Abstract
There are defined hypothalamic functions in the genesis of thirst, but little is known of the cortical processes subserving consciousness of thirst notwithstanding the medical disorders that occur in psychiatric illness, addiction, and the attested decline of thirst with aging. In 10 adult males, positron emission tomography scans were made (i) during genesis of moderate thirst by infusion of i.v. hypertonic saline 0.51 M, (ii) after irrigation of the mouth with water to remove the sensation of dryness, and (iii) 3, 14, 45, and 60 minutes after drinking water to fully satiate thirst. The correlation of regional cerebral blood flow with thirst score showed the major activation to be in the posterior cingulate. Maximum thirst sensation evoked 13 highly significant activations and 9 deactivations in cingulate and parahippocampal gyri, insula, thalamus, amygdala, and mesencephalon. It is possible that cingulate sites (Brodmann’s areas 32, 24, and 31) that persisted with wet mouth but disappeared immediately after drinking to satiation may have an important role in the consciousness of thirst. Consciousness of thirst, a primal vegetative emotion, and satiation of thirst appear to be subserved by phylogenetically ancient brain regions. This is salient to current discussion on evolutionary emergence of primary consciousness.
In the face of dehydration (increase of extracellular sodium concentration) and osmotic pressure, animals actively seek water, often by using memory of the sources. This is consonant with J. Z. Young’s (1) emphasis that a general definition of intentionality is a property of mental life that refers to entities that are not observable, that is, not immediately present at the time. Longuet Higgins (2) has suggested that for an animal to have an intention, to have a goal, may require it to form an internal model—an image of the external world. Thus, thirst behavior with search for water can be interpreted as evidence of consciousness. Water deprivation with progression of thirst to the point of desire totally occupying the stream of consciousness is an exemplar of a basic vegetative system. Rullier (3) in 1821 in the Dictionaire des Sciences Medicales par une Societé de Médicins et Chirurgiens said of thirst “le sentiment le plus vif et le plus impérieux de la vie.”
The phylogeny of drinking behavior has been comprehensively analyzed by Fitzsimons (4, 5). Overall, comparative studies of drinking as physiologically apt behavior suggest thirst sensation and intentional seeking of water might have emerged very early in vertebrates, with implications in the phylogeny of consciousness. Other evidence has suggested to biologists that primal awareness emerged in animals lower in the phylogenetic scale than mammals (6–10).
The primacy of structures in the anterior wall of the third ventricle (11), the role of osmoreceptors in the circumventricular organs (12, 13), and sodium sensors within the blood–brain barrier (14) in the genesis of thirst has been reviewed in the initial report of this work (15), which concerned the correlation of changes in regional cerebral blood flow (rCBF) with change of plasma Na concentration.
Emergent knowledge of the primary hypothalamic genesis of thirst displaced the Cannon theory (16) that dry mouth was the primary cause. Sensory inflow from the dry mouth areas does, however, contribute to thirst sensation.
Disorders of thirst are important in clinical medicine. Polydipsia, which may lead to serious hyponatremia, is seen in psychiatric disorders (17, 18), as for example in schizophrenic inpatients (19, 20). Analysis of “rush” experienced by heroin and cocaine addicts showed that thirst was one of the highest of 20 ranked sensations (21) along with excitement, pleasure, and strength. Narcotics like morphine and pethidine have a large dipsogenic effect 2–6 hours after injection into rats (22). Conversely, the established decline in thirst sensation and attendant drinking behavior with aging (23–25) is conducive to hypernatremia. This is often much amplified in confused states, cerebrovascular disease, and Alzheimer’s disease (26).
A major issue in the field of genetically programmed ingestive behavior is the fact that an animal or human, when dehydrated, will drink rapidly to satiation over 3–10 minutes. Thereupon, thirst and the motivation to drink disappears (27), long before the fluid drunk could be absorbed from the gut and correct any chemical changes—systemic or in brain fluids—that might be generative of thirst. This was confirmed experimentally (15). Striking alterations in consciousness of thirst occurred independent of any change of plasma Na concentration ([Na]) or osmolality. This process of rapid satiation, albeit with variation between species in relation to whether immediate correction of the total deficit occurs, carries high survival value because it allows animals to gratify desire and exit from situations, e.g., waterholes, where they are particularly vulnerable to predators (27). Thus, the study with the behavioral sequence contrived and the changes in consciousness of thirst so entrained addressed issues of broad biological importance.
In the positron emission tomography (PET) experiments described here, thirst was contrived in 10 male volunteers by an attested method of rapid intravenous infusion of hypertonic 0.51 M NaCl, which increased plasma [Na] by 4 mmol/liter. After control scans, scans were made during increasing plasma [Na] and when thirst reached an apogee (mean of 43 min after the end of the i.v. infusion). Thereupon, the subjects rinsed their mouth with water without swallowing, which removed the “dry mouth” component, although the thirst sensation remained. After scanning this condition, in which the mouth was wet but thirst persisted, they were permitted to drink water to fully satiate thirst, and were scanned sequentially at 3, 14, 45, and 60 minutes after the act of satiation.
METHODS
Subject Preparation.
