 68 | 1.71 | |
| S4 | TOL | 80 | 9.50 ** |
| SENS | 73 | −1.95 | |
| S5 | TOL | 75 | 9.00 ** |
| SENS | 81 | −2.43 * |
Positive or negative t statistic indicates that the mean was greater or less than zero, respectively;
p<0.05;
p<0.001.
Genotyping
The breeders and animals selected as backup breeders from each generation were genotyped using a PCR protocol with simple sequence length polymorphism (SSLP) DNA markers, as previously described (Radcliffe et al., 2004). Subjects were scored as being either homozygous IHAS1, ILAS1 or heterozygous. Genotype was determined by visual comparison to a water control and to control DNA from IHAS1, ILAS1, and IHAS1 X ILAS1 F1 rats each of which was included in each PCR plate.
Data Analysis
ANOVA procedures were used to test for significant effects of line, selection generation, or sex. Significant differences in allele frequencies were determined as described by Belknap et al. (1997). Briefly, the frequency of marker alleles has two potential sources of random variation in a short-term selection: (1) genetic drift and (2) estimation error. Variance due to genetic drift was calculated according to the equation Vardt = p0q0[1 - (1 − 1/(2N))t] = p0q0Ft, where N is the number of breeding individuals per line (14), p0 and q0 are the frequencies of the IHAS1 and ILAS1 alleles, respectively, in the founding F2 population, and Ft is the coefficient of inbreeding at generation t. Estimation error was calculated as follows: Varqt = [(ptqt)/2n]H + [(ptqt)/2n]L, where n is the number of genotyped individuals in the high or low lines in generation t, and p and q are allele frequencies as above. The overall variance, Varδ, was determined by adding Vardt and Varqt. The standard deviation of allele frequency differences at any generation t(SDδt) was given by the square root of Varδ and this term was used to test for significance by the equation zt =δt/SDδt, whereδt is the difference in IHAS allele frequencies between the high and the low lines (qH - qL) and zt is the normal deviate at any generation t. A nominal α of p < 0.05 (one-tailed) was set as the level of significance which is much less conservative than that suggested by Lander and Kruglyak (1995) for the determination of a significant QTL. This level of significance was deemed acceptable because the current study was an independent analysis conducted to provide support for our earlier mapping studies. Further, the Lander and Kruglyak (1995) criteria are based on a full genome scan, but our scan was limited to only five linkage groups which were selected a priori based on the hypothesis that these specific regions contained QTL related to dBEC. QTL effect size (h2QTL), or the proportion of the phenotypic variance due to the QTL in the F2, was calculated as described by Belknap et al. (1997) as follows: h2QTL = s2QTL/s2P = 2δ2t/i2Pt2. Data from the latest generation that showed an approximately linear response of q in both lines was used for these calculations; later generations would have underestimated h2QTL (Belknap et al., 1997).
Results
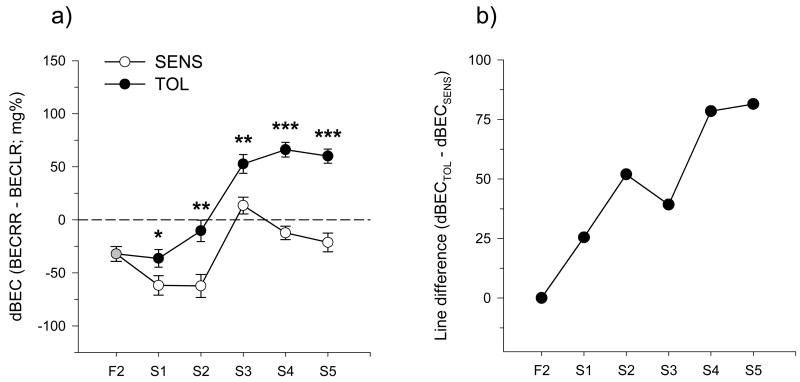
The phenotypic response to selection for dBEC is shown in figure 1. Three-way ANOVA indicated highly significant main effects of line, selection generation, and sex (p<0.0001). The TOL line showed an increase in dBEC across generations which leveled off at S4 while the SENS line was initially well below the F2 mean, but then increased between generations S2 and S3 (figure 1-a). Post hoc analysis indicated that the lines were significantly different at every generation. Note that after S3 the phenotypic mean started coming down again in the SENS line as reflected by a significant line-by-generation interaction (p<0.0001). There was also a significant line-by-generation-by-sex interaction (p<0.05) which was driven partly by a lower dBEC in females compared to males in the F2 and within each selection line, although not at every generation (generations S1 and S4 for the TOL line and S1 and S5 for the SENS line). The phenotypic differences between the lines, shown in figure 1-b, were used to calculate realized heritability for dBEC (Belknap et al., 1997; Falconer, 1989). Realized heritability, an estimate of the fraction of the phenotypic variance due to additive genetic factors, was 0.11, 0.14, 0.08, 0.13, and 0.11 for generations S1 through S5, respectively.
