Abstract
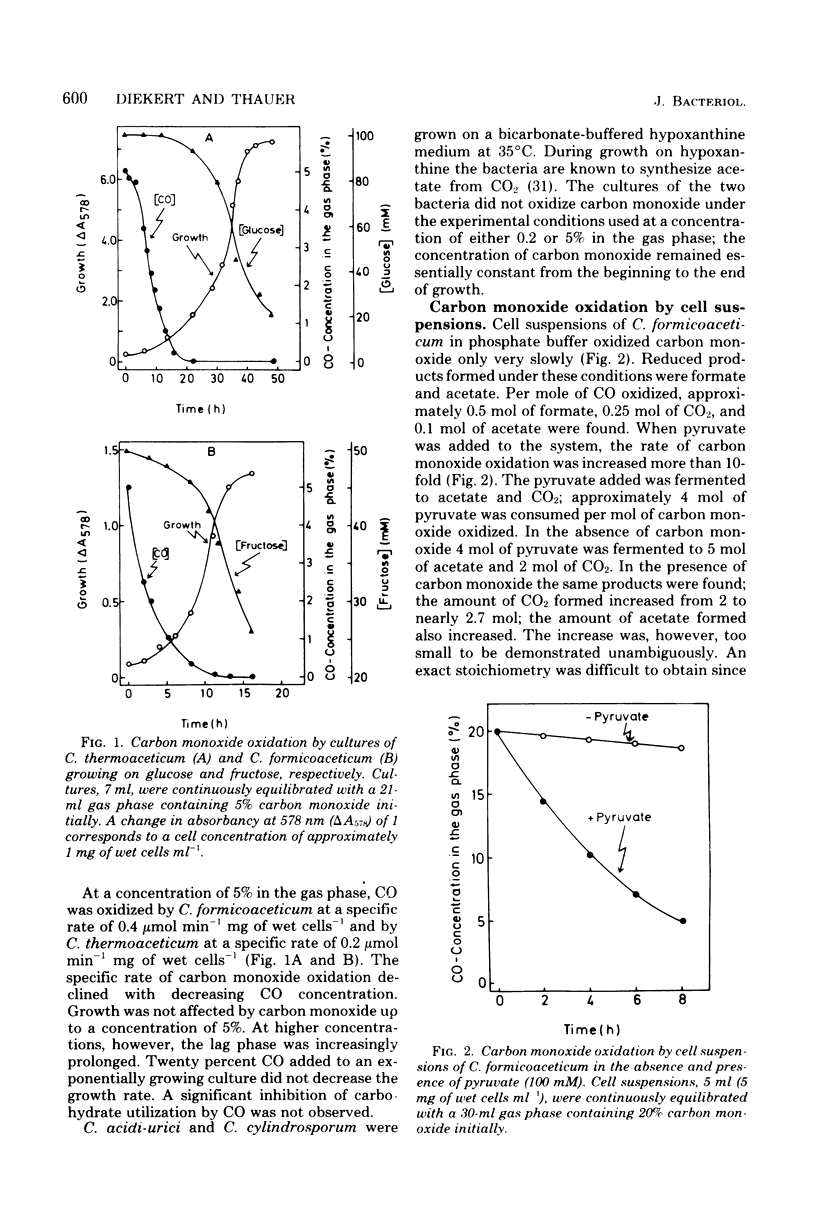
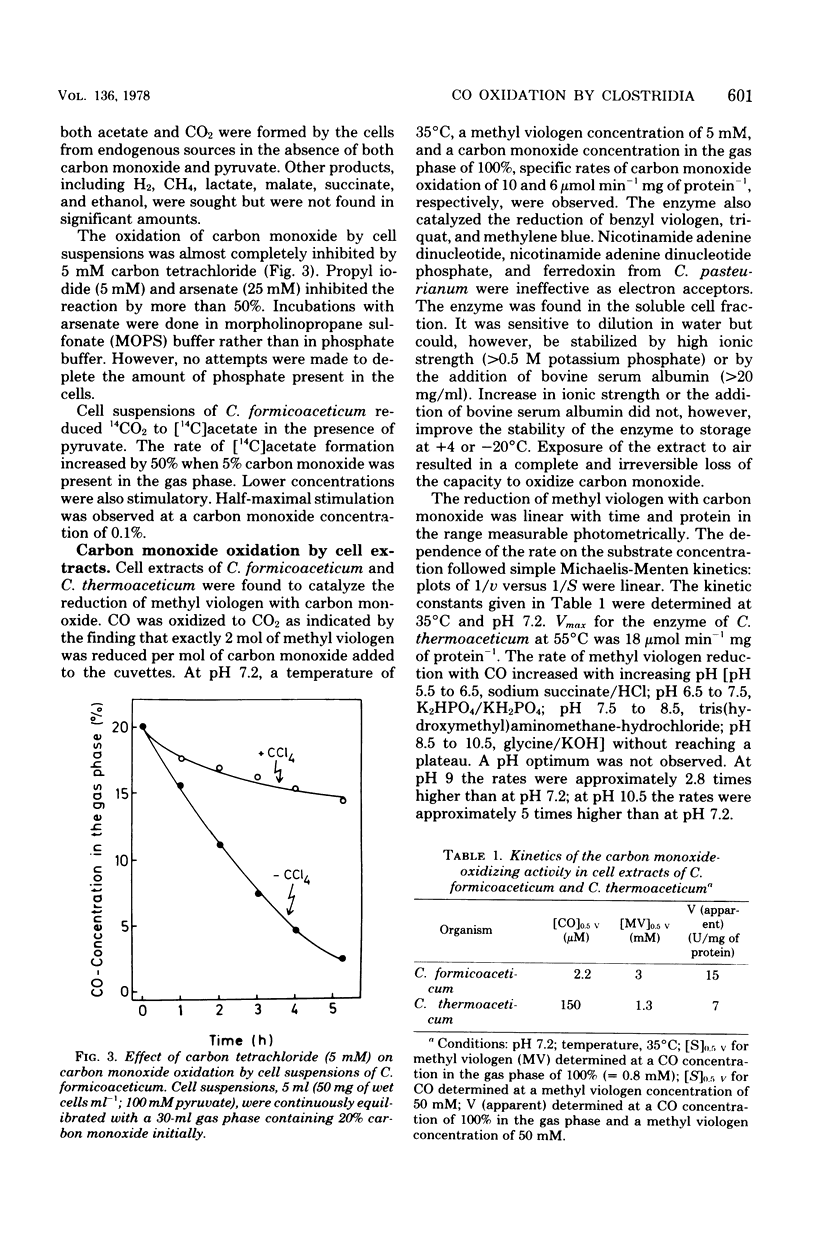
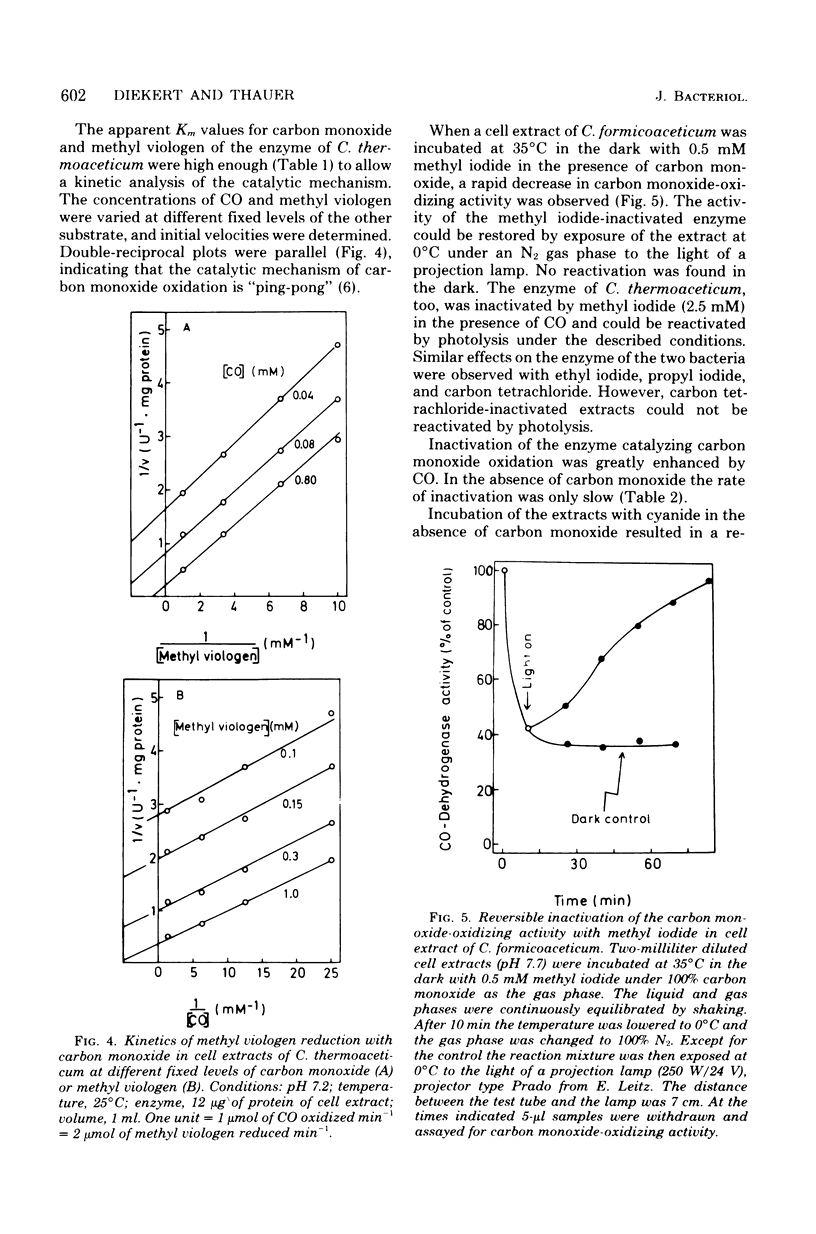
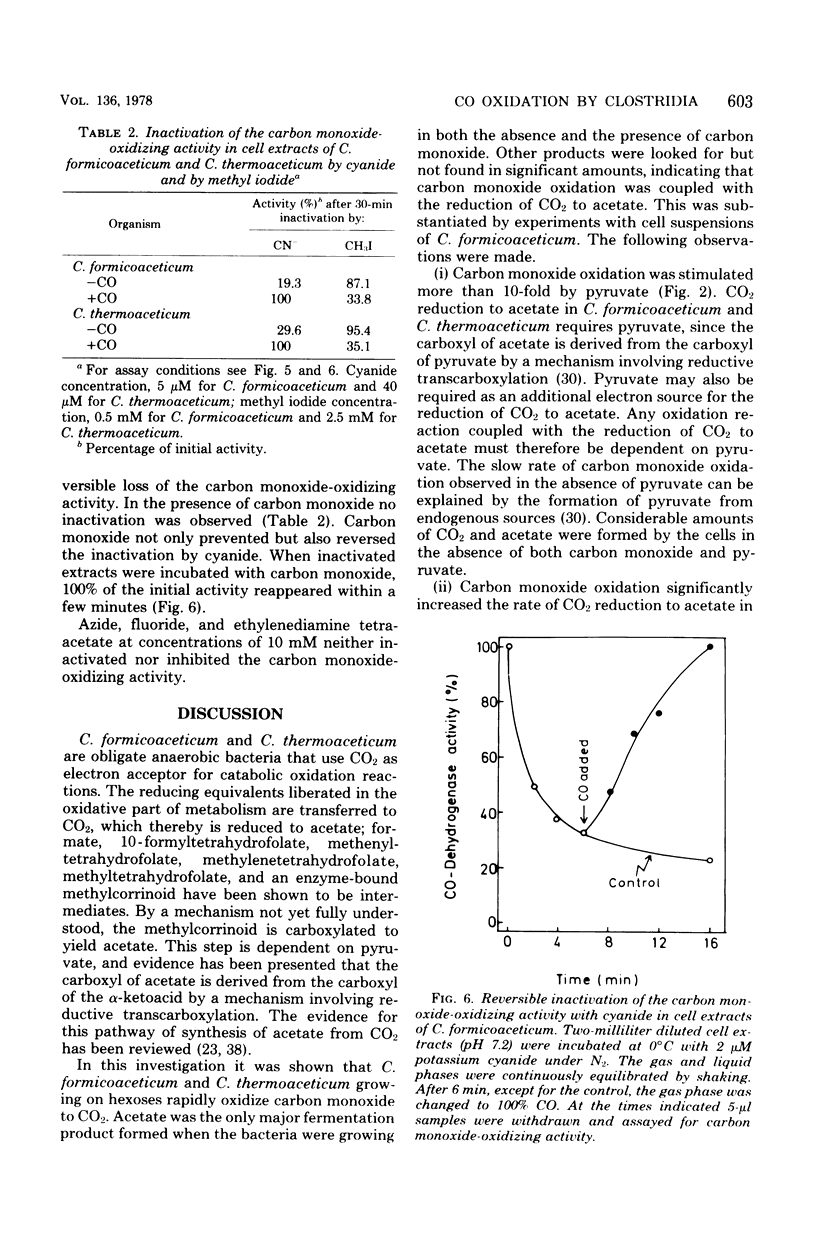
Cultures of Clostridium formicoaceticum and C. thermoaceticum growing on fructose and glucose, respectively, were shown to rapidly oxidize CO to CO2. Rates up to 0.4 μmol min−1 mg of wet cells−1 were observed. Carbon monoxide oxidation by cell suspensions was found (i) to be dependent on pyruvate, (ii) to be inhibited by alkyl halides and arsenate, and (iii) to stimulate CO2 reduction to acetate. Cell extracts catalyzed the oxidation of carbon monoxide with methyl viologen at specific rates up to 10 μmol min−1 mg of protein−1 (35°C, pH 7.2). Nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate and ferredoxin from C. pasteurianum were ineffective as electron acceptors. The catalytic mechanism of carbon monoxide oxidation was “ping-pong,” indicating that the enzyme catalyzing carbon monoxide oxidation can be present in an oxidized and a reduced form. The oxidized form was shown to react reversibly with cyanide, and the reduced form was shown to react reversibly