Abstract
The cystic-fibrosis transmembrane conductance regulator (CFTR) functions as a cAMP-regulated Cl− channel and as a regulator of other membrane conductances. cAMP-dependent activation of CFTR inhibits epithelial Na+ channels (ENaC). The specificity of interaction between CFTR and ENaC was examined by coexpression of ENaC and ATP-binding cassette (ABC) proteins other than CFTR. In addition, we identified domains within CFTR that are of particular importance for the inhibition of ENaC. To that end, two-electrode voltage-clamp experiments were performed on Xenopus oocytes coexpressing ENaC together with CFTR, the multidrug resistance protein MDR1, the sulfonyl urea receptor SUR1, or the cadmium permease YCF1. Except for CFTR, none of the other ABC proteins were able to inhibit ENaC. Several truncated versions of CFTR were examined for their inhibitory effects on ENaC. In fact, it is shown that C-terminal truncated CFTR is able to inhibit ENaC on activation by intracellular cAMP. Moreover, the data also show that an intact first-nucleotide binding domain (NBF-1) is important for inhibition of ENaC. We conclude that NBF-1 of CFTR contains a CFTR-specific regulatory site that down-regulates ENaC. It is speculated that this regulatory site also is needed for CFTR-mediated interactions with other membrane proteins and that it is not present in NBF-1 of other ABC proteins.
Keywords: Xenopus oocytes, MDR1, YCF1, SUR1
The epithelial Na+ conductance (ENaC) in luminal membranes of airway epithelial cells is enhanced in cystic fibrosis (CF; refs. 1–3). It has been shown that this enhancement is caused by the inability of mutant CF transmembrane conductance regulator (CFTR) to inhibit ENaC (3, 4). In non-CF airways, ENaC is down-regulated during activation of CFTR, thereby switching the epithelium from absorption to secretion of NaCl. A defect in this process may contribute significantly to the pulmonary disease in CF. Previous measurements of the rectal potential difference of patients with CF also suggested an enhanced amiloride-sensitive Na+ conductance for intestinal epithelium (5). This suggestion is supported by subsequent studies identifying this defect in the intestinal tract of CFTR (−/−) knockout mice (6). In fact, recent experiments performed on human intestinal biopsies indicated enhanced amiloride-sensitive short-circuit currents in the CF intestinal epithelium that are caused by a defect in CFTR-dependent inhibition of ENaC (M.M., M. Bleich, J. Kühr, M. Brandis, R.G., and K.K., unpublished work).
Interaction of CFTR with other ion channels seems to be a common mechanism realized in various epithelial tissues. Several cellular processes are influenced by CFTR, such as the outwardly rectifying Cl− channel, which is activated by CFTR (7–10). Whether this activation occurs exclusively through an autocrine release of ATP or by additional mechanisms is not currently clear (11, 12). CFTR also has been shown to bind sulfonyl urea compounds and to confer glybenclamide sensitivity to ATP-gated K+ channels (13). Both regulation of outwardly rectifying Cl− channels by CFTR and interaction with KATP channels essentially depend on the presence of the first-nucleotide binding domain (NBF-1) of CFTR (12, 14). In the present article, we show that the ability of CFTR to inhibit ENaC in a cAMP-dependent manner requires the NBF-1, which cannot be replaced by NBF-1 of other ABC proteins.
METHODS
cDNA for ABC Proteins and ENaC.
cDNAs encoding wild-type human CFTR, mouse multidrug resistance protein MDR1 (15), hamster sulfonyl urea receptor SUR1 (16), and yeast cadmium permease factor YCF1‡ were subcloned into pBluescript vectors (Stratagene) and amplified in Escherichia coli (XL1-Blue, Stratagene). cDNAs for SUR1, MDR1, and YCF1 were kindly provided by L. Aguilar-Bryan (Baylor College of Medicine, Houston), J. Riordan (Mayo Clinic, Scottsdale, AZ), and D. Thiele (University of Michigan, Ann Arbor, MI), respectively. cDNAs encoding the α-, β-, and γ-subunits of the rat amiloride-inhibitable ENaC (kindly provided by B. Rossier, Pharmacological Institute, Lausanne, Switzerland) were subcloned into pBluescript (pBSSK−).
CFTR cDNA Truncations and Domains.