The subjects were male, aged 24–36 years, and preparation, image analysis, and institutional consent procedures for this experiment have been reported in an earlier report (15). The experimental sequence involved two control rest scans, scanned during and at the end of rapid infusion of 0.51 M NaCl, when maximum thirst developed a mean of 43 min later, after irrigation of the mouth with water, and at 3, 14, 45, and 60 min after drinking water to satiation. The principal contrasts reported are of maximum thirst, wetting the mouth, and 3 and 14 min after drinking to satiation where each condition is compared to the first preinfusion nonthirsty baseline condition. PET and MRI scans were acquired with each subject supine and with his head supported in a foam-padded, hemicylindrical head holder, with eyes closed and in a quiet room. Each of these four primary comparisons achieved significance (P < 0.001) by a histogram based, nonregional omnibus statistic. The significance of the brain activations and deactivations responsible for the omnibus effects were tested with voxel-based Z statistic analyses. Two statistical thresholds were used: one for large clusters, another for smaller clusters. Large clusters (≥60 contiguous voxels) were required to have a less rigorous Z threshold (Z > 1.96; uncorrected P < 0.01), as there is less likelihood of large clusters occurring by chance. Smaller clusters (<60 voxels) were required to have a higher Z threshold (Z > 3.27; uncorrected P < 0.0005).
A correlational analysis also was undertaken (15), whereby rCBF was correlated against the thirst scores reported by each subject for each scan. Significant activations were chosen as those with Z scores >3.27.
A second independent analysis of the data also was undertaken. An automated image registration algorithm (AIR 3.0) (28, 29) was used to align the 10 PET images of each subject, using a linear transformation, to a standard average rCBF PET image located in Talairach space. Each PET image was then resampled at a 2.0 × 2.0 × 4.0 mm voxel size. Each subject’s MRI was aligned to a standard MRI located in Talairach space, and an average of the 10 individual MRIs was computed. The PET images were smoothed by using a three-dimensional 12-mm Full-Width Half-Maximum Gaussian blurring function (AIR 3.0). Statistical parametric mapping (SPM96) (30) was used to identify voxels that had statistically significant relative rCBF changes. Subtraction analyses comparing maximum thirst, wet mouth, and 3 and 14 minutes after drinking water to satiation to the first preinfusion scan were made. Also, a subtraction analysis was made between the three conditions involving a high state of thirst (100% infusion, maximum thirst, and wet mouth) and the six conditions where little or no thirst was experienced by the subjects—namely, the initial two resting scans and the scans taken 3, 14, 45, and 60 min after drinking to satiation. Significant activations were chosen as those having Z scores >2.33 and >20 voxels.
RESULTS
As reported (15), plasma [Na] increased by 4 mmol/liter at the end of the 0.51 M NaCl infusion (0.2 ml⋅kg−1⋅min−1 for 25 or 50 min), and the increased concentration persisted over the remainder of the experiment. The subjects evaluated thirst score on the basis of 10 being equivalent to the worst thirst the subject had experienced, and 0 being no sensation of thirst. The thirst score increased during the i.v. infusion, reached apogee of 5.25 ± 0.90 (mean ± SEM) with a mean of 43 ± 2.5 min after the infusion finished. Thirst score decreased (3.55 ± 0.64) but still remained highly significantly elevated after irrigating the mouth with water, and then fell to near baseline (0.85 ± 0.43) within 3 min of drinking water to satiation. It remained near baseline for the remainder of the experiment (15).
Correlation of Thirst Score and rCBF.
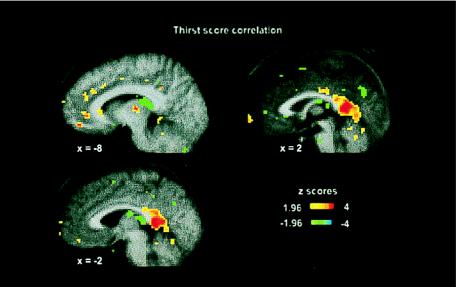
The principal brain area that correlated with thirst score is shown in Fig. 1 (0, −44, 8; cluster size 113, Z = 3.76). This posterior cingulate activation area involving Brodmann’s area (BA) 29 and BA 26 was bilateral, extending from x = +6 and −6. A number of subsignificant activations were observed in the left mediodorsal nucleus of the thalamus (Z = 3.07) and the left pulvinar (x = −18, y = −24, z = −2; cluster size 42; Z = 3.16), and in the anterior cingulate in BA 24 and BA 32 (Fig. 1).
Figure 1.
The correlation of rCBF and the change-of-thirst score derived from 99 PET scans from 10 subjects during the experimental sequence. The sections are at x = −8, x = −2, and x = + 2 and show activations (red-yellow) and deactivations (blue-green). The color coding of Z scores is shown in the figure. The bilateral posterior cingulate activations are evident.
Subtraction Analysis of Maximum Thirst.
The salient change in the brain associated with the development of moderate-to-severe thirst (maximum thirst minus baseline) occurred predominantly in the cingulate gyrus (10 areas with Z score > 3.08, Figs. 2 and 3). There also were highly significant activations in parahippocampal, insula, and thalamic areas (Table 1 and Fig. 2). The cingulate areas involved anterior (Table 1) and two less strong activations in the posterior cingulate region, i.e., BA 29 (x = −4, y = −42, z = 20; cluster size 33, Z = 3.17; and x = −2, y = −44, z = 8; cluster size 51; Z = 3.11). There were also six foci in the midcingulate (y = −4 to y = −16). Overall, BA 24, 9, 32, and 29 were involved. Cingulate, amygdala, parahippocampus, thalamic, and two brainstem areas, one in the region of the paranigral pigmented and parabrachial nucleus (31) were contemporaneously highly significantly deactivated (Figs. 2 and 3). Maximum thirst was associated with parietal activation; the strongest focus was in the mouth region of the post-central gyrus and disappeared with wetting the mouth (Table 1, Fig. 2).
