Abstract
We implemented an experimentally observed orthogonal arrangement of theta and gamma generation circuitry in septotemporal and lamellar dimensions is a two-dimensional model of hippocampus. The model includes three types of cells: pyramidal, basket, and oriens lacunosum-moleculare (OLM) neurons. In this reduced model, application of continuous electric fields allowed us to switch between theta, gamma and mixed theta–gamma regimes without additional pharmacological manipulation. Electric field effects on individual neurons were modeled based on experimental data. Network simulation results predict a flexible experimental technique, which would employ adaptive subthreshold electric fields to continuously modulate neuronal ensemble activity, and can be used for testing cognitive correlates of oscillatory rhythms as well as for suppressing epileptiform activity.
1. Introduction.
Oscillatory rhythms are thought to play a significant role in hippocampal cognitive functions. For example, theta and gamma rhythms influence learning and spatial navigation [9,8,3]. A definitive test of causative relationships, however, is complicated by the nonspecific nature of the pharmacological or electrical manipulations used to modify or eliminate these rhythms. Systemic antagonist injections affect the cortex in addition to the hippocampus. An electric stimulation as in [18] would “reset” and transiently inactivate hippocampal neuronal populations.
A more flexible experimental technique employs adaptive subthreshold electric fields to continuously modulate neuronal ensemble activity for control of epileptic seizures [6]. Advantages of such fields versus standard stimulation techniques include more precise control at lower stimulation energies. We are conducting intracellular experiments testing the effects of subthreshold electric fields at the single cell level [1]. The present study incorporates experimental single-cell polarization results in a reduced network to demonstrate an effective way of switching between hippocampal oscillatory regimes.
2. Methods
2.1. Effects of electric fields on individual neurons
An electric field aligned with the main neuronal axis hyperpolarizes neuronal somata when the positive electrode is close to the soma and depolarizes them in the opposite configuration [17, 2] (Fig.1). Interneurons with more symmetric somato-dendritic trees, or neurons aligned orthogonally to the field, are predicted to show small or no effect [17].
Figure 1.
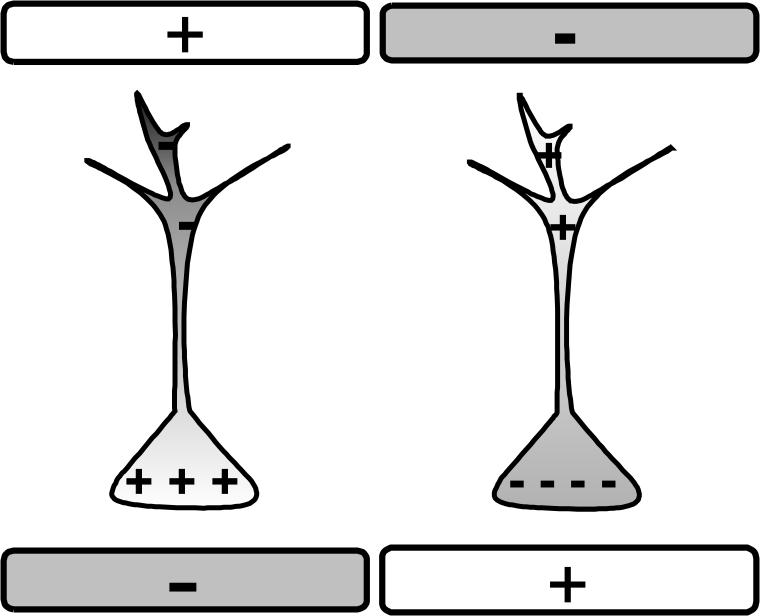
Schematic of neuronal polarization. In our naming convention, negative field depolarizes the soma (left) and positive hyperpolarizes it (right).
To explore electric field effects in hippocampal slice recordings, a pair of electrodes creating a uniform field is located outside of the alveus of areas CA1 and CA3. In this orientation the somatodendritic axis of most pyramidal cells is aligned with the field. In the whole animal preparation, one electrode can be positioned within the body of hippocampus and another outside, creating a radial field configuration. Visualized whole-cell patch-clamp recordings in the presence of the applied electric field are done with respect to an equipotential extracellular reference electrode [1, 2].
One of the effects of the electric field includes changes in membrane potential (Vm) similar to the injection of de- or hyperpolarizing current steps. The amplitude of this effect depends on individual cell morphology, and ionic channel density and distribution. While for some cells the polarization is as high as 2mV per 10mV/mm of the applied field, a typical pyramidal cell effect is about 1mV per 10 mV/mm [1, 2] as measured at the soma. Both pyramidal cells and interneurons with somatodendritic axes aligned with electric field (such as perisomatic basket cells) are polarized in a similar way. Although such small shifts in membrane potential typically do not bring neurons above firing threshold from rest, they readily modify spike latency [13]. Similarly, a shift in Vm will also change synaptic driving force, and due to the shape of polarization profile [17, 2] distal synapses will be more substantially affected than perisomatic synapses.