The following CFTR constructs and truncations were generated by using PCR fragments. (i) NBF-1 + R domain + two transmembrane-spanning domains on each side (M284/I942X) was generated with primers 5′-ATGATTGAAAACTTAAGACAAACAG-3′ (sense, s) and 5′-AATTAGAGTATGCACCAGTGGTA-3′ (antisense, as). (ii) NBF-1 (W401M/D651X) was generated with a NcoI and ATG containing 5′-GCCGCCACCATGGAGGAGGGATTTGGG-3′ (s) and a stop codon and XhoI containing 5′-GACCAGCTCGAGCTAGAAAGAATCACATCCC-3′ (as). (iii) The N-terminal CFTR truncation including the N terminus and the first six transmembrane helices (E402X) was generated with 5′-CCCGAGGTACCATGCAGAGGTC-3′ (s) and 5′-CTACTCGAGCTACCAGAAGGCTGTTACATTC-3′ (as). (iv) The N-terminal CFTR truncation including the N terminus, the first six transmembrane helices, and NBF-1 (C590X) was obtained as a SpeI/KpnI CFTR cDNA fragment. (v) The N-terminal CFTR truncation including the N terminus, the first six transmembrane helices, and an extended NBF-1, according to a remodeled NBF-1 (ref. 17; D651X), was generated with a SpeI/BamHI CFTR fragment (M1-A457), and the PCR product was generated with 5′-GAGGAGGGATTTGGGGAAT-3′ (s) and 5′-TTAGAAAGAATCACATCCCATGA-3′ (as). (vi) The N-terminal truncation including the N terminus, the first six transmembrane helices, NBF-1, and the R domain (E831X) was obtained by ligating a BamHI/KpnI fragment (G458/E831X) with a SpeI/BamHI fragment (M1-A457). (vii) The C-terminal half of CFTR including the R domain but not NBF-1 (M595-C) was obtained by using the primer 5′-TTTGAAAGCTGTGTCTGT-3′ (s) and 5′-GCTCTTGTGGACAGTAATATATCG-3′ (as) and was cloned in pBluescript (Stratagene). A HpaI/KpnI fragment was removed and was replaced by a HpaI/KpnI fragment of CFTR. All fragments were subcloned in an oocyte expressing vector pTLN that uses the Xenopus β-globin untranslated regions to boost expression in oocytes (kindly provided by T. J. Jentsch, Hamburg, Germany; ref. 18). All PCR products and truncations were confirmed for correct sequence by dideoxynucleotide-termination DNA sequencing (Thermo Sequenase I, Amersham Pharmacia) by using a 373A DNA sequencer (Applied Biosystems).
cRNA and Microinjection.
All cDNAs were linearized by either NotI or KpnI, and cRNA was transcribed in vitro by using T7 or T3 polymerases and a 5′ cap (mCAP mRNA capping kit, Stratagene). Isolation and microinjection of oocytes have been described (19). In brief, after isolation from adult Xenopus laevis female frogs, oocytes were dispersed and defolliculated by a 0.5-h treatment with collagenase (type A; Boehringer Mannheim). Subsequently, oocytes were rinsed and kept in ND96 buffer (in mmol/liter; 96 NaCl/2 KCl/1.8 CaCl2/1 MgCl2/5 Hepes/2.5 Na pyruvate, pH 7.55), supplemented with theophylline (0.5 mmol/liter) and gentamycin (5 mg/liter) at 18°C. Oocytes of identical batches were injected with cRNA of α, β, and γ-ENaC (10 ng of each subunit), ABC transporters (20 ng), and CFTR truncations (20 ng), respectively, after dissolving cRNAs in about 50 nl of double-distilled water (PV830 pneumatic pico pump, WPI Instruments, Berlin). Oocytes injected with 50 nl of double-distilled water alone served as controls.
Electrophysiological Analysis.
After injection (2–4 days), oocytes were impaled with two electrodes (Clark Electromedical Instruments, Pangbourne, U.K.) that had resistances of 1 MΩ when filled with 2.7 mol/liter KCl. A flowing (2.7 mol/liter) KCl electrode served as a bath reference to minimize junction potentials. Membrane currents were measured by voltage clamping of the oocytes (OOC-1 amplifier, WPI Instruments) in intervals from −90 to +30 mV, in steps of 10 mV, each for 250–1,000 ms. Current data were filtered at 400 Hz (OOC-1 amplifier). Between intervals, oocytes were voltage clamped to their membrane voltage for 20 s. Data were collected continuously, displayed, and analyzed by using the programs chart and scope (MacLab, Milford, MA; AD Instruments, Hastings, U.K.; Macintosh). Conductances were calculated for the voltage-clamp range of −90 to +30 mV according to Ohm’s law. During the whole experiment, the bath was perfused continuously at a rate of 5–10 ml/min. Statistical analysis was performed according to Student’s t test. P values <0.05 were accepted to indicate statistical significance (∗). All experiments were conducted at room temperature (22°C). All compounds used for the experiments were of highest grade of purity available. Forskolin, 3-isobutyl-1-methylxanthine (IBMX), and amiloride were all from Sigma.