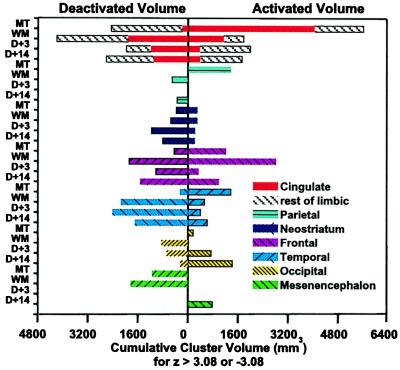
Figure 2.
The volume (mm3) of the regional activations and deactivations observed in each brain region during maximum thirst, 5 min after wetting the mouth, 3 min after drinking to satiation, and 14 min after drinking to satiation are shown as a stacked bar graph. “Rest of limbic” refers collectively to the parahippocampal and insula cortex and amygdala and also includes the thalamus. The activation volumes were determined by adding all voxels with Z score >3.08 and deactivation by adding voxels with Z score <−3.08. MT, maximum thirst; WM, wet mouth; D + 3, 3 min after drinking water to satiation; D + 14, 14 minutes after drinking water to satiation.
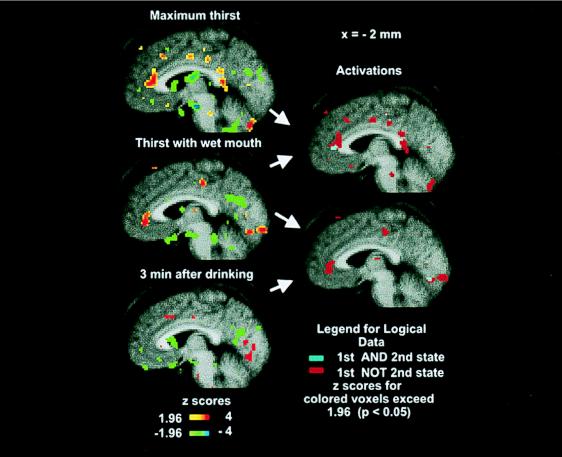
Figure 3.
(Left) The PET activations (saggital section x = −2) of the average brain of the 10 subjects where initial resting non-thirsty state is compared with maximum thirst (Top), 5 min after wetting the mouth (Middle), and 3 min after drinking to satiation (Bottom). Only significant activations (Z > 1.96) and deactivations (Z < −1.96) are shown as per color bars. (The color coding of Z scores is shown on the figure.) (Right) Logical images formed by logical AND (cyan: present in both) of two activation statistical images, and logical NOT (red: present in first but not second) of the two activation images as indicated by arrows. Each logical image is formed from two statistical images as indicated by the arrows on the right of the statistical images.
Table 1.
Changes in rCBF relative to resting PET scans
| Maximum thirst
|
Wet mouth
|
3 min. after satiation
|
14 min. after satiation
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment Hemisphere | Gyrus or region | BA | x | y | z | Cluster size, mm3 | Z score | Segment Hemisphere | Gyrus or region | BA | x | y | z | Cluster size, mm3 | Z score | Segment Hemisphere | Gyrus or region | BA | x | y | z | Cluster size, mm3 | Z score | Segment Hemisphere | Gyrus or region | BA | x | y | z | Cluster size, mm3 | Z score |
| Positive activations | |||||||||||||||||||||||||||||||
| Limbic | Limbic | Limbic | Limbic | ||||||||||||||||||||||||||||
| L | Ci | 24 | −6 | −10 | 35 | 80 | 3.91 | R | Ci | 24 | 16 | −12 | 40 | 65 | 3.63 | L | I | — | −32 | −26 | 10 | 63 | 3.85 | R | Ci | 24 | 14 | 4 | 38 | 48 | 4.17 |
| R | Ci | 24 | 14 | −16 | 42 | 66 | 3.57 | L | AC | 32 | −2 | 36 | −3 | 41 | 3.44 | L | VL | — | 20 | −22 | 0 | 69 | 3.63 | R | PL | — | 20 | −16 | 12 | 50 | 3.32 |
| L | Ci | 24 | −10 | −4 | 36 | 35 | 3.54 | R | PCi | 31 | 8 | −50 | 22 | 37 | 3.66 | Neostriate | R | VL | — | 21 | −22 | 4 | 78 | 4.41 | |||||||
| L | AC | 32 | −4 | 22 | 37 | 34 | 3.28 | Neostriate | R | Pu | — | 16 | 6 | 4 | 29 | 3.35 | Frontal | ||||||||||||||
| L | AC | 24 | −1 | 30 | 10 | 73 | 3.23 | R | CH | — | 8 | 20 | 10 | 38 | 3.85 | Frontal | R | MF | 6 | 13 | −18 | 44 | 47 | 3.32 | |||||||
| L | P | 28 | −18 | −10 | −22 | 63 | 5.13 | Frontal | R | MF | 11 | −14 | 42 | −10 | 43 | 3.68 | Temporal | ||||||||||||||
| R | I | 13 | 40 | −26 | 16 | 60 | 3.62 | L | MF | 47 | −16 | 40 | −10 | 71 | 4.29 | Temporal | R | ST | 22 | 51 | −38 | 18 | 55 | 3.69 | |||||||
| R | PL | — | 20 | −16 | 12 | 46 | 3.62 | R | MF | 47 | 38 | 36 | −6 | 63 | 3.63 | R | TT | 41 | 36 | −26 | 18 | 70 | 3.38 | L | ST | 38 | −47 | 17 | −28 | 23 | 3.46 |
| Parietal | R | IF | 47 | 34 | 31 | −4 | 52 | 3.49 | Occipital | Occipital | |||||||||||||||||||||
