Abstract
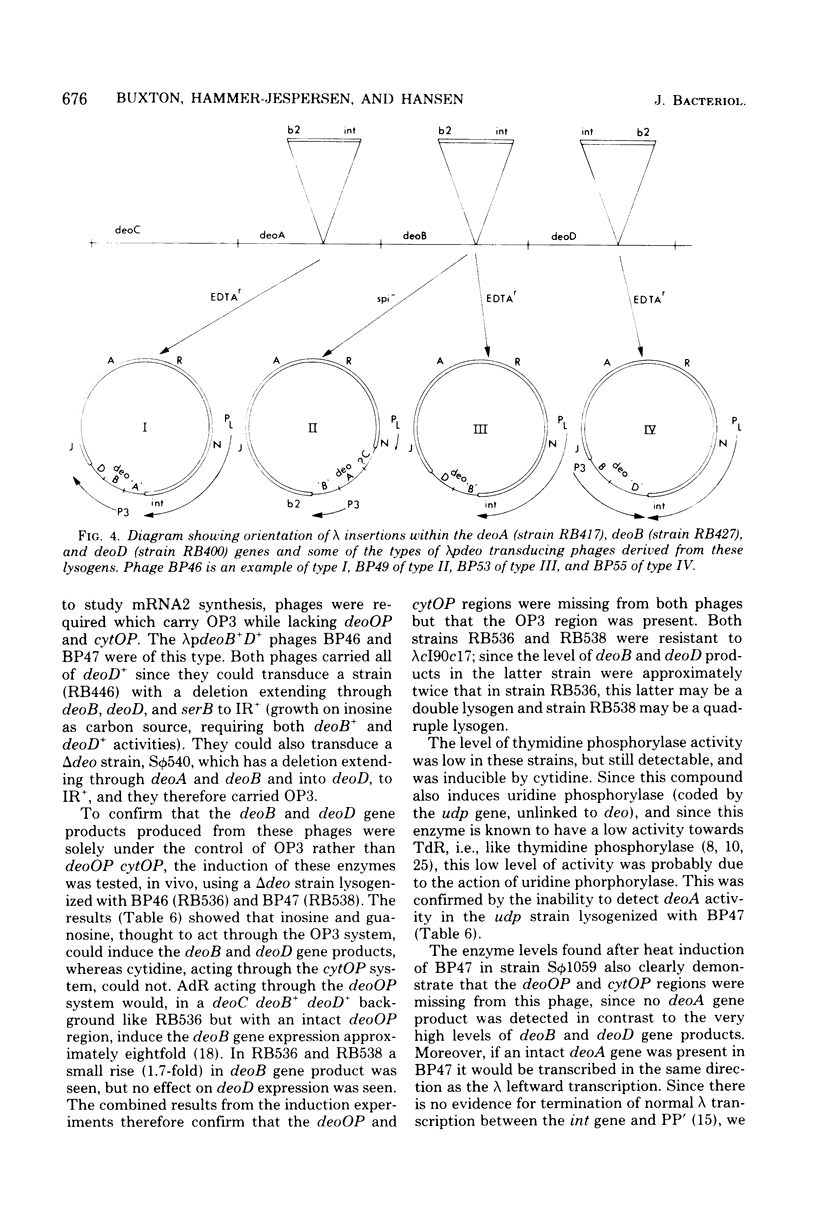
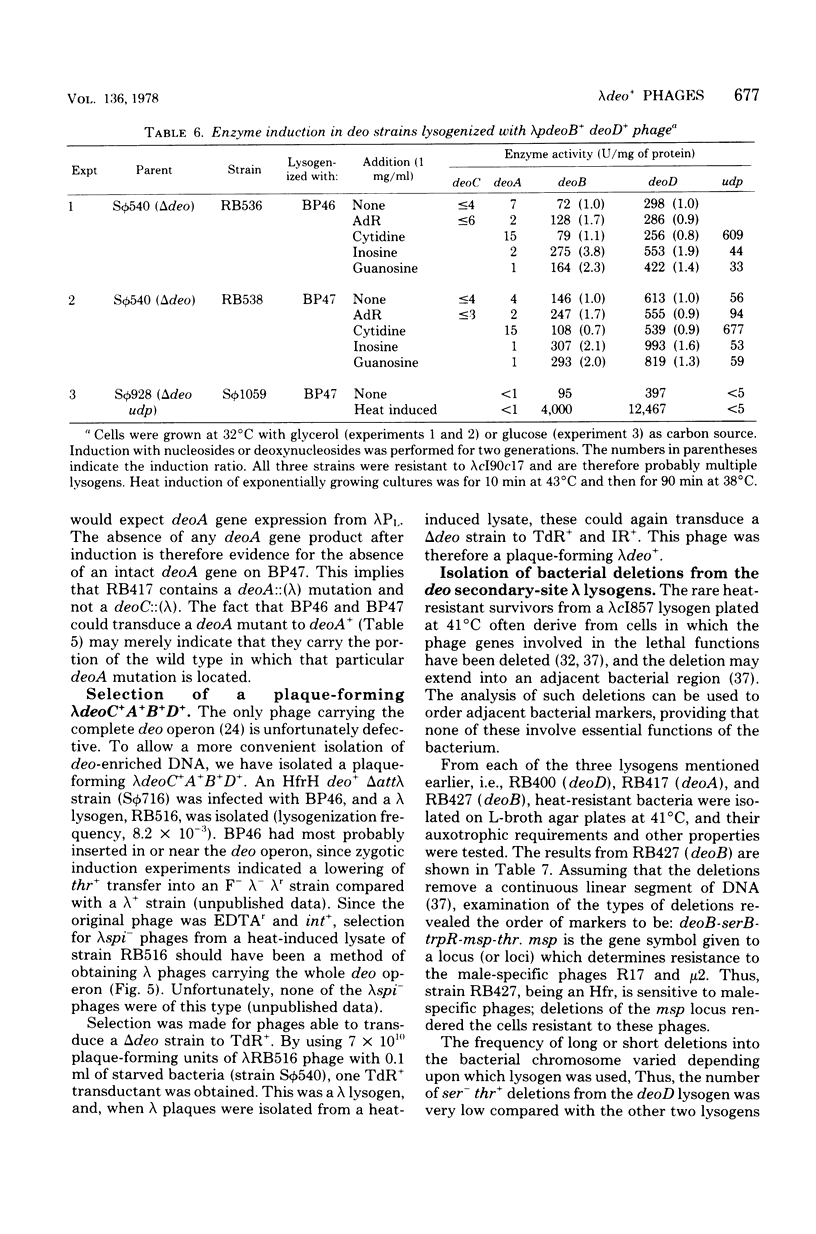
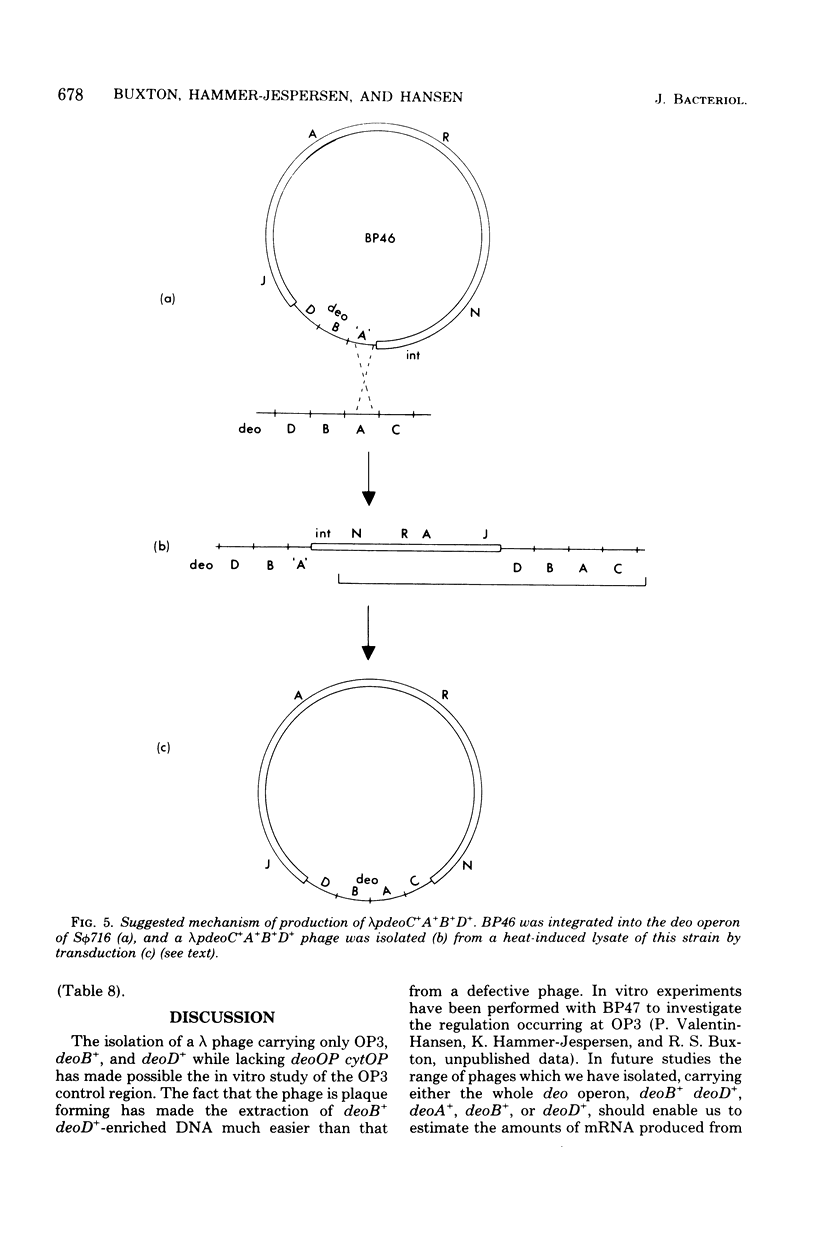
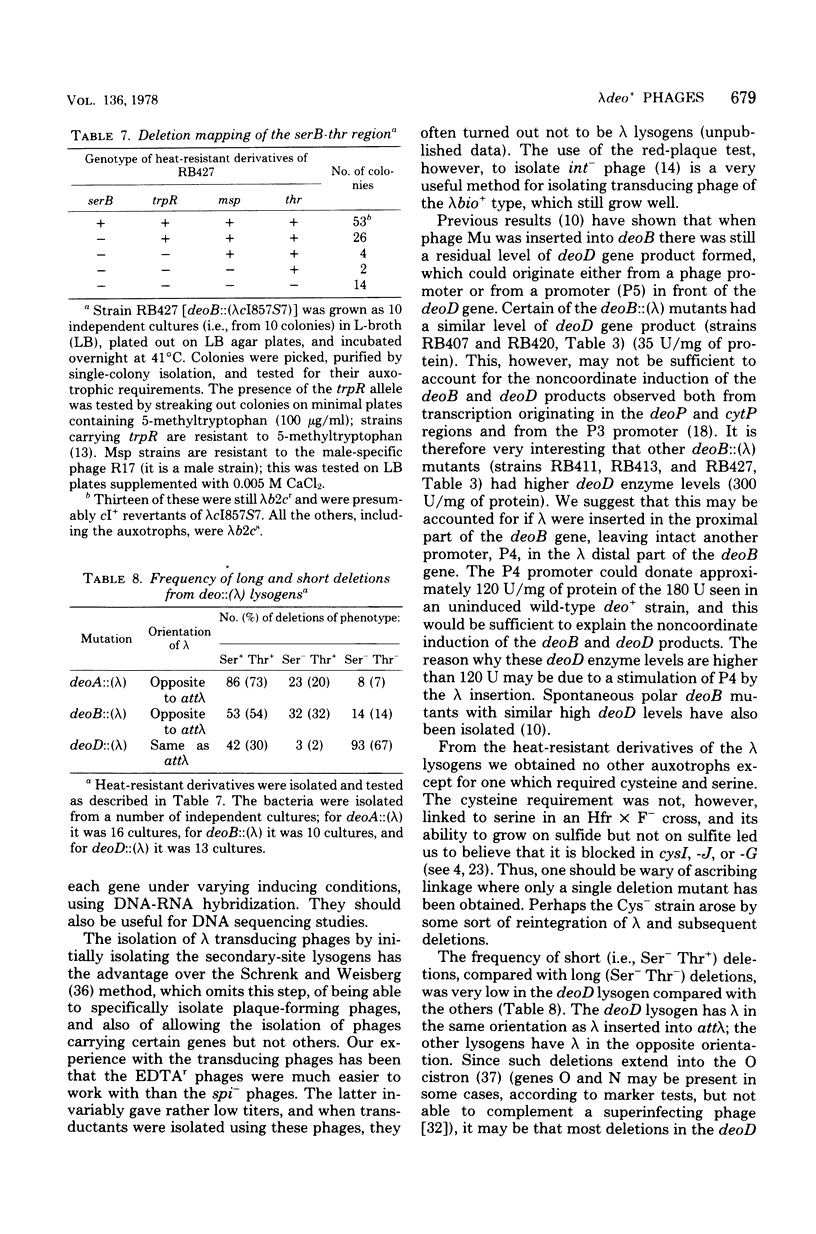
A procedure has been devised to isolate plaque-forming lambda cI857S7 transducing bacteriophage which carry the internal promoter, P3, of the deo operon of Escherichia coli and the deoB and deoD genes, while lacking the deoP and cytP promoters of the same operon, in order to study, specifically, regulation at the P3 site. This has been accomplished by selecting for the insertion of bacteriophage lambda into the deoA gene in a strain deleted for the normal lambda attachment site (delta att lambda) and isolating from this lysogen lambda spi- and lambda EDTAr phage. Among these, lambda pdeoB+D+ phage were identified by their transducing abilities. From in vivo enzyme induction experiments performed on a delta deo strain lysogenized with such phage, they were shown to carry the P3 promoter while lacking the deoP and cytP promoters. A lambdapdeo B+D+ phage phage was used to lysogenize a deo+ delta att lambda