Figure 1.
Mean values for dBEC collapsed across sex in the tolerant (filled symbols) and sensitive (open symbols) lines (panel a). Panel b shows the selection differential for dBEC between the tolerant and sensitive lines. Asterisks indicate significant effects of selection line at each generation: ***, p<0.001; **, p<0.01; *, p<=0.05
Mean dBEC for the founding F2 population and the S1 generation for both the TOL and SENS lines was significantly less than zero indicating acute sensitization (table 1). By generation S3 and through S5, the TOL showed significant acute tolerance. In addition to S1, the SENS line showed significant acute sensitization in generations S2 and S5. The dBEC measure was not significantly different from zero in generations S3 and S4 for the SENS line, and in generation S2 in the TOL line.
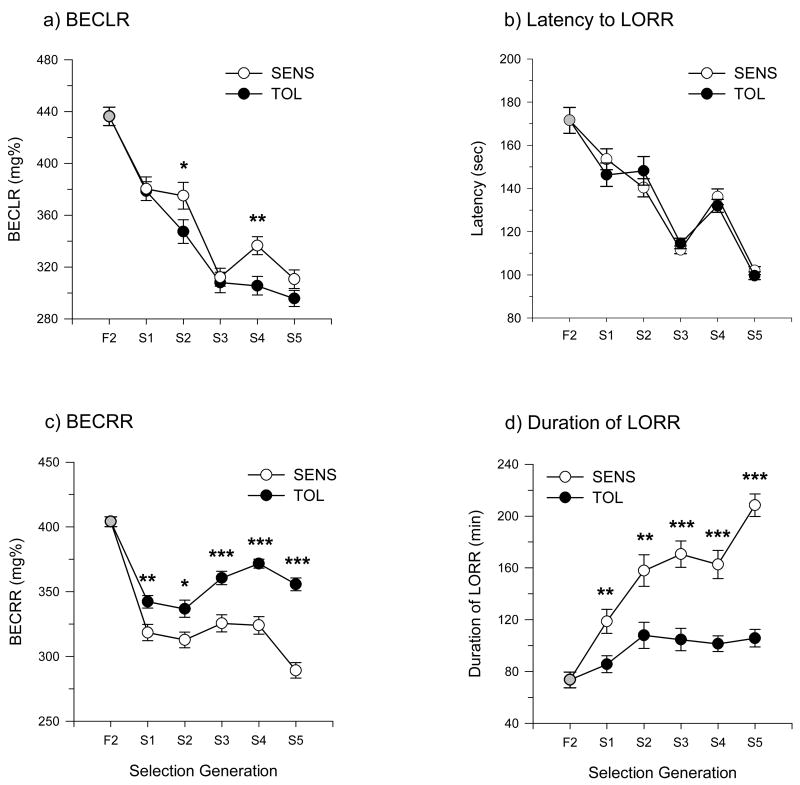
BECLR, the latency to lose the righting reflex, BECRR, and duration of LORR for the TOL and SENS lines are shown in figure 2. The results of three-way ANOVA for these variables are show in table 2. The TOL line was overall less sensitive than the SENS line as measured by BECRR and duration of LORR, and post hoc analyses indicated that this was the case for every generation (figures 2-c and 2-d). The main effect of line for BECLR was driven by a lower BECLR in the TOL line as compared to SENS, although post hoc analysis indicated significant differences at only generations S2 and S4 (figure 2-a). Note that there was no main effect of line for latency to LORR (figure 2-b); however, latency showed a similar decline across generations as BECLR and these two variables were significantly correlated (r = 0.21, p<10−7).
Figure 2.
Mean values for BEC at loss of the righting reflex (a), latency to LORR (b), BEC at regain of the righting reflex (c), and duration of loss of righting reflex (d) collapsed across sex in the tolerant (filled circles) and sensitive (open circles) lines. Asterisks indicate significant effects of selection line at each generation: ***, p<0.001; **, p<0.01; *, p<=0.05
Table 2.
