Abstract
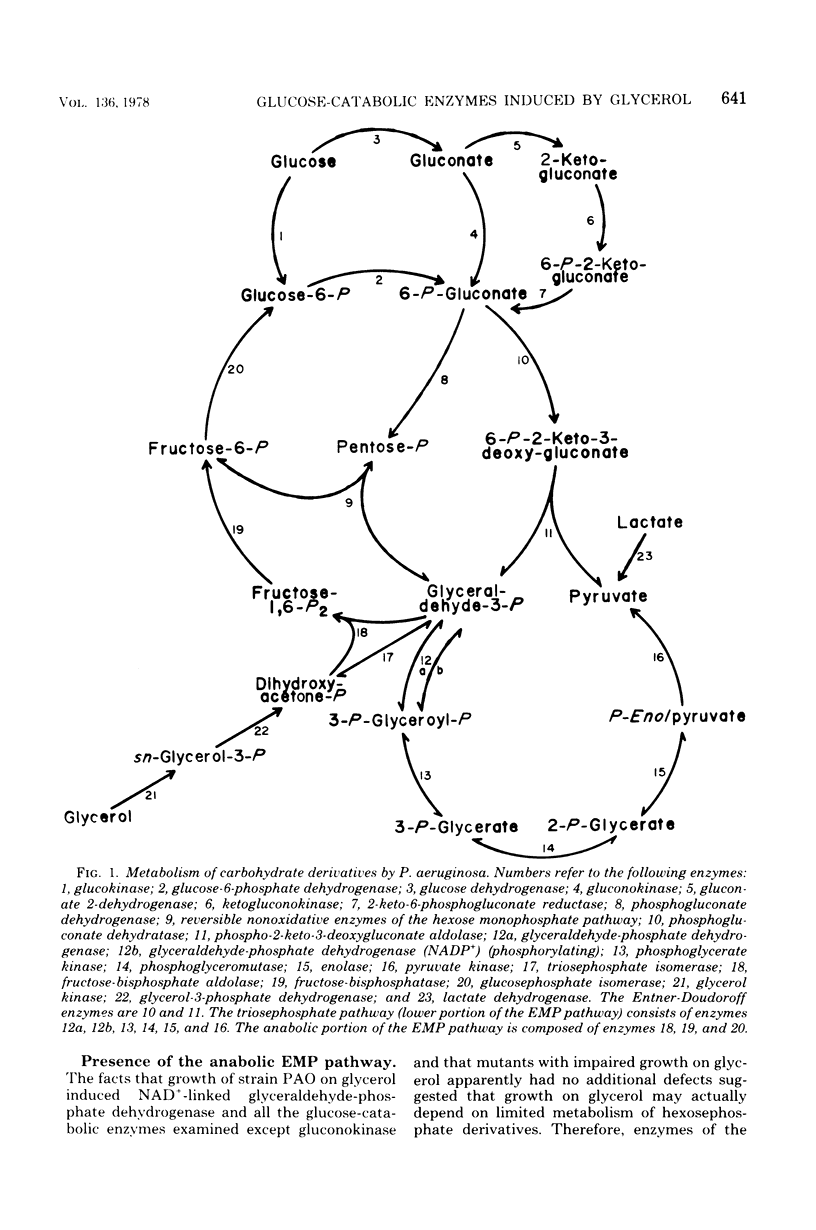
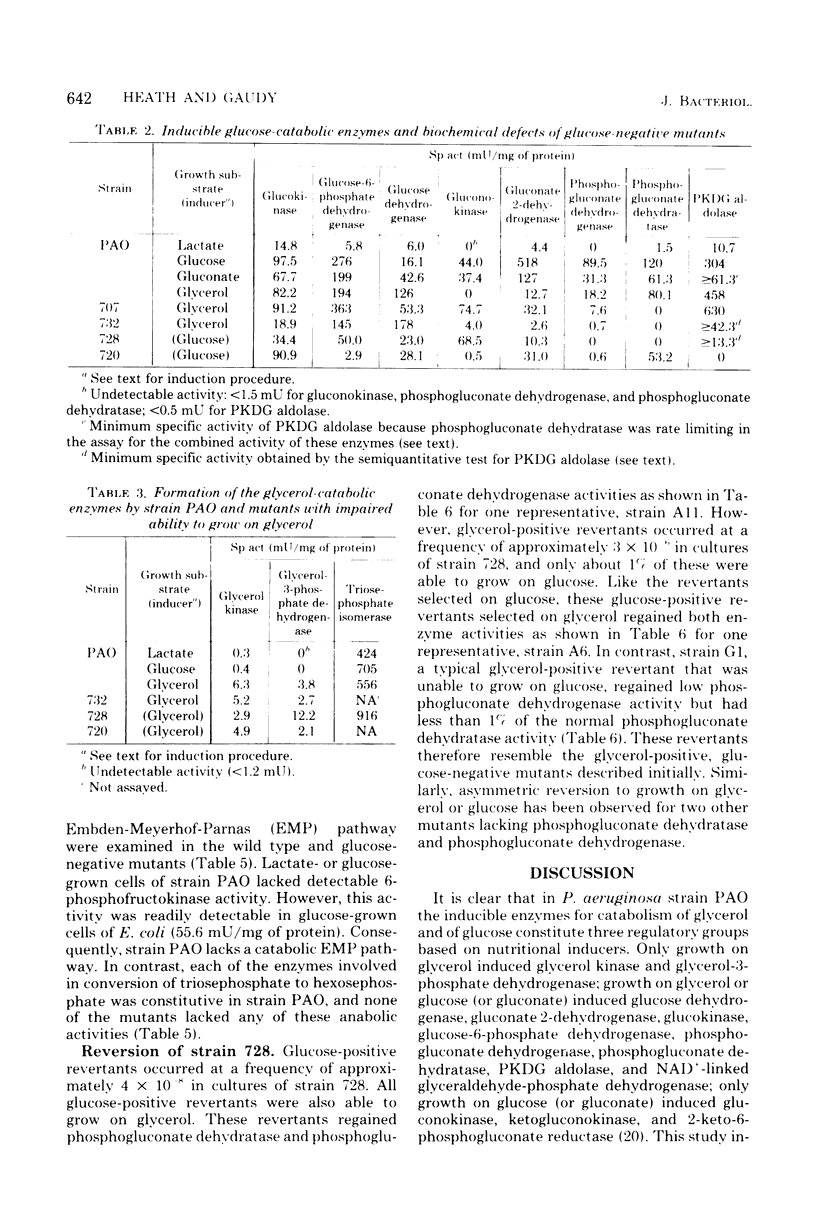
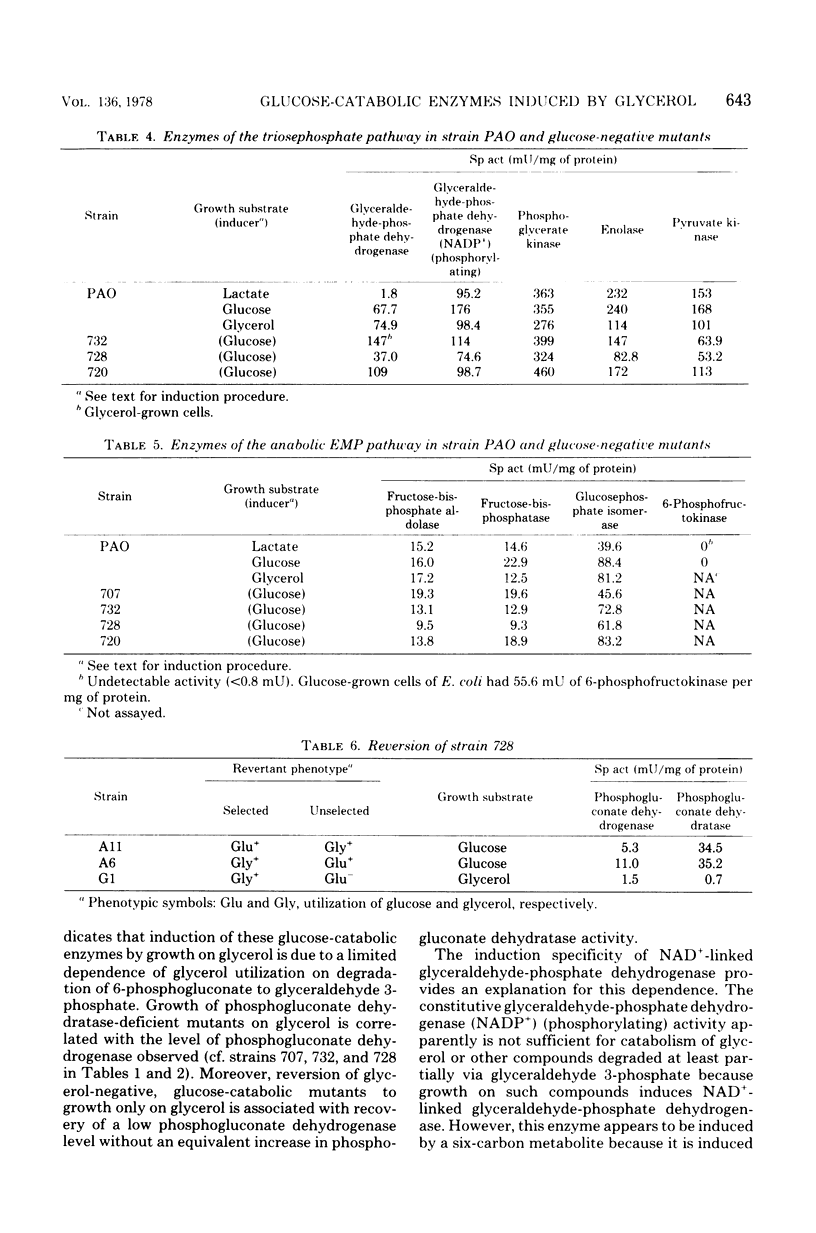
The relationship between catabolism of glycerol and metabolism of hexosephosphate derivatives in Pseudomonas aeruginosa was studied by comparing the growth on glycerol and enzymatic constitution of strain PAO with these characteristics of glucose-catabolic mutants and revertants. Growth of strain PAO on glycerol induced a catabolic oxidized nicotinamide adenine dinucleotide-linked glyceraldehyde-phosphate dehydrogenase and seven glucose-catabolic enzymes. The results indicated that these enzymes were induced by a six-carbon metabolite of glucose. All strains possessed a constitutive anabolic Embden-Meyerhof-Parnas pathway allowing limited conversion of glycerol-derived triosephosphate to hexosephosphate derivatives, which was consistent with induction of these enzymes by glycerol. Phosphogluconate dehydratase-deficient mutants grew on glycerol. However, mutants lacking both phosphogluconate dehydrogenase and phosphogluconate dehydratase were unable to grow on glycerol, although these strains possessed all of the enzymes needed for degradation of glycerol. These mutants apparently were inhibited by hexosephosphate derivatives, which originated from glycerol-derived triosephosphate and could not be dissimilated. This conclusion was supported by the fact that revertants regaining only a limited capacity to degrade 6-phosphogluconate were glycerol positive but remained glucose negative.