with alkyl halides: cyanide inactivated the enzyme only in the absence of carbon monoxide, and alkyl halides inactivated it only in the presence of carbon monoxide. Extracts inactivated by alkyl halides were reactivated by photolysis. The findings are interpreted to indicate that carbon monoxide oxidation in the two bacteria is catalyzed by a corrinoid enzyme and that in vivo the reaction is coupled with the reduction of CO2 to acetate. Cultures of C. acidi-urici and C. cylindrosporum growing on hypoxanthine were found not to oxidize CO, indicating that clostridia mediating a corrinoid-independent total synthesis of acetate from CO2 do not possess a CO-oxidizing system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- BROT N., WEISSBACH H. ENZYMATIC SYNTHESIS OF METHIONINE. CHEMICAL ALKYLATION OF THE ENZYME-BOUND COBAMIDE. J Biol Chem. 1965 Jul;240:3064–3070. [PubMed] [Google Scholar]
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich W. Uber die Reaktion von Kohlenmonoxid mit Corrinoiden. Z Naturforsch B. 1970 Dec;25(12):1431–1434. [PubMed] [Google Scholar]
- Fuchs G., Schnitker U., Thauer R. K. Carbon monoxide oxidation by growing cultures of Clostridium pasteurianum. Eur J Biochem. 1974 Nov 1;49(1):111–115. doi: 10.1111/j.1432-1033.1974.tb03816.x. [DOI] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. Photosynthetic bacterium growing under carbon monoxide. Nature. 1968 Feb 10;217(5128):555–556. doi: 10.1038/217555a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Postgate J. Carbon monoxide as a basis for primitive life on other planets: a comment. Nature. 1970 Jun 6;226(5249):978–978. doi: 10.1038/226978a0. [DOI] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Schrauzer G. N., Lee L. P. The reduction of vitamin B 12a by carbon monoxide. Arch Biochem Biophys. 1970 May;138(1):16–25. doi: 10.1016/0003-9861(70)90278-x. [DOI] [PubMed] [Google Scholar]
- Schrauzer G. N., Michaely W. J. Uber die Methylenblau-Hemmung der Reduktion des Hydroxocobalamins durch Kohlenoxid. Z Naturforsch B. 1972 May;27(5):577–578. doi: 10.1515/znb-1972-0523. [DOI] [PubMed] [Google Scholar]
- Schrauzer G. N., Sibert J. W. Electron transfer reactions catalyzed by vitamin B 12 and related compounds: the reduction of dyes and of riboflavin by thiols. Arch Biochem Biophys. 