Results of three-way ANOVA (line-by-generation-by-sex) for BECLR, latency, BECRR, and duration of LORR.
| Main effects (F ratio)a | Interaction effects (F ratio)a | ||||||
|---|---|---|---|---|---|---|---|
| Line | Generation | Sex | Line-by-generation | Line-by-sex | Generation-by-sex | Line-by-generation-by-sex | |
| BECLR | 14.6*** | 40.9*** | 153.0*** | n.s. | n.s. | n.s. | n.s. |
| Latency to LORR | n.s. | 42.5*** | 19.6*** | n.s. | n.s. | n.s. | n.s. |
| BECRR | 123.0*** | 9.5*** | 50.2*** | 5.9*** | n.s. | 4.8** | 3.6** |
| Duration of LORR | 130.5*** | 14.1*** | 163.8*** | 6.7*** | 7.2** | 2.9* | n.s. |
Significance indicated by:
, p<0.05;
, p<0.01;
, P<0.001
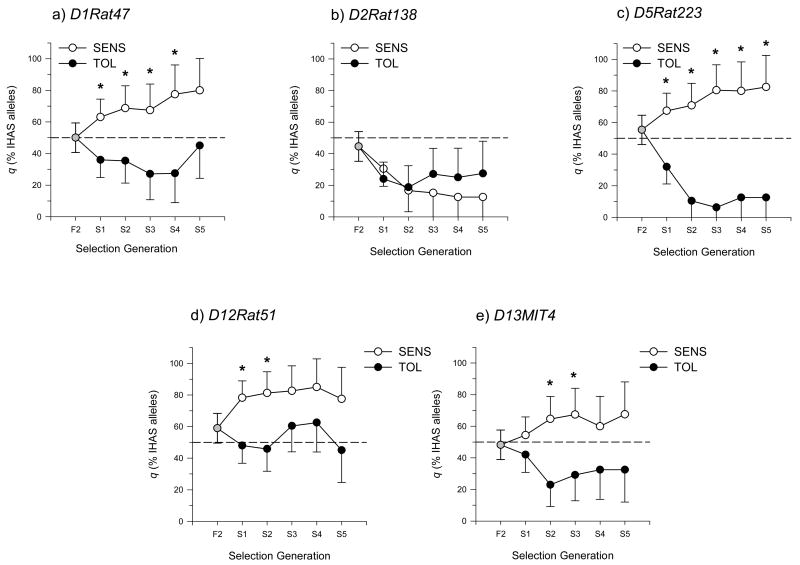
Previous mapping studies in IHAS X ILAS F2 rats and congenic lines suggested the presence of QTL for duration of LORR and/or BECRR on chromosomes 1, 2, 5, 12, and 13 (Radcliffe et al., 2004; Radcliffe et al., 2006a). To test the possibility that the pharmacological basis for these QTL could be related to the dBEC phenotype, the breeders and backup breeders from each generation were genotyped using markers positioned near the QTL on these chromosomes (n = 14 per line for F2; n = 20–25 per line for all other generations). From 3 to 7 markers were tested for each chromosome with an intermarker distance that ranged from 5 to 22 cM with an average of 11.6 ± 1.4 cM. The number of markers was dependent on the size of the confidence interval of the original QTL. The allele frequency (IHAS vs. ILAS) of at least one marker from each of chromosomes 1, 5, 12, and 13 was found to be significantly different for two or more generations of selection. Markers with the most significant effect are shown in figure 3. The frequency of ILAS alleles was higher in the TOL line while the SENS line had a higher frequency of IHAS alleles for these four chromosomes. However, both the TOL and SENS lines tended to accumulate ILAS alleles for all markers on chromosome 2 with no significant difference between the lines. This is illustrated with the marker D2Rat138 which showed the biggest effect of any other chromosome 2 marker in past F2 studies (figure 3-b) (Radcliffe et al., 2004; Radcliffe et al., 2006a).
Figure 3.
Percentage of IHAS DNA marker alleles (q) in high and low dBEC selected lines for the marker showing the largest effect on each chromosome. Error bars are the estimated standard deviation calculated for each line as the square root of the sum of Varqt for the line and the overall Vardt (see Methods). Significant difference between allele frequencies (within selection generation) is indicated by an asterisk (*, p<0.05).