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blevins W. T., Feary T. W., Phibbs P. V., Jr 6-Phosphogluconate dehydratase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1975 Mar;121(3):942–949. doi: 10.1128/jb.121.3.942-949.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. E., Hellebust J. A., Watson S. W. Reductive pentose phosphate cycle in Nitrosocystis oceanus. J Bacteriol. 1966 Mar;91(3):1178–1185. doi: 10.1128/jb.91.3.1178-1185.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., STANIER R. Y. Studies on the oxidation of glucose by Pseudomonas fluorescens. J Bacteriol. 1951 Aug;62(2):181–186. doi: 10.1128/jb.62.2.181-186.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Butters S. J., Quay S. C., Friedman S. B. Glucose uptake and phosphorylation in Pseudomonas fluorescens. J Bacteriol. 1974 Oct;120(1):147–153. doi: 10.1128/jb.120.1.147-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin J. E., Fraenkel D. G. 2-keto-3-deoxygluconate 6-phosphate aldolase mutants of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1277–1283. doi: 10.1128/jb.108.3.1277-1283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood L. B., Tabenkin B., Ward G. E. The Production of Gluconic Acid and 2-Keto-Gluconic Acid from Glucose by Species of Pseudomonas and Phytomonas. J Bacteriol. 1941 Jul;42(1):51–61. doi: 10.1128/jb.42.1.51-61.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necásek J., Pikálek P., Drobník J. The mutagenic effect of prolonged treatment with ethyl methanesulfonate. Mutat Res. 1967 Jul-Aug;4(4):409–413. doi: 10.1016/0027-5107(67)90003-6. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Rosemeyer M. A. 6-Phosphogluconate dehydrogenase from human erythrocytes. Methods Enzymol. 1975;41:220–226. doi: 10.1016/s0076-6879(75)41051-5. [DOI] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Feary T. W., Blevins W. T. Pyruvate carboxylase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1974 Jun;118(3):999–1009. doi: 10.1128/jb.118.3.999-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Friedman S. B., Eisenberg R. C. Gluconate regulation of glucose catabolism in Pseudomonas fluorescens. J Bacteriol. 1972 Oct;112(1):291–298. doi: 10.1128/jb.112.1.291-298.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L., Berman S. M., Cánovas J. L., Berman R. H. Pathways of D-fructose catabolism in species of Pseudomonas. Arch Microbiol. 1977 Feb 4;112(1):49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Abramsky T. 6-phosphogluconate dehydrogenase from Neurospora crassa. Methods Enzymol. 1975;41:227–231. doi: 10.1016/s0076-6879(75)41052-7. [DOI] [PubMed] [Google Scholar]