| R | PG | 3 | 36 | −22 | 38 | 59 | 3.62 | R | IF | 45 | 51 | 16 | 10 | 49 | 3.44 | R | LG | 18 | 4 | −86 | −8 | 66 | 3.65 | R | LG | 18 | 4 | −68 | −2 | 87 | 4.2 |
| L | PG | 40 | −34 | −22 | 24 | 30 | 3.45 | R | SC | 11 | 13 | 22 | −12 | 41 | 3.33 | L | Cu | 17 | −18 | −82 | 6 | 36 | 3.38 | ||||||||
| Frontal | Diencephalon–Mesencephalon | ||||||||||||||||||||||||||||||
| L | O | 11 | −15 | 24 | −10 | 33 | 3.37 | L | M | — | −6 | −28 | −4 | 34 | 3.4 | ||||||||||||||||
| Temporal | R | Po | — | 20 | −34 | −30 | 64 | 3.19 | |||||||||||||||||||||||
| R | MT | 37 | 37 | −62 | 7 | 63 | 3.82 | ||||||||||||||||||||||||
| R | TT | 41 | 40 | −26 | 16 | 60 | 3.62 | ||||||||||||||||||||||||
| R | MT | 21 | 56 | 2 | −14 | 50 | 3.51 | ||||||||||||||||||||||||
| Negative activations | |||||||||||||||||||||||||||||||
| Limbic | Limbic | Limbic | Limbic | ||||||||||||||||||||||||||||
| R | A | — | 16 | −6 | −12 | 40 | −3.75 | L | Ci | 32 | −14 | 11 | 36 | 26 | −3.42 | L | PCi | 30 | 0 | −66 | 16 | 67 | −3.91 | L | PCi | 18 | −22 | −66 | 8 | 65 | −3.34 |
| R | DM | — | 5 | −16 | 16 | 75 | −3.95 | L | PCi | 29 | −10 | −44 | 20 | 88 | −3.67 | L | PCi | 30 | −12 | −61 | 16 | 82 | −3.19 | R | DN | — | −5 | −10 | 14 | 47 | −3.39 |
| L | DM | — | −2 | −12 | 14 | 73 | −3.81 | R | PCi | 31 | 8 | −60 | 12 | 80 | −3.81 | L | I | — | −34 | 18 | 1 | 30 | −3.6 | ||||||||
| L | P | 19 | −22 | −48 | 4 | 62 | −3.76 | ||||||||||||||||||||||||
| R | P | 36 | 26 | −24 | −24 | 27 | −3.67 | ||||||||||||||||||||||||
| R | AN | — | 6 | −8 | 14 | 51 | −3.51 | ||||||||||||||||||||||||
Positive activations (increase in rCBF) relative to the control (resting) PET scan with Z score >3.27 or cluster size >60 mm3. The four states scanned were maximum thirst associated with the sense of dryness in mouth; after irrigating the mouth with water but not swallowing (wet mouth); 3 min. after drinking water to satiate thirst; and 14 min. after drinking water to satiate thirst. Data are shown for the cingulate and hippocampal gyrus, amygdala, and thalamus (termed limbic system–Papez); the parietal, frontal, temporal, and occipital lobes; the neostriate; and the diencephalon–mesencephalon. Negative activations (decrease in rCBF) relative to the control PET scan with Z score <−3.27 or cluster size >60 mm3. For the same four states, but only limbic system–Papez deactivations are shown. Ci, cingulate; P, parahippocampal; AC, anterior cingulate; I, insula; PL, posterolateral nucleus of thalamus; PG, postcentral gyrus; MT, midtemporal gyrus; TT, transverse temporal gyrus; A, amygdala; DM, dorsomedial nucleus of thalamus; CH, head of caudate nucleus; MF, medial frontal gyrus; IF, inferior frontal gyrus; SC, subcallosal gyrus; VL, ventrolateral posterior nucleus of thalamus; Pu, putamen; LG, lingual gyrus; Cu, cuneus; M, midbrain; Po, pons; DN, dorsal nucleus of thalamus; PCi, postcingulate; AN, anterior nucleus of thalamus; ST, superior temporal gyrus; O, orbital frontal gyrus.
Wetting the Mouth During Maximum Thirst.
Irrigating the mouth with water caused reduction of cingulate activation, although highly significant foci persisted in the anterior, mid-, and posterior cingulate (Table 1) (BA 24, 32, and 31), which disappeared immediately after drinking to satiation (Fig. 3; Table 1). Wetting the mouth caused a number of strong frontal activations to appear, particularly in BA 47 (Table 1). Also, deactivation foci appeared (Table 1), particularly in the posterior cingulate and parahippocampal regions (BA 31, 30, 19, 36).