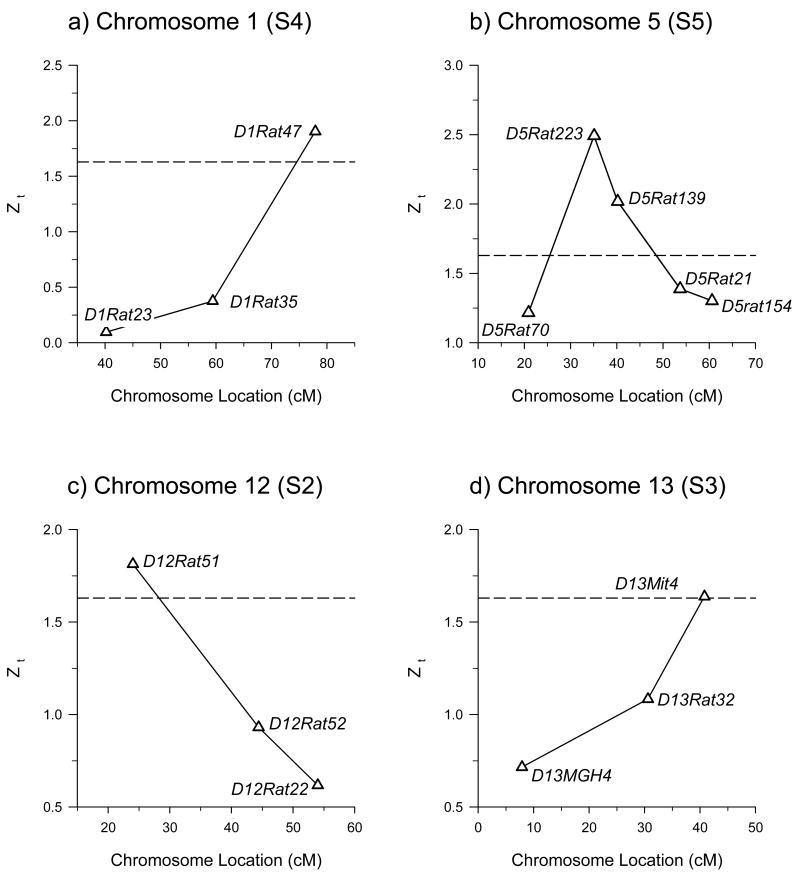
The normal deviate (Zt), which is the measure of statistical significance for differences in allele frequency between the lines (δ), can be used in a point analysis to estimate QTL position. This is shown in figure 4 in which Zt is plotted as a function of marker location for all of the markers that were tested on chromosomes 1, 5, 12, and 13. For each chromosome, the latest generation that showed a significant effect of δ is shown (see figure 3). Similar to point mapping using other measures of statistical significance, the marker with the highest Zt is linked to the most likely QTL position. Estimates of the amount of phenotypic variance accounted for by each QTL (h2QTL) are shown in table 3.
Figure 4.
QTL maps for dBEC on chromosomes 1, 5, 12, and 13. Marker position is shown as a function of the normal deviate (Zt) of δ for the most advanced generation that showed a significant difference in δ (generation shown in parentheses). Horizontal dashed line is the nominal significance threshold (Zt=1.63, p<0.05). Marker positions are from the Rat Genome Database (rgd.mcw.edu), SHRSP X BN cross.
Table 3.
Summary of the short-term selection QTL mapping results.
| Chrom. | Peak marker | Generationa | Peak locationb | h2QTL |
|---|---|---|---|---|
| 1 | D1Rat47 | S1 | 77.9 cM | 0.06 |
| 2 | n.s. | -- | -- | -- |
| 5 | D5Rat223 | S2 | 35.1 cM | 0.13 |
| 12 | D12Rat51 | S1 | 24.0 cM | 0.08 |
| 13 | D13Mit4 | S2 | 40.8 cM | 0.06 |
Generation for which q was approximately linear in both lines and from which h2QTL was calculated.
Location from the Rat Genome Database (rgd.mcw.edu), SHRSP x BN cross.
A comparison of the peak dBEC QTL locations for the short-term selection with the estimated location of previously mapped duration of LORR QTL (shown in table 4) indicates a high degree of correspondence. Note that the QTL on chromosome 12 did not overlap in the two F2 studies and therefore the LOD scores could not be combined (table 4) (Radcliffe et al., 2006a). The chromosome 12 QTL mapped in the current analysis is consistent with the second F2 study in which the QTL was significant. In addition, the chromosome 1 QTL peak was suggestive in the first F2 analysis and below the suggestive level in the second, but was confirmed in the current study.
Table 4.