RESULTS
Expression of ABC Transporters and Effects on ENaC.
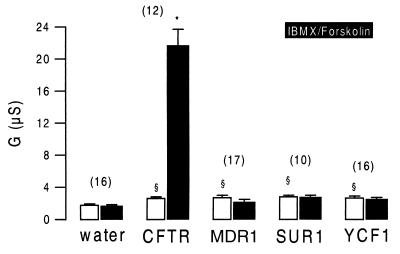
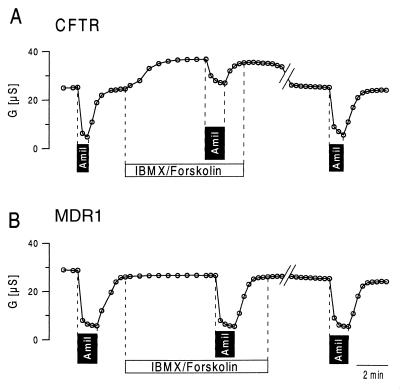
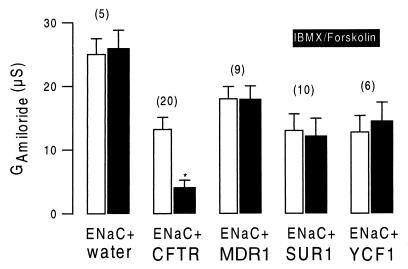
CFTR, MDR1, SUR1, and YCF1 were expressed in Xenopus oocytes. With the exception of a slight but significant increase in the nonspecific background conductance, no further changes in baseline properties could be observed when compared with water-injected cells. We examined whether stimulation of cRNA-injected oocytes with IBMX (1 mmol/liter) and forskolin (10 μmol/liter) had an effect on the whole-cell membrane conductance. Only CFTR-expressing oocytes experienced a significant increase in the whole-cell Cl− conductance on stimulation, and no effect could be observed for any of the other ABC transporters (Fig. 1). We examined whether either of these ABC transporters is able to inhibit ENaC. Oocytes coexpressing ENaC and CFTR had an amiloride-sensitive Na+ conductance under baseline conditions. Stimulation with IBMX and forskolin further increased the whole-cell conductance, caused by activation of CFTR Cl− currents, and attenuated the effects of amiloride, indicating inhibition of ENaC during activation of CFTR (Fig. 2A). After a 30-min washout of IBMX and forskolin, CFTR Cl− currents were deactivated, and ENaC currents were disinhibited as indicated by amiloride-sensitive whole-cell conductance approaching precontrol values. ENaC was not inhibited by any of the other ABC transporters on stimulation with IBMX and forskolin. As shown for the multidrug resistance protein MDR1, the effects of amiloride were similar before and after stimulation with IBMX and forskolin (Fig. 2B). The data for coexpression of ENaC with CFTR, MDR1, and the other ABC proteins are summarized in Fig. 3. It is shown that neither MDR1, SUR1, or YCF1 induced a change in the amiloride-sensitive Na+ conductance when stimulated with forskolin and IBMX, whereas ENaC currents were clearly inhibited on stimulation of CFTR. These data indicate that only CFTR but not the other ABC proteins are able to inhibit ENaC when stimulated via the cAMP-dependent pathway.
Figure 1.
Summary of the whole-cell conductances measured in Xenopus oocytes expressing human CFTR, mouse multidrug resistance protein MDR1, hamster sulfonyl urea receptor SUR1, and yeast cadmium factor YCF1. Water-injected oocytes served as controls. A marginal but significant increase in the nonspecific baseline conductance was observed in MDR1-, SUR1-, and YCF1-expressing oocytes. CFTR-expressing oocytes showed a slight but significant increase in baseline Cl− conductance that was largely increased on stimulation with 1 mmol/liter IBMX and 10 μmol/liter forskolin (black bars). No additional currents were activated in MDR1-, SUR1-, and YCF1-expressing oocytes. The numbers of experiments are shown in parentheses. ∗, Significant difference from control (P < 0.0001). §, Significant difference from water-injected oocytes (P < 0.01).