Satiation of Thirst.
By 3 min after drinking water to satiation, the major activations in the cingulate had disappeared (Table 1, Fig. 3). However, by 14 min, a new highly significant activation had appeared in the mid-cingulate in BA 24 (x = −14, y = 4, z = 38; Z = 4.17) (Table 1).
Whereas with the criterion of significance employed no activation sites were detected in mesencephalon and diencephalon with maximum thirst, two highly significant activation foci had emerged 14 min after drinking to satiation, one in the midbrain in the periaqueductal gray (x = −6, y = −28, z = −4) and one in the pons (Table 1).
In addition to the primary contrasts described in the preceding paragraphs, secondary contrasts were created by applying logical operators (AND, OR, NOT) to pairs of the statistical images. The purpose of this analysis was to highlight activations unique to one state or common between two or more states. Fig. 3 reflects such a saggital section at x = −2. Anterior cingulate cortex activity persisted after wetting of the mouth, an overlap (cyan) occurred in BA 32, and the anterior cingulate activity returned to baseline levels after drinking to satiation (Fig. 3). Overall, maximum thirst was uniquely associated with diffuse activation of the limbic and paralimbic cortex (Table 1).
As an independent appraisal, these data were also analyzed by using SPM (Methods). The major brain activations with maximum thirst relative to baseline were bilateral in the anterior cingulate at BA 32 (Z = 3.21; Z = 2.77) at Talairach coordinates (x = −6, y = 34, z = 24) and (x = 12, y = 34, z = 4), respectively and at postcingulate (x = 14, y = −44, z = 8; Z = 2.75) (BA 29). An activation also was observed in the left lobus paracentralis at BA 31 (x = −10, y = −26, z = 44; Z = 2.18). After wetting the mouth, brain activations were again observed bilaterally in the anterior cingulate (Z = 2.79; Z = 3.13) at Talairach coordinates (x = −12, y = 34, z = 4 and x = 6, y = 14, z = 36), respectively. Activations also were observed in the medial right frontal cortex, BA 11 (x = 21, y = 40, z = 12; Z = 3.21). Three minutes after drinking to satiation, the medial anterior cingulate regions were no longer activated; instead, the major activations were observed in the right precentral gyrus at BA 6 (x = 42, y = 2, z = 28; Z = 3.59) in the right lateral posterior thalamus (x = 18, y = −22, z = 16; Z = 3.39;), and in the right superior temporal gyrus at BA 22 (x = 64, y = −34, z = 8; Z = 2.8). Fourteen minutes after drinking to satiation, a new strong activation in the left cingulate gyrus (x = −14, y = −4, z = 32; Z = 3.48) was observed.
A categorical subtraction analysis (100% infusion, + maximum thirst + wet mouth) compared to (rest 1, + rest 2, + satiation at +3 min, +14 min, +45 min, + 60 min) showed major effects in BA 32 and BA 24 (namely, x = 10, y = 24, z = 32; cluster size 114, Z = 3.15, and x = 0, y = 20, z = 32; Z = 2.61) for BA 32, and (x = 12, y = −10, z = 44; cluster size 35, Z = 2.74) for BA 24. Bilateral strong claustrum activation also occurred (x = 32, y = −20, z = 8; cluster size 57; Z = 3.26); and (x = −32, y = −14, z = 12; cluster size 37; Z = 2.82).
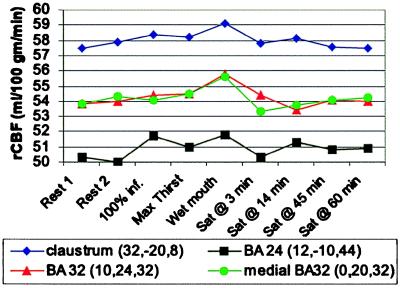
The changes in rCBF at the four strongest activations from this subtraction analysis show a progressive rise in rCBF during the infusion scans, with maximum value occurring with wet mouth, and an obvious decrease of rCBF occurred by 3 min after drinking to satiation. The final rCBF (scans 9 and 10) was equivalent to the initial (scans 1 and 2) (Fig. 4).
Figure 4.
The mean rCBF for the 10 subjects at the location of the four strongest activations identified in the subtraction analysis involving the high state of thirst (100% infusion, maximum thirst, and wet mouth) compared with the six scans in which thirst was absent or minimal. The maximal rCBF was reached with the wet mouth scan, and a clear decrease of rCBF occurred 3 min after drinking water to satiation, and by 45 and 60 min, it had returned to the initial rCBF level.
The analysis with the two independent methods highlighted predominance of the cingulate as the site of the most robust activations.
DISCUSSION
General Biological Aspects.
Three results with biomedical importance emerge from this study. First, the genesis of a compelling consciousness of thirst involving a contemporaneous awareness of dryness of the mouth caused striking activation and inhibitory processes in phylogenetically ancient brain regions. The areas involved—parahippocampus, amygdala, and thalamus—are present in reptiles and amphibians (32). Together with cingulate areas and insula, the cortical elements include allocortex and transitional cortex that differ structurally from the isocortex characteristic of the association areas, which contain six more or less pronounced layers (33–35). The five-layered cortex of the cingulate regions appears in the earliest mammals (36), but there are differing views (35, 37, 38) regarding the phylogeny of these limbic areas. Butler and Hodos (38) state that the current evidence indicates that the limbic system evolved long before the advent of any amniote vertebrates, let alone early mammals. Overall, the findings are consonant with the concept of thirst as a primitive vegetative function with much of its circuitry emergent early in vertebrate evolution.