Summary of QTL mapping results for duration of LORR in IHAS X ILAS F2 studies.
| Study 1a | Study 2a | ||||||
|---|---|---|---|---|---|---|---|
| Chrom. | Peak location | LODb | 95% CIc | Peak location | LODb | 95% CIc | Combined |
| 1 | 68 cM | 2.22* | 47–89 cM | 76 cM | 1.40 | 45–94 cM | 2.65* |
| 2 | 87 cM | 3.5* | 76–107 cM | 92 cM | 2.93* | 80–104 cM | 5.30** |
| 5 | 37 cM | 2.80* | 17–51 cM | 59 cM | 1.71* | 34–83 cM | 3.35* |
| 12 | 53 cM | 2.41* | 45–53 cM | 24 cM | 4.86** | 24–33 cM | -- |
| 13 | 39 cM | 2.93* | 33–41 cM | 33 cM | 3.69** | 22–39 cM | 5.48** |
Study 1: Radcliffe et al. 2004; Study 2: Radcliffe et al., 2006a.
Peak LOD; suggestive (*), significant (**).
95% confidence interval calculated using WinQTLCart (Wang et al. 2006).
LOD scores were combined using Fishers method (Sokal and Rohlf, 1981); scores were not combined for chromosome 12 because the 95% confidence interval for the two QTL did not overlap (Radcliffe et al., 2006a).
Discussion
The original selection of the HAS and LAS rats was based on duration of the loss of the righting reflex (LORR) and, as expected, blood ethanol concentration at regain of righting response (BECRR) co-segregated with duration of LORR (Draski et al., 1992). This result indicates that the phenotypic difference in duration of LORR was primarily the result of differences in acute CNS sensitivity to ethanol rather than to pharmacokinetic factors which is also supported by the absence of a line difference in ethanol clearance (Draski et al., 1992). Strain differences in acute adaptive processes (acute tolerance or sensitization) during the LORR test previously had been observed for the HAS and LAS (unpublished observations), as well as for the Inbred Long and Short Sleep mice (ILS, ISS) which were selectively bred for the same trait as the HAS and LAS (Keir and Deitrich, 1990; Radcliffe et al., 2006b). These results, along with the current results in which the duration of LORR co-segregated with dBEC suggest that acute adaptive processes are responsible for a large portion of the genetic variance in duration of LORR in rodent models.
It is not clear the extent to which acute sensitization actually occurred in the SENS line and the F2 founders. This is because the measure of BECLR is imprecise since BEC is rising rapidly following intraperitoneal administration and the elapsed time between the loss of the righting reflex and taking of the blood sample tends to cause an overestimation of BECLR. The measure of dBEC thus tends to be underestimated and may actually be negative which is indicative of acute sensitization. Using a modified version of the LORR test which included a more efficient measure of BECLR, the ILS selected mouse strain showed significant acute sensitization (Radcliffe et al., 2006b). Although this was not observed in other inbred mouse strains (Ponomarev and Crabbe, 2004), it is possible that selection pressure for high sensitivity in ILS mice, HAS rats, and SENS rats forced the accumulation of “sensitization alleles” in these lines.
The question of whether acute sensitization is a real effect was addressed indirectly by the BECLR response which dropped rather dramatically between generations S1 and S3 in both the SENS and TOL lines (figure 2-a). The reason for this is uncertain, although the most reasonable explanation is that it was the result of unknown environmental factors; e.g., seasonal effects or increased experimenter efficiency at quickly obtaining a blood sample at the loss of function. Whatever the cause, the decline in BECLR across generations appears to have had minimal effect on the relative dBEC difference between the SENS and TOL lines. In generation S5, BECLR is at its lowest point for both lines and not significantly different, yet dBEC shows its maximal difference at this generation. This result suggests that the response shown by the SENS line may have been true acute sensitization rather than an artifact of delayed sampling of BECLR.
It was expected that the selection process would have produced differences in BECLR (down in TOL, up in SENS) as well as in BECRR (up in TOL, down in SENS). In general, BECRR showed the expected response, but BECLR differed between the lines only at generations S2 and S4 (fig. 2). As such, this study can be viewed as a selection based primarily on BECRR with the measure of LORR duration secondary. The magnitude of the difference in duration of LORR between the TOL and SENS is very similar to that observed by generation 8 in the original HAS/LAS selection (Draski et al., 1992). It would thus appear that the current selection essentially replicated the original selection of the HAS and LAS further supporting a close relationship between duration of LORR, BECRR, and acute adaptations to ethanol.