Figure 2.
Time course of the whole-cell conductance measured in oocytes coexpressing CFTR and α-, β-, and γ-ENaC (A) or MDR1 and α-, β-, and γ-ENaC (B). Amiloride (Amil; 10 μmol/liter) blocked most of the whole-cell conductance when applied under control conditions. In CFTR-expressing oocytes, the effect of amiloride was attenuated after stimulation with IBMX and forskolin. After washout of IBMX/forskolin (30 min), the initial effect of amiloride was recovered. In MDR1-expressing oocytes the effects of amiloride were independent of cAMP-dependent stimulation.
Figure 3.
Summary of amiloride-sensitive whole-cell conductances measured in oocytes coexpressing α-, β-, and γ-ENaC and different ABC transporters. In oocytes expressing only ENaC (ENaC + water) or coexpressing ENaC with either MDR1, SUR1, or YCF1, no changes of the amiloride-sensitive ENaC conductance were observed on stimulation with IBMX and forskolin, whereas activation of CFTR significantly inhibited ENaC conductance. The numbers of experiments are shown in parentheses. ∗, Significant difference from control (P < 0.0001).
Expression of CFTR Truncations.
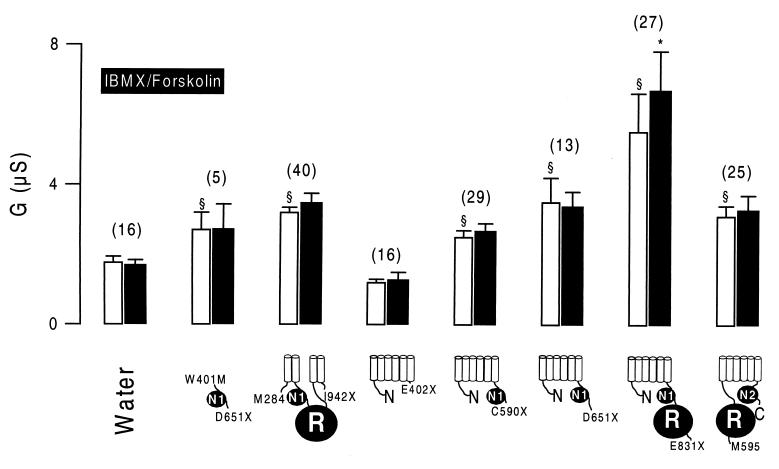
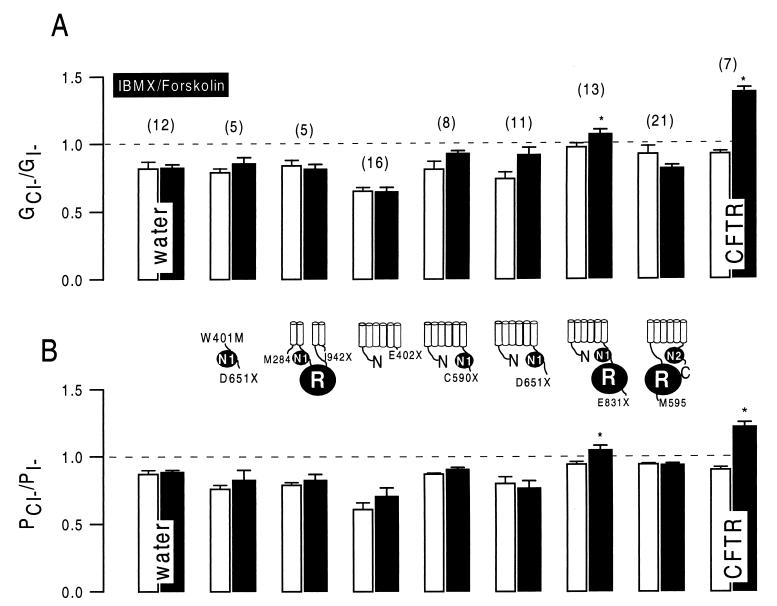
Several truncated versions of CFTR were generated to identify CFTR domains essential to the down-regulation of ENaC: (i) the central cytosolic part of CFTR including two transmembrane helices on each site (M284/I942X); (ii) NBF-1 (W401M/D651X); (iii) the N-terminal half of CFTR without NBF-1 (E402X); (iv) the N-terminal half of CFTR including NBF-1 (C590X); (v) the N-terminal half of CFTR including an additional stretch of 61 amino acids, as described in a recent study (ref. 17; D651X); (vi) the N-terminal half of CFTR including NBF-1 and the R domain (E831X); and (vii) the C-terminal half including the R domain but not NBF-1 (M595-C). With the exception of E402X, expression of all of these constructs enhanced baseline whole-cell Cl− conductance when compared with water-injected control oocytes (Fig. 4). Replacement of extracellular Cl− by gluconate inhibited outward currents and shifted reversal potentials to more depolarized values (data not shown). When stimulated by IBMX and forskolin, only oocytes expressing the N-terminal half protein including the R domain (E831X) increased their whole-cell conductance, and no change could be observed for the other CFTR truncations (Fig. 4). Anion selectivity of the whole-cell currents was determined under control conditions after stimulation with IBMX and forskolin. To that end, extracellular Cl− was replaced isotonically by I−, and permeability ratios were calculated according to the shift in the individual reversal potentials (ref. 20; Fig. 5B). In addition, conductances were measured with either Cl− or I− present in the extracellular bath solution, and individual GCl/GI ratios were assessed (Fig. 5A). A significant change in halide permeability and conductivity ratios could be observed only for full-length CFTR and E831X, indicating a shift from I− > Cl− toward Cl− > I−. These results suggest that only E831X is able to form a cAMP-regulated Cl− conductance, and the other CFTR truncations are not.