Second, Bernard’s (39) experiments on copious drinking with an open esophageal fistula relegated “dry mouth” to a secondary role in thirst genesis. The data here showed reduction of activation foci with wetting of the mouth, not only in parietal sensory areas, but in the limbic system and particularly the cingulate foci. However, the data showed persistence of some cingulate foci after mouth wetting. The results support Cannon’s view (16) of the importance of dry mouth in thirst, but, like the open fistula experiment and the avid drinking in dehydrated ruminants that have continuous salivary secretion (27), they vitiate any notion of a dominant causal role.
Third, the consummatory act of rapid drinking was associated with contemporaneous loss of thirst and the major of the anterior and posterior cingulate activations within 3 min of completion. Characteristically, gratification occurred without any alteration in plasma [Na] or osmolality (27, 40). A new activation appeared caudally in the midcingulum 14 min after drinking to satiation (BA 24). A strong active focus appeared in the midbrain close to the aqueduct, which is of interest in the light of attested cingulate pathways to periaqueductal gray. Satiation also caused several strong limbic deactivations—mainly posterior cingulate, insula, and thalamic. Further experiments will be required to evaluate significance of these regions in the consciousness of gratification. The trio of sensory inflow from mouth, esophageal monitoring of volume swallowed, and gastric distension appears to be jointly sufficient and severally necessary to abolish thirst (27).
Paramount Role of the Limbic System.
The outstanding cerebral effect of significant thirst and dry mouth was a general functional change in the limbic system and entities involved in the Papez circuit (41). The term “limbic system” is used here as a loose construct to imply that limbic cortex and its primary brainstem connections constitute a system involving functional integration (35). Later studies have doubted the value of the concept of a limbic system and stressed the complexity of cortical connections of the hypothalamus (42, 43).
Significant thirst may be termed a primal compelling emotion. In terms of difference from some other emotional states, thirst, when severe, is relatively inaccessible to cognitive amelioration, in contrast to the situation to a variable degree with, for example, anger, hatred, love, sexual desire, territoriality, and fear. Underlying this putative dichotomy may be the fact that the latter emotions involve situational perceptions based on distance receptor input. Thirst, however, is interoceptor-driven and initiated through mechanisms in the phylogenetically ancient basal brain, as with hunger for air. The relative inaccessability of the primal emotion to higher cognitive amelioration may reside in this fundamental of brain organization.
The anterior cingulate receives extensive inputs from the remainder of the limbic system and other regions of the cerebral cortex, especially from areas frequently designated as associative (33, 34, 46, 47). In monkeys, BA 32 receives afferents from BA 9, the temporal pole, and the orbitofrontal cortex (47). The anterior cingulate was the site of activations correlating with change of plasma [Na] in these experiments (15) and some of the wide ranging connections of this region as described by Vogt and others (46, 47) were noted there. There is evidence that the anterior and posterior cingulate are functionally and anatomically coupled through an intracingulate neural network (46, 47). Whether the predominant correlation of the anterior cingulate foci with changes in plasma [Na] (15), and whether the posterior cingulate region with thirst reflects such a functional interconnection subserving genesis of thirst, awaits further investigation.
The posterior cingulate cortex is involved in processes underlying spatial memory and visual association areas, and somatosensory association areas send afferents to the posterior as well as anterior part of the cingulate cortex (34, 48). Afferents also come from the hippocampus (34, 46) and anterior thalamic nuclei. The subtraction studies in which the six low thirst score images were subtracted from conditions of highest thirst score (i.e., end of infusion, maximum thirst, and wet mouth) also showed major activations in the anterior and middle cingulate—BA 32 and BA 24. The bilateral activations also seen in the claustrum were interesting because, inter alia, it receives afferents from the hypothalamus and intralaminar nucleus of the dorsal thalamus (38). Some caution is noted in attributing this bilateral activation to the claustrum in light of its close proximity to the deep insula, which has involvement in salivation and mouth movement.
In relation to major effects in other lobes, the frontal activations (BA 47, 45, and 11) that appeared with wetting the mouth and disappeared with drinking to satiation were striking, and of particular interest in the light of the data of Rolls in monkeys showing there are neurons in the orbitofrontal cortex that respond to taste of water (49). Furthermore, the activity of these neurons was greatest when the monkeys were thirsty, whereas, following satiation, their firing reduced to baseline (49). In our experiments, the large frontal response to the wetting of the mouth while thirst persisted and the disappearance of this effect when the mouth was wet (but wet as a result of drinking water to satiation) was consistent with Rolls’ data. Clinical data does not suggest removal or damage of frontal lobes deranges thirst (33). Vascular lesions damaging the anterior cingulate cause mutism with or without akinesia (51), which are clinical conditions in which thirst derangement may not be evident without systematic study of blood chemistry.