There was a significant main effect of sex for all of the measured traits and significant interactions involving sex for BECRR, duration of LORR, and dBEC. For the duration of LORR phenotype, sex differences are often attributable to pharmacokinetic parameters; i.e., sex differences in fat composition (ethanol compartmentalization) and possibly metabolism. However, females tended to be less sensitive than males in both the TOL and SENS line for not only duration of LORR, but also for BECLR and BECRR indicating sex differences in CNS sensitivity. Previous studies have attempted to identify sex-specific QTL for the duration of LORR, but the results have been inconclusive (see Radcliffe et al., 2006a). Nonetheless, it is possible if not likely that sex-specific genetic factors contributed to the current results. Theoretically, this could be determined for the QTL regions that were investigated. For example, if a particular QTL was male specific, it would be expected that the change in allele frequency in the females would lag slightly behind the change in the males as the selection progressed. This effect, however, would be subtle and would require a much greater number of breeders and offspring for it to be detected.
A short-term selection can be used to replicate and confirm previous QTL findings (Belknap et al., 1997). The present study was conducted differently in that the selection trait (dBEC) was postulated to contribute to the phenotype for which the original QTL were mapped (duration of LORR). A significant segregation of DNA marker alleles was observed for four of the five QTL regions that were tested (fig. 4). Further, the location of the most significant marker for each of the four intervals was in good agreement with the peak QTL location determined from interval mapping procedures conducted in the original F2 QTL experiments (Radcliffe et al., 2004; Radcliffe et al., 2006a). The co-segregation of dBEC, duration of LORR, and BECRR along with the QTL results provide evidence that genetic variance for these traits is under the control of a largely overlapping set of genes. It should be noted, however, that genotyping was limited to only regions that were previously identified in the original duration of LORR mapping studies. It is possible that other genomic loci contain moderate to large effect AFT/sensitization QTL that would not have been detected in this study. Moreover, it is also possible that other AFT/sensitization alleles exist in the nNIH heterogeneous stock from which the HAS and LAS were selected.
The results for the QTL on chromosome 2 in this study are puzzling since it was significant in the studies based on duration of LORR and it was expected that the IHAS and ILAS alleles would have segregated similarly as the other QTL (fig. 3-b) (Radcliffe et al., 2004; Radcliffe et al., 2006a). It is possible that the interval contains two loci which interact with one or more loci on other chromosomes (epistasis) and the specific genetic structure of the F2 breeding pairs may have facilitated the relatively rapid segregation of the appropriate combination of alleles leading to the odd result for chromosome 2. General support for this hypothesis has been obtained from studies of marker-assisted congenic lines developed for the chromosome 2 interval. When a large ILAS segment from chromosome 2 was introgressed onto an IHAS background, duration of LORR was decreased as expected (Radcliffe et al., 2006a). Chromosome 2 sub-congenic lines containing a smaller ILAS segment derived from the distal region of the larger interval were also found to be less sensitive, as expected (decreased duration of LORR). However, lines that contained the more proximal region of the larger interval actually had an increased duration of LORR (unpublished observations). Previous studies indicated that the chromosome 2 QTL effect size was from 0.03 to 0.04 and the smallest QTL effect size detected in the current study was 0.06. It is thus possible that there was insufficient statistical power to detect the chromosome 2 QTL, although if this was true, at least a partial segregation of alleles should have occurred. In this case, the alleles simply did not segregate. Finally, since the current study was investigating a different trait than the earlier mapping experiments, it is possible that the chromosome 2 QTL is specific for duration of LORR and thus it would not have been detected for dBEC.
The results with chromosome 1 are of interest. The QTL was not replicated between the two F2 studies (table 4); however, the effect in this short-term selection was significant. In another F2 QTL mapping study using the Alcohol Tolerant and Alcohol Non-Tolerant (AT and ANT) rats, a highly significant QTL for both duration of LORR and BECRR was observed in this same region of chromosome 1 (in preparation). Preliminary results with AT/ANT chromosome 1 congenic lines have confirmed this QTL. These rats were developed from a completely different genetic background and it is therefore uncertain if the same genes are involved as the apparent HAS/LAS QTL.