- Silverberg M., Dalziel K. 6-Phospho-D-gluconate dehydrogenase from sheep liver. Methods Enzymol. 1975;41:214–220. doi: 10.1016/s0076-6879(75)41050-3. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Enzymatic control of the metabolic activity of Pseudomonas aeruginosa grown in glucose or succinate media. Biochim Biophys Acta. 1969 Dec 30;192(3):395–401. doi: 10.1016/0304-4165(69)90388-2. [DOI] [PubMed] [Google Scholar]
- Tsay S. S., Brown K. K., Gaudy E. T. Transport of glycerol by Pseudomonas aeruginosa. J Bacteriol. 1971 Oct;108(1):82–88. doi: 10.1128/jb.108.1.82-88.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M., Cánovas J. L. Glucolysis in Pseudomonas putida: physiological role of alternative routes from the analysis of defective mutants. J Bacteriol. 1973 Nov;116(2):908–914. doi: 10.1128/jb.116.2.908-914.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tigerstrom M., Campbell J. J. The tricarboxylic acid cycle, the glyoxylate cycle, and the enzymes of glucose oxidation in Pseudomonas aeruginosa. Can J Microbiol. 1966 Oct;12(5):1015–1022. doi: 10.1139/m66-136. [DOI] [PubMed] [Google Scholar]
- WANG C. H., STERN I. J., GILMOUR C. M. The catabolism of glucose and gluconate in Pseudomonas species. Arch Biochem Biophys. 1959 Apr;81(2):489–492. doi: 10.1016/0003-9861(59)90229-2. [DOI] [PubMed] [Google Scholar]