Thalamic activations and deactivations were prominent in the experiment, and connections of the thalamus to the anterior and posterior cingulate, brain stem, hypothalamus, and amygdala are well established (50). The thalamus is a relay of sensory afferent inflow as would have occurred with the dry mouth. The left parahippocampus (BA 28), very strongly activated in maximum thirst, projects to the cingulate, and there is cingulate–parahippocampal continuity rounding the splenium of the corpus callosum.
Evolutionary Considerations.
In relation to current general discussion of the phylogenetic emergence of consciousness, Edelman (7) has proposed that it is the evolutionary development of the ability to create a scene that led to the emergence of primary consciousness. He says, “The word ‘scene’ is meant to convey the idea that responses to roughly contemporaneous events in the world are connected by a set of re-entrant processes… we experience primary consciousness as a ‘picture’ or ‘mental image’ of ongoing categorized events.” Alternate to this primarily distance receptor concept, a theory that has been advanced is that primary awareness might have emerged with the basic vegetative systems, and, accordingly, from interoceptor-initiated brain events (11). That is, the evolutionary origin might have come from sensations and primal emotions arising from sensors and receptors, both internal and surface, signaling that the existence of the creature was immediately threatened—for example, hunger for air, thirst, hunger, pain, and extreme temperature change (13). The basic vegetative systems have control centered in the phylogenetically ancient areas of the brain (mesencephalon and diencephalon), which is also the case with the elemental processes of arousal and of sleep. It is salient, however, to Edelman’s view that the optic tectum is a major anatomical feature in, e.g., the lamprey, alligator, shark, and pigeon. The optic tectum is the recipient of inflow from optic nerves, and in many fishes it is larger than the cerebral hemispheres (32).
The results in this study underscore that thirst is subserved by a complex distributed pattern of activation and deactivation, the functional changes being mainly in phylogenetically ancient brain areas. The data suggests that the anterior and posterior cingulate, as well as the anterior wall of the third ventricle, are major elements of a pattern including thalamic, hippocampal, orbitofrontal, insula, and midbrain sites that subserve the genesis of consciousness of thirst when plasma [Na] increases. A prototypical primal vegetative emotion like thirst is likely in humans to be evocative of memories of sources of fluid and of the pleasures of gratification, thus giving rise to a complex conscious experience, and this possibly might have contributed to some activity in association areas seen here. It is feasible the complex pattern of activations and deactivations found here represent a “dynamic core” (52) of functionally integrated, but anatomically distributed, processes subserving consciousness of thirst.
Acknowledgments
The authors appreciate discussion and bibliographic suggestion from Dr. Helen Mayberg, Dr. Fred Plum, Dr. John McKenzie, Professor Fred Mendelsohn, and Professor David Copolov. This work was supported by the Robert J. Jr. and Helen C. Kleberg Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, The Howard Florey Biomedical Foundation of the U.S., and the National Health and Medical Research Council of Australia.
ABBREVIATIONS
- BA
Brodmann’s area
- rCBF
regional cerebral blood flow
- PET
positron emission tomography
References
- 1.Young J Z. Philosophy and the Brain. London: Oxford Univ. Press; 1986. [Google Scholar]
- 2.Kenny A J P, Longuet Higgins H C, Lucas J R, Waddington C H. The Nature of Mind. Edinburgh: Edinburgh Univ. Press; 1973. [Google Scholar]
- 3.Rullier S. Dictionaire des Sciences Medicales par une Societé de Médecines et de Chirurgiens. 1821;51:448–490. [Google Scholar]
- 4.Fitzsimons J T. The Physiology of Thirst. Cambridge, U.K.: Cambridge Univ. Press; 1979. [Google Scholar]
- 5.Fitzsimons J T. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 6.Smith H W. From Fish to Philosopher. Brown,Boston: Little; 1953. [Google Scholar]
- 7.Edelman G. Bright Air, Brilliant Fire. London: Penguin; 1992. [Google Scholar]
- 8.Griffin D R. The Question of Animal Awareness: Evolutionary Continuity of Mental Experience. Los Altos, CA: Kaufman; 1981. [Google Scholar]
- 9.Denton D A. The Pinnacle of Life. San Francisco: Harper Collins; 1994. [Google Scholar]
- 10.Pitts G C. Perspect Biol Med. 1994;37:275–284. doi: 10.1353/pbm.1994.0085. [DOI] [PubMed] [Google Scholar]
- 11.Denton D A, McKinley M J, Weisinger R S. Proc Natl Acad Sci USA. 1996;93:7397–7404. doi: 10.1073/pnas.93.14.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verney E B. Proc R Soc London Ser B. 1947;135:25–106. [PubMed] [Google Scholar]