Figure 4.
Summary of whole-cell conductances observed in oocytes expressing various truncated versions of CFTR. All truncations induced enhanced baseline Cl− conductance. When stimulated with IBMX and forskolin, only oocytes expressing the N-terminal half of CFTR including the R domain (E831X) responded with an increase of the whole-cell conductance. The numbers of experiments are shown in parentheses. ∗, Significant difference from control (P < 0.002). §, Significant difference from water-injected oocytes (P < 0.01).
Figure 5.
Cl−/I− conductivity and permeability ratios for water-injected control oocytes and for oocytes expressing wild-type CFTR and truncated CFTR, respectively. When stimulated with IBMX and forskolin, only oocytes expressing either full-length CFTR or E831X had a change of the conductivity (GCl/GI) and permeability (PCl/PI) ratios toward Cl− > I−, indicating activation of CFTR typical Cl− conductance. The numbers of experiments are shown in parentheses. ∗, Significant difference from control (P < 0.01).
Inhibition of ENaC by Truncated CFTR.
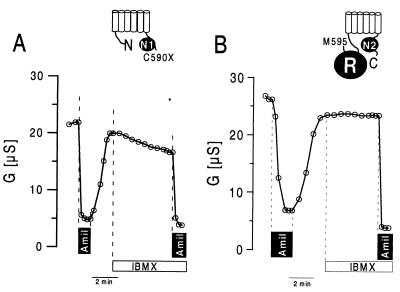
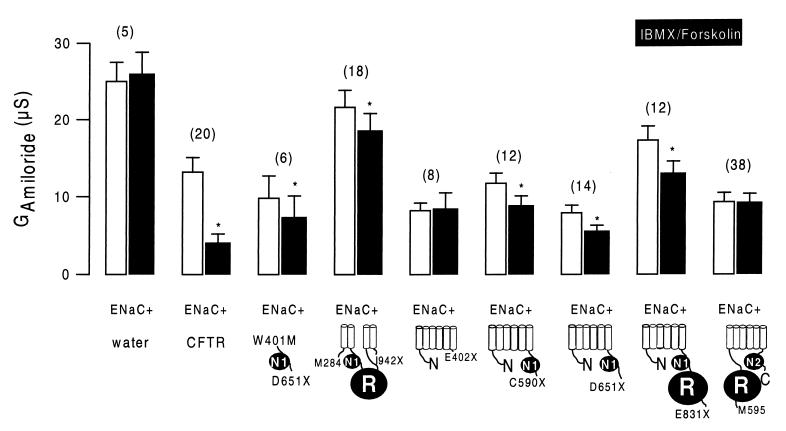
The various CFTR truncations were examined for their ability to down-regulate ENaC. As shown as an example in Fig. 6A, the N-terminal half of CFTR without the R domain (C590X) is able to inhibit ENaC. Stimulation with IBMX and forskolin slightly but significantly inhibited the whole-cell conductance in these oocytes. Consequently, the amiloride-sensitive whole-cell conductance was attenuated after activation of the cAMP-dependent pathway (Fig. 7). In contrast, the C-terminal half of CFTR including the R domain but not NBF-1 (M595-C) was not able to inhibit ENaC on cAMP-dependent stimulation (Fig. 6B). Neither the total nor the ENaC-specific amiloride-sensitive portion of the whole-cell conductance was inhibited during stimulation with IBMX and forskolin. The results of these and the other constructs are summarized in Fig. 7, showing amiloride-sensitive Na currents before and after increase of intracellular cAMP by IBMX and forskolin. Variable amounts of ENaC currents were detected during coexpression with different CFTR truncations. However, the results indicate that, except for M595-C and E402X, all CFTR truncations inhibited ENaC conductances slightly (about 25%) but significantly (P < 0.01) during stimulation. Because M595-C and E402X are the only CFTR truncations that do not contain NBF-1, these results suggest that CFTR’s ability to inhibit ENaC relies on the presence of an intact NBF-1. The results also show that, although activation of a Cl− conductance by cAMP largely augments the inhibitory effect of CFTR on ENaC (19), it is not an absolute prerequisite for CFTR-dependent down-regulation of ENaC.