strain, integration of lambda occurring within the region of homology, and, from a heat-induced lysate of this strain, a plaque-forming lambda+ phage carrying the complete deo operon was obtained. Phage lambda was also inserted into the deoB and deoD genes and into the tdk gene. By isolating lambdaspi- and lambdaEDTAr phage from the deo::(lambda) mutants and determining which bacterial genes they carried and whether they retained the int gene of lambda, it was found that lambda had inserted into deoD with the same orientation as lambda inserted into attlambda, whereas lambda inserted into deoA and deoB had the opposite orientation. Deletions extending from the site of lambda insertion into the bacterial chromosome were isolated by selecting for heat-resistant revertants. These confirmed the order of markers to be deo-serB-trpR-thr and also placed a locus, msp, determining sensitivity or resistance of male strains to male-specific phages, between trpR and thr. For some reason unknown, but which may be related to the orientation of the lambda prophages, short deletions rendering the bacterium Ser- Thr+ were of much lower frequency from the deoD::(lambda) lysogen than from the other two lysogens. From an examination of the residual deoD enzyme levels in deoB::(lambda) mutants, it was deduced that there may be two promoter sites within the deoB::(lambda) mutants, it was deduced that there may be two promoter sites within the deoB gene, transcription from one of these being sufficient to account for the noncoordinate nature of the induction of deoB and deoD gene products.
Full text
PDF
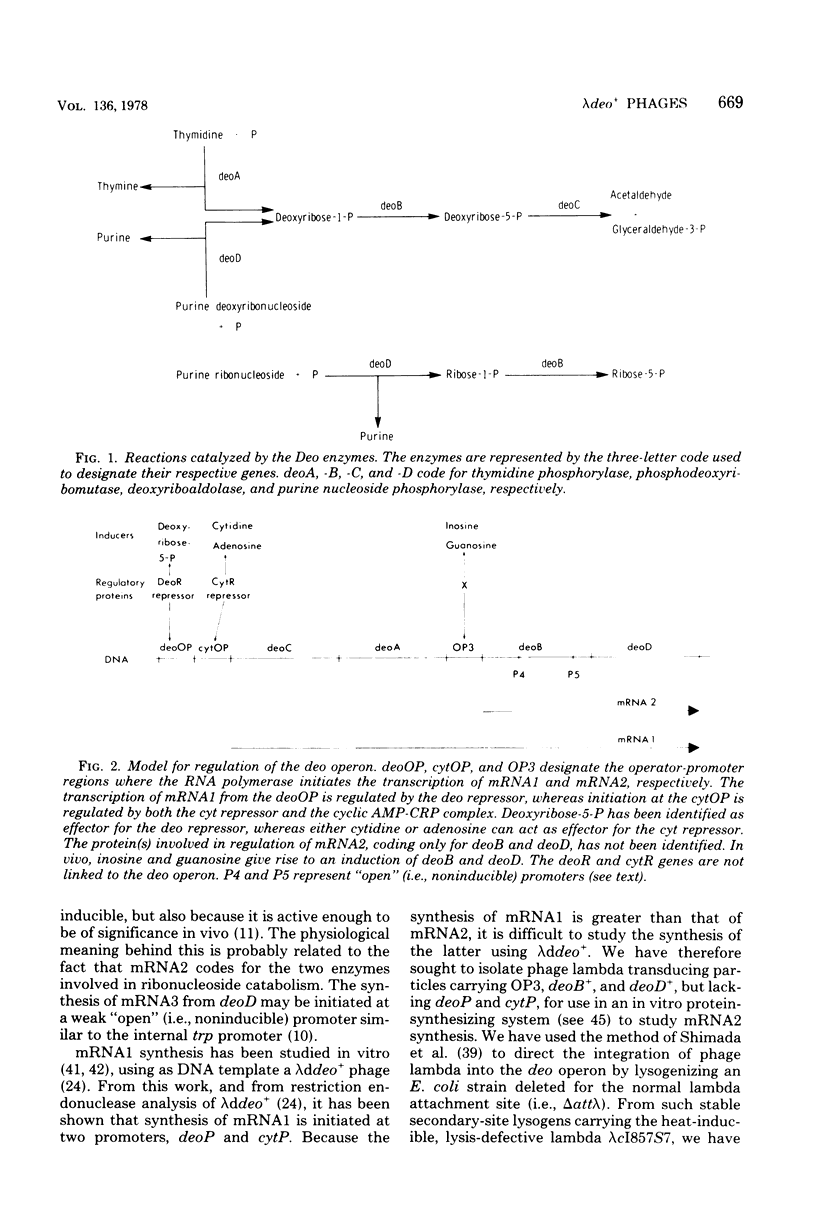



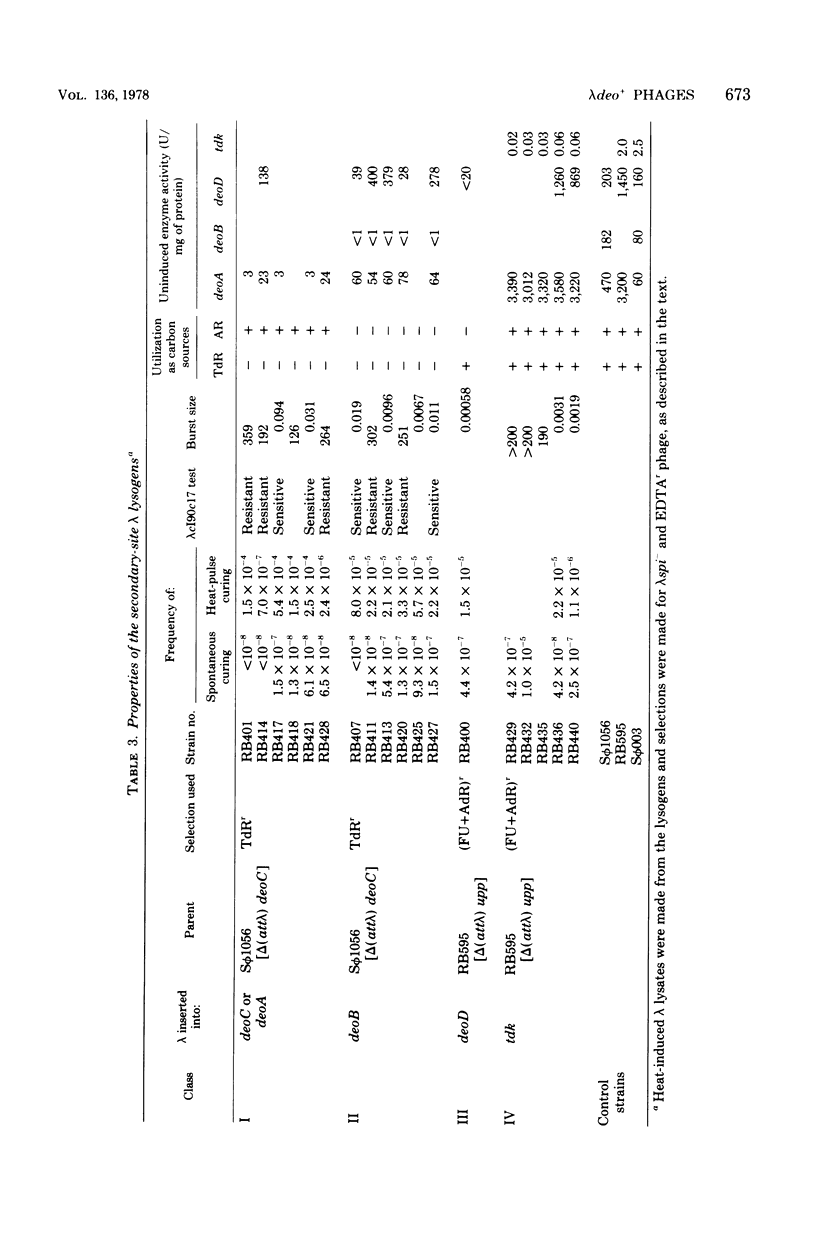
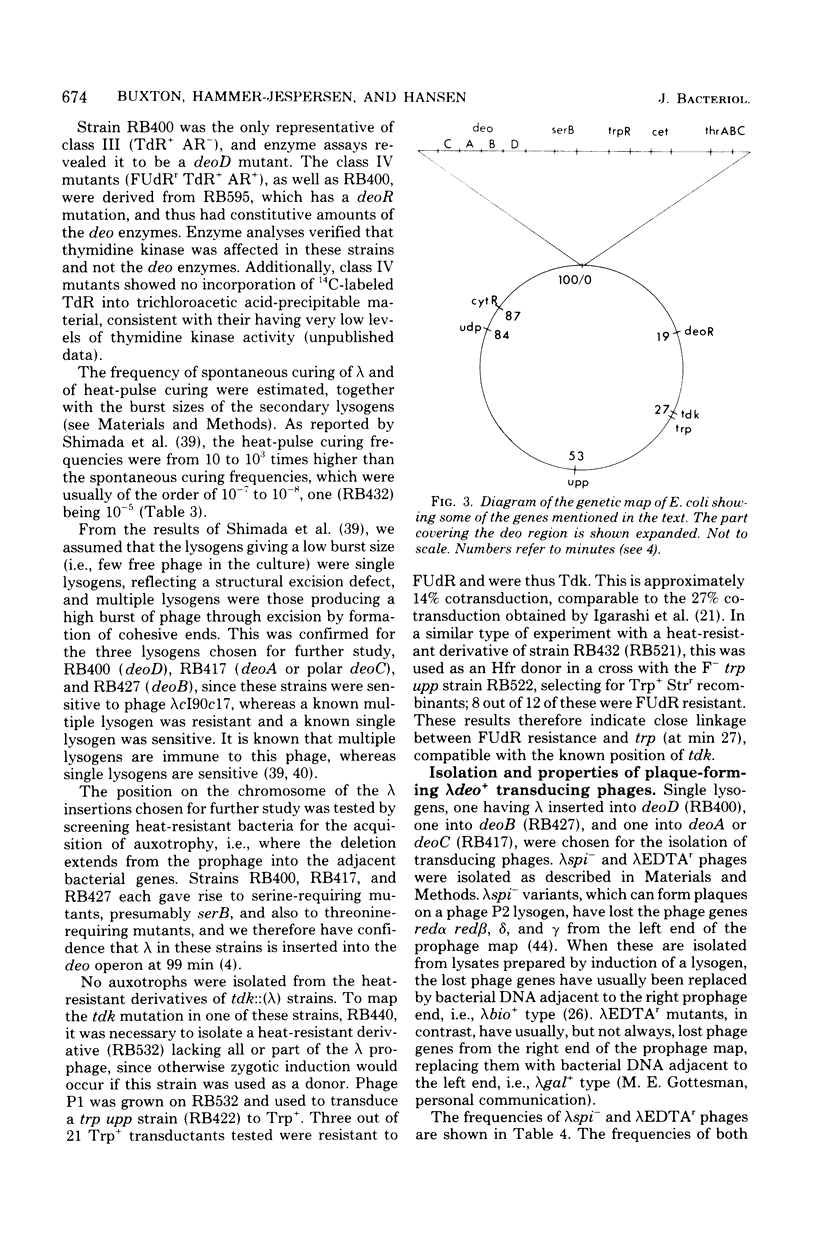
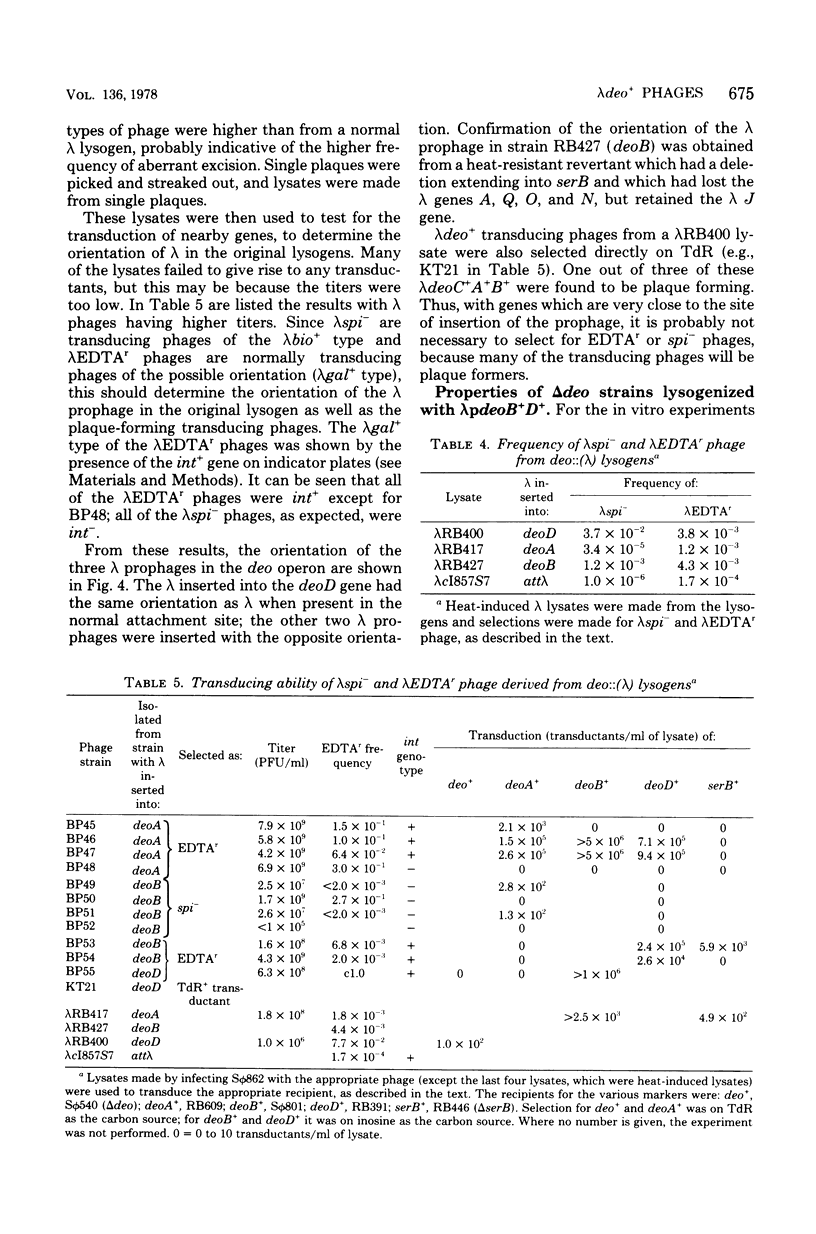


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A regulatory mutant affecting the synthesis of enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1971;111(1):77–83. doi: 10.1007/BF00286556. [DOI] [PubMed] [Google Scholar]
- Albrechtsen H., Hammer-Jespersen K., Munch-Petersen A., Fiil N. Multiple regulation of nucleoside catabolizing enzymes: effects of a polar dra mutation on the deo enzymes. Mol Gen Genet. 