1969 Mar;130(1):257–266. doi: 10.1016/0003-9861(69)90032-0. [DOI] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Schulman M., Parker D., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO 2 . V. Determination by mass analysis of the different types of acetate formed from 13 CO 2 by heterotrophic bacteria. J Bacteriol. 1972 Feb;109(2):633–644. doi: 10.1128/jb.109.2.633-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Wäscher C., Thauer R. K. A rapid procedure for the purification of ferredoxin from Clostridia using polyethyleneimine. FEBS Lett. 1978 May 15;89(2):219–222. doi: 10.1016/0014-5793(78)80221-x. [DOI] [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Propylation characteristics with the use of a chemical reducing system and purified enzyme. J Biol Chem. 1967 Apr 10;242(7):1509–1516. [PubMed] [Google Scholar]
- Taylor R. T., Whitfield C., Weissbach H. Chemical propylation of vitamin-B12 transmethylase: anomalous behavior of S-adenosyl-L-methionine. Arch Biochem Biophys. 1968 Apr;125(1):240–252. doi: 10.1016/0003-9861(68)90658-9. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Käufer B., Schnitker U. Carbon-monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974 Jun 15;45(2):343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Jungermann K. Separation of 14C-formate from CO2 fixation metabolites by isoionic-exchange chromatography. Anal Biochem. 1970 Dec;38(2):461–468. doi: 10.1016/0003-2697(70)90471-9. [DOI] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976 Jun;40(2):403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H., Taylor R. T. Roles of vitamin B 12 and folic acid in methionine synthesis. Vitam Horm. 1970;28:415–440. doi: 10.1016/s0083-6729(08)60905-x. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Propylation and purification of a B12 enzyme involved in methane formation. Biochemistry. 1966 Nov;5(11):3598–3603. doi: 10.1021/bi00875a031. [DOI] [PubMed] [Google Scholar]
- YAGI T. Enzymic oxidation of carbon monoxide. Biochim Biophys Acta. 1958 Oct;30(1):194–195. doi: 10.1016/0006-3002(58)90263-4. [DOI] [PubMed] [Google Scholar]
- YAGI T., TAMIYA N. Enzymic oxidation of carbon monoxide. III. Reversibility. Biochim Biophys Acta. 1962 Dec 17;65:508–509. doi: 10.1016/0006-3002(62)90454-7. [DOI] [PubMed] [Google Scholar]