Figure 6.
Time course of the whole-cell conductance measured in oocytes coexpressing C590X and α-, β-, and γ-ENaC (A) or M595-C and α-, β-, and γ-ENaC (B). Amiloride (Amil; 10 μmol/liter) blocked most of the whole-cell conductance when applied under control conditions. In C590X-expressing oocytes, whole-cell conductances were inhibited, and the effect of amiloride was attenuated after stimulation with IBMX and forskolin. In M595-C-expressing oocytes the effect of amiloride was independent of cAMP-dependent stimulation.
Figure 7.
Summary of amiloride-sensitive whole-cell conductances (GAmiloride) measured in oocytes coexpressing α-, β-, and γ-ENaC and different truncated versions of CFTR. GAmiloride values measured under control conditions (white bars) were significantly smaller after stimulation of the oocytes with IBMX and forskolin (black bars), except for E402X and M959-C. The numbers of experiments are shown in parentheses. ∗, Significant difference from control (P < 0.01).
DISCUSSION
CFTR’s function as a cAMP-regulated Cl− channel has been well documented (21). However, recent experiments point to another property of CFTR, namely, its ability to regulate other ion conductances. In this respect, CFTR has been shown to activate another type of Cl− channel, i.e., the outwardly rectifying Cl− channel of intermediate conductance (ICOR) during activation of the cAMP-dependent pathway (11, 22). It was suggested that activation of ICOR by CFTR occurs via an autocrine mechanism that involves ATP permeation through CFTR (11). The same group of researchers analyzed various truncated versions of CFTR for their ability to regulate ICOR and were able to identify domains within CFTR that are essential for either the Cl− channel function or the property to regulate ICOR (12). According to their report (12), the first transmembrane domain of CFTR is essential for Cl− permeation, whereas another part of CFTR that includes NBF-1 and the R domain is essential for activation of ICOR, probably by somehow facilitating the release of ATP. Another study suggested that GTP-binding proteins participate in the regulation of ICOR by CFTR (23). Moreover, it was shown in other reports that CFTR is also able to replace the sulfonyl urea receptor (SUR) to some degree and is thus able to confer glybenclamide sensitivity to KATP channels (13, 24, 25). Using truncated versions of CFTR that were similar to those described here, the authors arrived at the conclusion that NBF-1 is essential for the interaction of CFTR with KATP (14).
The results of these studies are in striking agreement with the data presented here. Our results point out the importance of the NBF-1 of CFTR for the inhibition of ENaC. Moreover, the results from the present experiments are in congruence with the previously described down-regulation of ENaC by a larger cytosolic domain of CFTR comprising NBF-1 and the R domain (26). Some data from two-hybrid analysis even suggested a direct interaction of this domain with ENaC. Thus far, however, we have not been able to coimmunoprecipitate both ENaC and CFTR (R.S. and K.K., unpublished results). Thus, additional, still unidentified proteins may be involved in the down-regulation of ENaC by CFTR. In this respect, PDZ1-binding domains were identified very recently in the C-terminal tail of CFTR that bind to an apical membrane protein in human airways (27, 28). It was suggested that the function of CFTR may be linked to other membrane proteins such as ENaC by means of these PDZ1-binding domains.