1976 Jul 23;146(2):139–145. doi: 10.1007/BF00268082. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Eisenstark A., Barth P. T., Pritchard R. H. Deoxynucleoside-sensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1968;102(2):112–127. doi: 10.1007/BF01789138. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Pritchard R. H. The role of nucleoside phosphorylases in the degradation of deoxyribonucleosides by thymine-requiring mutants of E. coli. Mol Gen Genet. 1971;110(4):289–298. doi: 10.1007/BF00438271. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Bradford R. M. Inability of low thymine-requiring mutants of Escherichia coli lacking phosphodeoxyribomutase to be induced for deoxythymidine phosphorylase and deoxyriboaldolase. J Bacteriol. 1968 Jun;95(6):2434–2435. doi: 10.1128/jb.95.6.2434-2435.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Albrechtsen H., Hammer-Jespersen K. Overlapping transcriptional units in the deo operon of Escherichia coli K-12. Evidence from phage Mu-1 insertion mutants. J Mol Biol. 1977 Aug 15;114(3):287–300. doi: 10.1016/0022-2836(77)90251-0. [DOI] [PubMed] [Google Scholar]
- Buxton R. S. Genetic analysis of thymidine-resistant and low-thymine-requiring mutants of Escherichia coli K-12 induced by bacteriophage Mu-1. J Bacteriol. 1975 Feb;121(2):475–484. doi: 10.1128/jb.121.2.475-484.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Holland I. B. Genetic studies of tolerance to colicin E2 in Escherichia coli K-12. I. Re-location and dominance relationships of cet mutations. Mol Gen Genet. 1973 Dec 14;127(1):69–88. doi: 10.1007/BF00267784. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda. Virology. 1976 Jul 1;72(1):147–153. doi: 10.1016/0042-6822(76)90319-6. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A. Mutants of Escherichia coli unable to metabolize cytidine: isolation and characterization. Mol Gen Genet. 1973 Nov 2;126(2):177–186. doi: 10.1007/BF00330992. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A., Schwartz M., Nygaard P. Induction of enzymes involed in the catabolism of deoxyribonucleosides and ribonucleosides in Escherichia coli K 12. Eur J Biochem. 