An important and well-supported hypothesis in alcoholism research is that acute ethanol sensitivity early in an individual’s drinking career is a contributing factor to, or at least a predictor of, genetic risk for future alcohol use disorders (Schuckit, 1994). Yet acute sensitivity can be partitioned into at least two genetically distinct components, initial sensitivity and acute neuroadaptive processes like AFT, which can make interpretation of acute sensitivity experiments problematic. The findings presented here suggest that acute neuroadaptation makes substantial contributions to genetic variance in acute ethanol sensitivity. We observed little effect of selection for dBEC on initial sensitivity – i.e., sensitivity during the rising phase of ethanol distribution (BECLR) – but others have noted genetic differences in this measure (Ponomarev and Crabbe, 2004; Radcliffe et al., 2006b). It is certainly not clear which of these pharmacological parameters are responsible for the relationship between acute sensitivity and genetic risk alcoholism. It may be both. For example, an inherently low sensitivity as manifested through low initial sensitivity and rapid development of AFT may permit an individual to drink more which could promote heavy drinking and a more rapid development of tolerance or dependence (Schuckit et al., 2004). Another possibility is that other traits that are pleiotropic to acute sensitivity may be the root cause of the association with alcoholism. For example, studies have shown a relationship between AFT and contextual learning and also between ethanol sensitization and contextual learning, suggesting a common mechanistic basis for ethanol-induced plasticity and learning and memory (Radcliffe et al., 1998; Quadros et al., 2003). Recent microarray studies support such a relationship in the selectively bred ILS and ISS mouse strains (unpublished observations). Addictive processes have an important learning component (Everitt and Robbins, 2005; Hyman, 2005) and it is possible that low acute sensitivity contributes to alcoholism through pleiotropy with learning-related mechanisms.
In summary, the current results indicate that genetic loci that contribute to variance in adaptive responses to acute ethanol overlap with previously identified QTL for the duration of LORR suggesting a mechanistic and genetic relationship between these responses. Ongoing studies are being directed towards identification of the specific genes that mediate these responses and of the pathways in which they take part. Knowledge of these responses will contribute to a deeper understanding of genetic risk for human alcoholism in the context of acute responses to ethanol.
Acknowledgments
The authors would like to thank Dr. John Belknap for helpful discussions, Ms. Tina Fay for expert technical assistance, and Dr. Charles Goodlett and the reviewers of this paper for their excellent advice and suggestions. This work was supported by USPHS grants AA13177 (RAR) and AA011464 (RAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan AM, Spuhler KP, Harris RA. Gamma-aminobutyric acid-activated chloride channels: relationship to genetic differences in ethanol sensitivity. J Pharmacol Exp Ther. 1988;244:866–870. [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Draski LJ, Deitrich RA, Wood WG. Chronic ethanol consumption alters effects of ethanol in vitro on brain membrane structure of high alcohol sensitivity and low alcohol sensitivity rats. Alcoholism. 1995;19:886–891. doi: 10.1111/j.1530-0277.1995.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Richards SP, O’Toole LA, Helms ML. Short-term selective breeding as a tool for QTL mapping: Ethanol preference drinking in mice. Behav Genet. 1997;27:55–66. doi: 10.1023/a:1025615409383. [DOI] [PubMed] [Google Scholar]
- Bennett B, Beeson M, Gordon L, Carosone-Link P, Johnson TE. Genetic dissection of quantitative trait loci specifying sedative/hypnotic sensitivity to ethanol: Mapping with interval-specific congenic, recombinant lines. Alcohol Clin Exp Res. 2002;26:1615–1624. doi: 10.1097/01.ALC.0000037136.49550.B3. [DOI] [PubMed] [Google Scholar]
- Bennett B, Downing C, CarosoneLink P, Ponicsan H, Ruf C, Johnson TE. Quantitative trait locus mapping for acute functional tolerance to ethanol in the L x S recombinant inbred panel. Alcohol Clin Exp Res. 2007;31:200–208. doi: 10.1111/j.1530-0277.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Crabbe JC, Phillips TJ. Sensitivity to ethanol-induced motor incoordination in FAST and SLOW selectively bred mice. Pharmacol Biochem Behav. 2000;66:241–247. doi: 10.1016/s0091-3057(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Collins AC. A review of research using short-sleep and long-sleep mice. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. Rockville, Maryland: NIAAA Research Monograph; 1981. pp. 161–170. [Google Scholar]
- Crabbe JC, Feller DJ, Dorow JS. Sensitivity and tolerance to ethanol-induced hypothermia in genetically selected mice. J Pharmacol Exp Ther. 1989;249:456–461. [PubMed] [Google Scholar]
- Crabbe JC, Rigter H, Uijlen J, Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–133. [PubMed] [Google Scholar]
- Deitrich RA, Baker RC. Genetic influences on alcohol metabolism and sensitivity to alcohol in animals. In: Begleiter H, Kissin B, editors. The Genetics of Alcoholism. Oxford University Press; 1995. pp. 139–164. [Google Scholar]