At any rate, the results reported here emphasize on the importance of an intact NBF-1 for CFTR-dependent inhibition of ENaC. Inhibition takes place even in the absence of a C-terminal PDZ1 domain. However, the inhibitory effects of the various truncations on ENaC were relatively small compared with full-length CFTR; although CFTR inhibited ≈70% of the ENaC conductance, the inhibitory effect of most truncations was in the range of ≈25%. This result suggests that other parts or functions of CFTR, such as those of a Cl− channel, participate in the down-regulation of ENaC. In fact, it has been shown that CFTR mutants producing only a little Cl− conductance such as R117H also inhibited in the range of 24% (19). The NBF-1 of the other ABC proteins examined here may have properties that do not allow for protein kinase A (PKA)-dependent inhibition of ENaC. However, although several putative PKA phosphorylation sites have been identified in each of these ABC transporters (15, 16, ‡), cAMP-dependent phosphorylation of these ABC transporters may not be adequate stimulus to induce inhibitory effects on ENaC. On the other hand, the results show that the R domain of CFTR, which contains several consensus and nonconsensus sites for PKA phosphorylation (29), is not essential for the inhibition of ENaC. Subsequent experiments will have to determine whether PKA-dependent phosphorylation of NBF-1 is required for inhibition of ENaC and whether binding and hydrolysis of ATP is required for blockage ENaC.
The present results raise some additional questions about the mechanism of CFTR-dependent inhibition of ENaC. According to one study, CFTR inverts the response of ENaC toward PKA-dependent stimulation from activation to inhibition (30). According to the present results, NBF-1 does contribute essentially to this process, either by direct interaction with ENaC or with the help of additional proteins. In that respect, GTP-binding proteins make good candidates (23, 31). As we have previously shown, the magnitude of Cl− currents through CFTR determines the degree of ENaC inhibition (19). In this study, only slight but significant inhibition of ENaC was observed with little Cl− currents. The CFTR truncations presented here enhanced only those baseline Cl− currents that were not activated further by PKA, except for E831X. Very similar observations have been reported (12). This cAMP-activated ion current showed an anion selectivity of Cl− > I−, similar to that of wild-type CFTR. Interestingly, this truncation showed the strongest inhibitory effect on ENaC. These results may indicate that a certain baseline or cAMP-dependent Cl− current is required for inhibition of ENaC in conjunction with a functional NBF-1.
Acknowledgments
We thank Mrs. H. Schauer and Mrs. P. Kindle for expert technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant Ku756/2-3, by the Zentrum klinische Forschung 1 (ZKF1), and by Fritz Thyssen Stiftung Grant 1996/1044. K.K. was supported by a Heisenberg Fellowship.
ABBREVIATIONS
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- ENaC
epithelial Na+ channel
- ABC
ATP-binding cassette
- NBF-1
first-nucleotide binding domain
- s
sense
- as
antisense
- IBMX
3-isobutyl-1-methylxanthine
- ICOR
intermediate conductance outwardly rectifying Cl− channel
- PKA
protein kinase A
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Lei, D. C., Kunzelmann, K., Koslowsky, T., Yezzi, M. J., Escobar, L. C., Xu, Z., Rommens, J. M., Tsui, L.-C., Tykocinski, M. & Gruenert, D. C. Eighth Annual North American CF Conference, Oct. 20–23, 1994, Orlando, FL (abstr.).