1971 Apr 30;19(4):533–538. doi: 10.1111/j.1432-1033.1971.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Ptersen A. Multiple regulation of nucleoside catabolizing enzymes: regulation of the deo operon by the cytR and deoR gene products. Mol Gen Genet. 1975;137(4):327–335. doi: 10.1007/BF00703258. [DOI] [PubMed] [Google Scholar]
- Hathaway B. G., Bergquist P. L. Temperature-sensitive mutations affecting the replication of F-prime factors in Escherichia coli K 12. Mol Gen Genet. 1973 Dec 31;127(4):297–306. doi: 10.1007/BF00267100. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. F., Bergquist P. L. Genetic mapping of chromosomal mutations affecting the replication of the F-factor of Escherichia coli. Mol Gen Genet. 1976 Oct 18;148(2):221–223. doi: 10.1007/BF00268388. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P., Collins J., Valentin-Hansen P. On the structure of the deo operon of Escherichia coli. Mol Gen Genet. 1977 Sep 21;155(1):93–102. doi: 10.1007/BF00268565. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leer J. C., Hammer-Jespersen K., Schwartz M. Uridine phosphorylase from Escherichia coli. Physical and chemical characterization. Eur J Biochem. 1977 May 2;75(1):217–224. doi: 10.1111/j.1432-1033.1977.tb11520.x. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Neubauer Z., Calef E. Immunity phase-shift in defective lysogens: non-mutational hereditary change of early regulation of lambda prophage. J Mol Biol. 1970 Jul 14;51(1):1–13. doi: 10.1016/0022-2836(70)90265-2. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Samson A. C.R., Holland I. B. Envelope protein changes in mutants of Escherichia coli refractory to colicin E2. FEBS Lett. 1970 Nov 9;11(1):33–36. doi: 10.1016/0014-5793(70)80485-9. [DOI] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Adhya S. L. The galactose operon of E. coli K-12. II. A deletion analysis of operon structure and polarity. Genetics. 1969 Jun;62(2):249–264. doi: 10.1093/genetics/62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Campbell A. Int-constitutive mutants of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):237–241. doi: 10.1073/pnas.71.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Rabideau K. Mechanism of lambda-c17cI virulence. J Mol Biol. 1969 Jun 28;42(3):385–400. doi: 10.1016/0022-2836(69)90231-9. [DOI] [PubMed] [Google Scholar]
- Surkova N. I., Malashenko A. M. Mutagennyi éffekt tioTEF u laboratornykh myshei. Soobshchenie II. Tsitogeneticheskii analiz povrezhdenii v kletakh kostnogo mozga. Genetika. 1974 Feb;10(2):81–88. [PubMed] [Google Scholar]
- Svenningsen B. A. Regulated in vitro synthesis of the enzymes of the deo operon of Escerichia coli. properties of the DNA directed system. Mol Gen Genet. 1975;137(4):289–304. doi: 10.1007/BF00703255. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Svenningsen B. A., Munch-Petersen A., Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978 Feb 16;159(2):191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Voytek P., Chang P. K., Prusoff W. H. Purification of deoxythymidine kinase by preparative disc gel electrophoresis and the effects of various halogenated nucleoside triphosphates on its enzymatic activity. J Biol Chem. 1971 Mar 10;246(5):1432–1438. [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]