- Deitrich RA, Spuhler KP. The development of a rat model for pharmacogenetic research in alcoholism. Alcohl Clin Exp Res. 1984;8:476–504. [Google Scholar]
- Deitrich RA, Spuhler KP, Baker RC, Erwin VG. Development and characteristics of rats selectively bred for sensitivty to ethanol. In: Kuriyama K, Takada A, Ishii H, editors. Biomedical and Social Aspects of Alcohol and Alcoholism. Elsevier Science; 1988. pp. 419–422. [Google Scholar]
- Draski LJ, Spuhler KP, Erwin VG, Baker RC, Deitrich RA. Selective breeding of rats differing in sensitivity to the effects of acute ethanol administration. Alcoholism. 1992;16:48–54. doi: 10.1111/j.1530-0277.1992.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K, Rusi M. Finnish selection studies on alcohol-related behaviors: General outline. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. Rockville, Maryland: NIAAA Research Monograph; 1981. pp. 87–117. [Google Scholar]
- Erwin VG, Deitrich RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996;279:1310–1317. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. New York: Wiley; 1989. [Google Scholar]
- Goldstein DB. Animal models developed by selective breeding: some questions raised and a few answered. In: Kiianmaa K, Tabakoff B, Saito T, editors. Genetic Aspects of Alcoholism. Helsinki: Finnish Foundation for Alcohol Studies; 1989. pp. 229–238. [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Keir WJ, Deitrich RA. Development of central nervous system sensitivity to ethanol and pentobarbital in short- and long-sleep mice. J Pharmacol Exp Ther. 1990;254:831–835. [PubMed] [Google Scholar]
- Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, Tabakoff B. Quantitative trait loci affecting initial sensitivity and acute functional tolerance to ethanol-induced ataxia and brain cAMP signaling in BXD recombinant inbred mice. J Pharmacol Exp Ther. 2002;302:1238–1245. doi: 10.1124/jpet.302.3.1238. [DOI] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissue. Methods Biochem Anal. 1959;7:217–251. [Google Scholar]
- Mellanby E. Alcohol: its absorption into and disappearance from the blood under different conditions. Med Res Comm Spec Rep (Lond) 1919;31:1–48. [Google Scholar]
- McClearn GE, Kakihana R. Selective breeding for ethanol sensitivity: Short-sleep and long-sleep mice. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. Rockville, Maryland: NIAAA Research Monograph; 1981. pp. 147–159. [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Palmer MR, Basile AS, Proctor WR, Baker RC, Dunwiddie TV. Ethanol tolerance of cerebellar purkinje neurons from selectively outbred mouse lines: in vivo and in vitro electrophysiological investigations. Alcohol Clin Exp Res. 1985;9:291–296. doi: 10.1111/j.1530-0277.1985.tb05752.x. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Characterization of acute functional tolerance to the hypnotic effects of ethanol in mice. Alcohol Clin Exp Res. 2004;28:991–997. doi: 10.1097/01.alc.0000131978.79857.5e. [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Souza-Formigoni MLO, Fornari RV, Nobrega JN, Oliveira MGM. Is behavioral sensitization to ethanol associated with contextual conditioning in mice? Behav Pharmacol. 2003;14:129–136. doi: 10.1097/00008877-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Bludeau P, Asperi W, Fay T, Deng XS, Erwin VG, Deitrich RA. Confirmation of quantitative trait loci for ethanol sensitivity and neurotensin receptor density in crosses derived from the inbred High and Low Alcohol Sensitive selectively bred rat lines. Psychopharmacology. 2006a;188:343–354. doi: 10.1007/s00213-006-0512-2. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG, Draski L, Hoffmann S, Edwards J, Deng XS, Bludeau P, Fay T, Lundquist K, Asperi W, Deitrich RA. Quantitative trait loci mapping for ethanol sensitivity and neurotensin receptor density in an F2 intercross derived from inbred high and low alcohol sensitivity selectively bred rat lines. Alcohol Clin Exp Res. 2004;28:1796–1804. doi: 10.1097/01.alc.0000148106.71801.d7. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG, Wehner JM. Acute functional tolerance to ethanol and fear conditioning are genetically correlated in mice. Alcohol Clin Exp Res. 1998;22:1673–1679. [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Lee MJ. Rapid ethanol tolerance mediated by adaptations in acute tolerance in inbred mouse strains. Pharmacol Biochem Behavior. 2006b;84:524–534. doi: 10.1016/j.pbb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Radlow R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology. 1994;114:1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Reaction to alcohol as a predictor or alcoholism. Clin Neuropharmacol. 1992;15(Suppl1):305A–306A. doi: 10.1097/00002826-199201001-00158. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiat. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiat. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk—a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: A pilot study and a comparison with sons of alcoholics. Alcohol Alcoholism. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. San Francisco: Freeman; 1981. [Google Scholar]
- Wang S, Basten CJ, Zeng Z-B. Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University; Raleigh, North Carolina: 2006. [Google Scholar]