Abstract
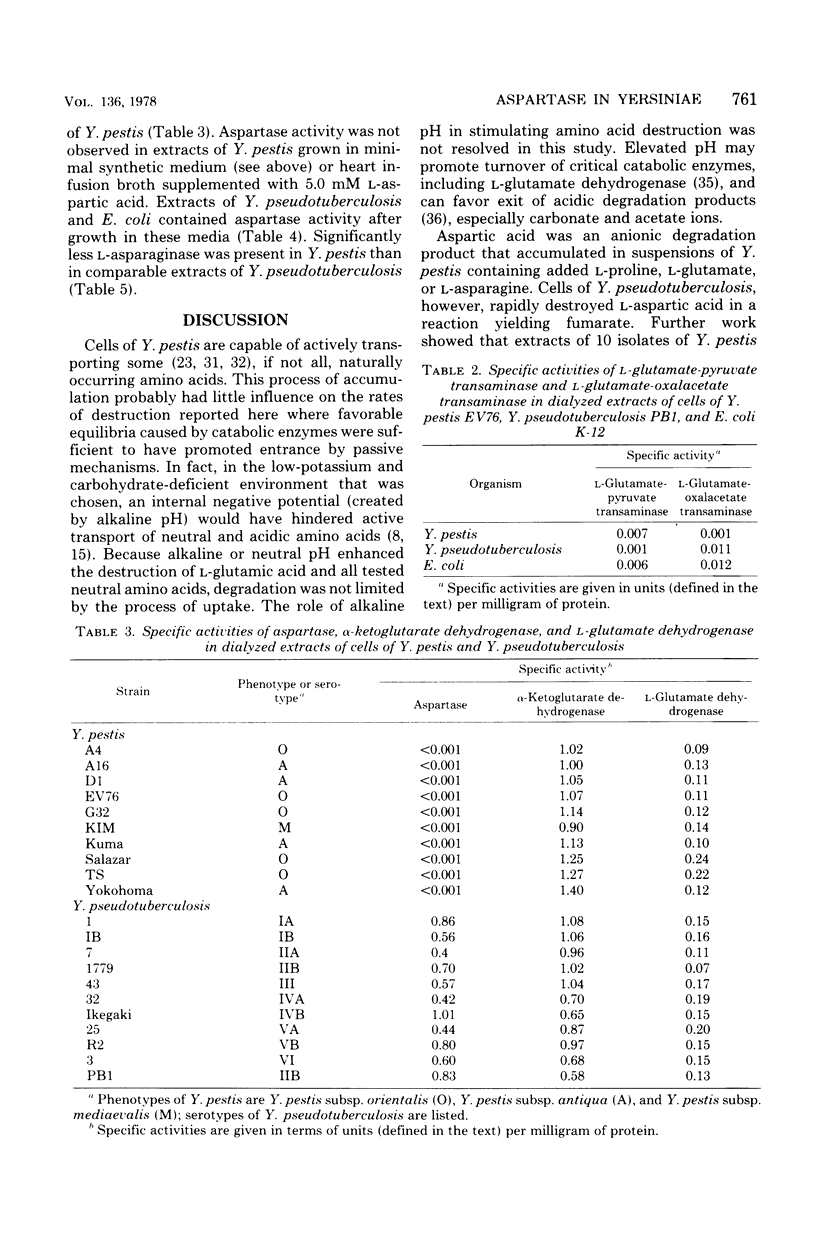
Growing cells of Yersinia pseudotuberculosis, but not those of closely related Yersinia pestis, rapidly destroyed exogenous L-aspartic and L-glutamic acids, thus prompting a comparative study of dicarboxylic amino acid catabolism. Rates of amino acid metabolism by resting cells of both species were determined at pH 5.5, 7.0, and 8.5. Regardless of pH, Y. pseudotuberculosis destroyed L-glutamic acid, L-glutamine, L-aspartic acid, and L-asparagine at rates greater than those observed for Y. pestis. Although rates of proline degardation were similar, its metabolism by Y. pestis at pH 8.5 resulted in excretion of glutamic and aspartic acids. Similarly, Y. pestis excreted aspartic acid when incubated with L-glutamic acid (pH 8.5) or L-asparagine (pH 5.5, 7.0, and 8.5). Aspartase activity was not detected in extracts of 10 strains of Y. pestis but was present in all 11 isolates of Y. pseudotuberculosis. The latter contained significantly more glutaminase, asparaginase, and L-glutamate-oxalacetate transminase activity than did extracts of Y. pestis; specific activities of L-glutamate dehydrogenase and alpha-ketoglutarate dehydrogenase were similar. The observed differences in dicarboxylic amino acid metabolism are traceable to asparatase deficiency in Y. pestis and may account for the slow doubling time of this organism relative to Y. pseudotuberculosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J Infect Dis. 1967 Dec;117(5):403–417. doi: 10.1093/infdis/117.5.403. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Interconversion of Purine Mononucleotides in Pasteurella pestis. Infect Immun. 1970 May;1(5):446–454. doi: 10.1128/iai.1.5.446-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Metabolism of carbohydrates by Pasteurella pseudotuberculosis. J Bacteriol. 1968 May;95(5):1698–1705. doi: 10.1128/jb.95.5.1698-1705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Sulen A. Mutations Influencing the Assimilation of Nitrogen by Yersinia pestis. Infect Immun. 1971 Apr;3(4):580–588. doi: 10.1128/iai.3.4.580-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Collins S. H., Hamilton W. A. Magnitude of the protonmotive force in respiring Staphylococcus aureus and Escherichia coli. J Bacteriol. 1976 Jun;126(3):1224–1231. doi: 10.1128/jb.126.3.1224-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., LEVY J. B. Induced synthesis of tricarboxylic acid cycle enzymes as correlated with the oxidation of acetate and glucose by Pasteurella pestis. J Bacteriol. 1955 Apr;69(4):418–431. doi: 10.1128/jb.69.4.418-431.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Mutation to rhamnose utilization in Pasteurella pestis. J Bacteriol. 1957 May;73(5):641–648. doi: 10.1128/jb.73.5.641-648.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Ingraham L. MEIOTROPHIC MUTANTS OF Pasteurella Pestis AND THEIR USE IN THE ELUCIDATION OF NUTRITIONAL REQUIREMENTS. Proc Natl Acad Sci U S A. 1957 May 15;43(5):369–372. doi: 10.1073/pnas.43.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A. N., Ganapathi K. Biochemistry of Pasteurella pestis. II. Metabolism of some amino acids. Indian J Biochem. 1964 Jun;1(2):80–82. [PubMed] [Google Scholar]
- LEVINE H. B., WEIMBERG R., DOWLING J. H., EVENSON M., ROCKENMACHER M., WOLOCHOW H. The oxidative dissimilation of serine by Pasteurella pestis. J Bacteriol. 1954 Mar;67(3):369–376. doi: 10.1128/jb.67.3.369-376.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Mapping of the aspartase gene in Escherichia coli K-12. Isr J Med Sci. 1969 May-Jun;5(3):413–415. [PubMed] [Google Scholar]