References
- 1.Boucher R C, Cotton C U, Gatzy J T, Knowles M R, Yankaskas J R. J Physiol (London) 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunzelmann K, Kathöfer S, Greger R. Pflügers Arch. 1995;431:1–9. doi: 10.1007/BF00374371. [DOI] [PubMed] [Google Scholar]
- 3.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall M, Hipper A, Greger R, Kunzelmann K. FEBS Lett. 1996;381:47–52. doi: 10.1016/0014-5793(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 5.Orlando R C, Powell D W, Croom R D, Berschneider H M, Boucher R C, Knowles M R. Gastroenterology. 1989;96:1041–1048. doi: 10.1016/0016-5085(89)91621-1. [DOI] [PubMed] [Google Scholar]
- 6.Grubb B R, Gabriel S E. Am J Physiol. 1997;273:G258–G266. doi: 10.1152/ajpgi.1997.273.2.G258. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel S E, Clarke L L, Boucher R C, Stutts M J. Nature (London) 1993;363:263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- 8.Egan M E, Flotte T, Afione S, Solow R, Zeitlin P L, Carter B J, Guggino W B. Nature (London) 1992;358:581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- 9.Jovov B, Ismailov I I, Berdiev B K, Fuller C M, Sorscher E J, Dedman J R, Kaetzel M A, Benos D J. J Biol Chem. 1995;270:29194–29200. doi: 10.1074/jbc.270.49.29194. [DOI] [PubMed] [Google Scholar]
- 10.Jovov B, Ismailov I I, Benos D J. J Biol Chem. 1995;270:1521–1528. doi: 10.1074/jbc.270.4.1521. [DOI] [PubMed] [Google Scholar]
- 11.Schwiebert E M, Egan M E, Hwang T-H, Fulmer S B, Allen S S, Cutting G R, Guggino W B. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 12.Schwiebert E M, Morales M M, Devidas S, Egan M E, Guggino W B. Proc Natl Acad Sci USA. 1998;95:2674–2679. doi: 10.1073/pnas.95.5.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNicholas C M, Guggino W B, Schwiebert E M, Hebert S C, Giebisch G, Egan M E. Proc Natl Acad Sci USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNicholas C M, Nason M W, Jr, Guggino W B, Schwiebert E M, Hebert S C, Giebisch G, Egan M E. Am J Physiol. 1997;273:F843–F848. doi: 10.1152/ajprenal.1997.273.5.F843. [DOI] [PubMed] [Google Scholar]
- 15.Tommasini R, Evers R, Vogt E, Mornet C, Zaman G J, Schinkel A H, Borst P, Martinoia E. Proc Natl Acad Sci USA. 1996;93:6743–6748. doi: 10.1073/pnas.93.13.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, Boyd A E, González G, Herrera-Sosa H, Nguy K, Bryan J, Nelson D A. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 17.Annereau J P, Wulbrand U, Vankeerberghen A, Cuppens H, Bontems F, Tummler B, Cassiman J J, Stoven V. FEBS Lett. 1997;407:303–308. doi: 10.1016/s0014-5793(97)00363-3. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz C, Pusch M, Jentsch T J. Proc Natl Acad Sci USA. 1996;93:13362–13366. doi: 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briel M, Greger R, Kunzelmann K. J Physiol (London) 1998;508.3:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hipper A, Mall M, Greger R, Kunzelmann K. FEBS Lett. 1995;374:312–316. doi: 10.1016/0014-5793(95)01132-x. [DOI] [PubMed] [Google Scholar]
- 21.Riordan J R. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- 22.Schwiebert E M, Flotte T, Cutting G R, Guggino W B. Am J Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 23.Ismailov I I, Jovov B, Fuller C M, Berdiev B K, Keeton D A, Benos D J. J Biol Chem. 1996;271:4776–4780. doi: 10.1074/jbc.271.9.4776. [DOI] [PubMed] [Google Scholar]
- 24.Ruknudin A, Schulze D H, Sullivan S K, Lederer W J, Welling P A. J Biol Chem. 1998;273:14165–14171. doi: 10.1074/jbc.273.23.14165. [DOI] [PubMed] [Google Scholar]
- 25.Ishida-Takahashi A, Otani H, Takahashi C, Washizuka T, Tsuji K, Noda M, Horie M, Sasayama S. J Physiol (London) 1998;508:23–30. doi: 10.1111/j.1469-7793.1998.023br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunzelmann K, Kiser G, Schreiber R, Riordan J R. FEBS Lett. 1997;400:341–344. doi: 10.1016/s0014-5793(96)01414-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Raab R W, Schatz P J, Guggino W B, Li M. FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 28.Short D B, Trotter K W, Reczek D, Kreda S M, Bretscher A, Boucher R C, Stutts M J, Milgram S L. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 29.Seibert F S, Tabcharani J A, Chang X B, Dulhanty A M, Mathews C, Hanrahan J W, Riordan J R. J Biol Chem. 1995;270:2158–2162. doi: 10.1074/jbc.270.5.2158. [DOI] [PubMed] [Google Scholar]
- 30.Stutts M J, Rossier B C, Boucher R C. J Biol Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 31.Manavalan P, Dearborn D G, McPherson J M, Smith A E. FEBS Lett. 1995;366:87–91. doi: 10.1016/0014-5793(95)00463-j. [DOI] [PubMed] [Google Scholar]