- 13.McKinley M J, Denton D A, Weisinger R S. Brain Res. 1978;141:89–103. doi: 10.1016/0006-8993(78)90619-4. [DOI] [PubMed] [Google Scholar]
- 14.Anderssen B, Olsson K. Cond Reflex. 1973;8:147–160. doi: 10.1007/BF03000495. [DOI] [PubMed] [Google Scholar]
- 15.Denton D A, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Proc Nat Acad Sci USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon W B. Proc R Soc London. 1919;90:283–301. [Google Scholar]
- 17.Vieweg V, Pandurangi A, Levenson J, Silverman J. Int J Psychiatry Med. 1994;24:275–303. doi: 10.2190/5WG5-VV1V-BXAD-805K. [DOI] [PubMed] [Google Scholar]
- 18.Jos C J, Evenson R C, Mallya A R. J Clin Psychiatry. 1986;47:368–370. [PubMed] [Google Scholar]
- 19.de Leon J, Dadvand M, Canuso C, Odom-White A, Stanilla J, Simpson G M. Biol Pyschiatry. 1996;40:28–34. doi: 10.1016/0006-3223(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 20.de Leon J, Verghese C, Tracy J I, Josiassen R C, Simpson G M. Biol Psychiatry. 1994;35:408–419. doi: 10.1016/0006-3223(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 21.Seecof R, Tennant F S. Am J Drug Alcohol Abuse. 1986;12:79–87. doi: 10.3109/00952998609083744. [DOI] [PubMed] [Google Scholar]
- 22.Arslan Y, Burckhardt R, Jawaharal K, Ornstein K, Peters G. In: The Physiology of Thirst and Sodium Appetite. de Caro G, Epstein A N, Massi M, editors. New York: Plenum; 1986. pp. 527–534. [Google Scholar]
- 23.Phillips P A, Johnston C I, Gray L. In: Thirst: Physiological and Pyschological Aspects. Ramsay D J, Booth D A, editors. London: Springer; 1991. pp. 403–410. [Google Scholar]
- 24.Anonymous. Lancet. 1984;2:1017–1018. [Google Scholar]
- 25.Crowe M J, Forslling M L, Rolls B J, Phillips P A, Ledingham J G G, Smith R F. Age Aging. 1987;16:285–293. doi: 10.1093/ageing/16.5.285. [DOI] [PubMed] [Google Scholar]
- 26.Albert S G, Nakra B R, Gronberg G T, Caminal E R. Int Psychogeriatr. 1994;6:79–86. doi: 10.1017/s104161029400164x. [DOI] [PubMed] [Google Scholar]
- 27.Denton D A. The Hunger for Salt. London: Springer; 1982. [Google Scholar]
- 28.Woods R P, Grafton S T, Holmes C J, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 29.Woods R P, Grafton S T, Watson J D G, Sicotte N L, Mazziotta J C. J Comput Assist Tomogr. 1997;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Friston K J, Homes A P, Worsley K J, Poline J-P, Frith C D, Frackowiak R S J. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 31.Schaltenbrand G, Bailey P. Einführung in die Stereo-taktischen Operationen mit Einem Atlas des Menschlichen Gehirns. Stuttgart: Thieme; 1959. [Google Scholar]
- 32.Pearson R, Pearson L. The Vertebrate Brain. London: Academic; 1976. [Google Scholar]
- 33.Damasio A R, Van Hoesen G W. In: Neuropsychology of Human Emotion. Heilman K M, Satz P, editors. New York: Guilford; 1983. pp. 85–111. [Google Scholar]
- 34.Roland P E. Brain Activation. New York: Wiley–Liss; 1993. [Google Scholar]
- 35.Maclean P D. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Basel: Birkhauser; 1993. pp. 1–18. [Google Scholar]
- 36.Rose M. J Pyschol Neurol. 1927;35:65–173. [Google Scholar]
- 37.Northcutt R G, Kaas J H. Trends Neurosci. 1995;18:373–378. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- 38.Butler A B, Hodos W. Comparative Vertebrate Neuroanatomy. New York: Wiley–Liss; 1996. [Google Scholar]
- 39.Bernard C. Lecons de Physiologie Experimentale Applique a la Medecine Faites au College de France. Vol. 2. Paris: Bailliere; 1856. [Google Scholar]
- 40.Rolls B J, Rolls E T. Thirst. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 41.Papez J W. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- 42.Brodal A. Neurological Anatomy. New York: Oxford Univ. Press; 1982. [Google Scholar]
- 43.Le Doux J. The Emotional Brain. Simon & Shuster, New York: Touchstone; 1996. [Google Scholar]
- 44.Hertzog A G, Van Hoesen G W. Brain Res. 1976;115:57–69. doi: 10.1016/0006-8993(76)90822-2. [DOI] [PubMed] [Google Scholar]
- 45.Pandya D N, Yeterian E. In: Cerebral Cortex. Peters A, Jones E, editors. New York: Plenum; 1985. pp. 3–61. [Google Scholar]
- 46.Van Hoesen G W, Morecraft R J, Vogt B G. In: Neurobiology of the Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1993. pp. 249–284. [Google Scholar]
- 47.Bentivoglio M, Kultas-Ilinsky K, Ilinsky I. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1993. pp. 71–122. [Google Scholar]
- 48.Olsson C R, Musil S Y, Goldberg M E. In: Neurobiology of Cingulate, Cortex and Limbic Thalamus. Vogt B A, Gabriel, editors. Boston: Birkhauser; 1993. pp. 366–380. [Google Scholar]
- 49.Rolls E T. The Brain and Emotion. London: Oxford Univ. Press; 1999. [Google Scholar]
- 50.Amaral D G, Price J L, Pilkaness A, Carmichael S T. In: The Amygdala. Appleton J P, editor. New York: Wiley–Liss; 1992. pp. 1–66. [Google Scholar]
- 51.Damasio A R, Anderson S W. In: Clinical Neurophysiology. Heilman K M, Valenstein E, editors. New York: Oxford Univ. Press; 1993. pp. 409–460. [Google Scholar]
- 52.Tononi H, Edelman G H. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]