2.2. Computational model
Even when effects of electric fields on single cells are known, the effect at the population level might be counterintuitive. For example, since both pyramidal and some basket cells are hyperpolarized by a positive electric field [6], disinhibition due to the hyperpolarization of interneurons might overcompensate the suppressive effect on excitatory cells by the field.
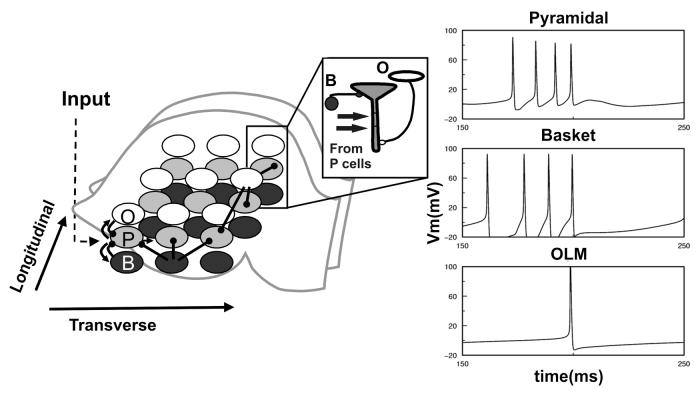
To better understand possible field effects at the network level, we have developed a two-dimensional spiking model of the hippocampal CA3 which includes pyramidal and OLM neurons as well as basket cells (based on [4,10,15]). Extending these models, we implement an orthogonal arrangement of theta and gamma rhythm-generating circuits with OLM axons arborizing anisotropically in a septotemporal orientation and CA3 collaterals and basket cell axons extending in a lamellar orientation (Fig.2, left). This is consistent with anatomical data [4,5] demonstrating a higher septotemporal/lamellar terminal arborization ratio of OLM cells (430/220 μm) vs basket cells (730/310 μm).
Figure 2.
Left. Network schematic (O- OLM, P- Pyramidal, B- Basket cells), arrows represent excitatory connections, filled circles - inhibitory. First cell in a row is externally stimulated (Input). Inset. Scheme of P cell synaptic inputs. Right. Firing of pyramidal soma (top), basket (middle) and OLM cells (bottom) at the same spatial location in the network during theta-gamma regime. Duration of only one theta cycle is shown to illustrate temporal interactions between network elements.
Simulations were carried out using KInNeSS software [7, available from www.kinness.net under GNU GPL]. The ensemble of channel types employed was the minimum required to reproduce theta, gamma and theta-gamma regimes in [4] with some adjustment in parameters. Basket and OLM cells were implemented as one-compartment and pyramidal cells as four-compartment neurons with fast Na, K, and leak currents, as well as excitatory (AMPA) and inhibitory (GABAA) synaptic currents. Additionally, pyramidal cells included NMDA excitatory channels and an afterhyperpolarizing current, while OLM neurons included persistent Na and h-currents [15].
Voltage in a typical compartment is described in a general form by:
| (1) |
where ΣIion is the sum of voltage-sensitive and leak currents, ΣIsyn is the sum of synaptic currents, Iconn is the current from the connecting compartment (for pyramidal cells), and Iapp is the applied current injection. Transient INa and IKdr had Hodgkin-Huxley dynamics (parameters for basket cells chosen from [4], parameters for pyramidal and OLM are from [15]), persistent sodium current had first order dynamics with respect to gating variable m, as in [15], and Ih was implemented as in [15].
Pyramidal cells were connected through recurrent collaterals, received somatic inhibition from basket cells, and dendritic inhibition from OLM cells. For a 4×4 network used here (Fig.2), connectivity kernels were represented by a gaussian
| (2) |
where i, j are cell positions (in lamellar and septotemporal dimensions, respectively) X,Y are target coordinates, and x0, y0 are kernel offsets. The excitatory kernel from pyramidal to pyramidal cells had a spatial extent ratio σx/σy = 1/0.2 (lamellar/septotemporal dimensions) and an offset x0=1 (reflecting the directionality of excitatory projections in the hippocampal trisynaptic circuit). The OLM inhibitory kernel had a ratio σx/σy = 0.2/2 (lamellar/septotemporal dimensions) with no offset, and the inhibitory kernel from basket cells had a ratio σx/σy = 0.75/0.2. Thus, pyramidal and basket cell axonal projections (σy < 1) are concentrated within lamella (represented by a row of cells in Fig.2), while OLM axonal projections project across lamellae (σy > 1).
The effects of an electric field aligned with the somatodendritic axis (Fig.1) were modeled as continuous periods of small positive or negative current injections at the pyramidal cell soma. Due to the orthogonal arrangement of their dendrites with respect to the field, OLM cells are not polarized. Experimentally, the electric field effects on basket cells are on average smaller in amplitude than those on pyramidal cells [1] and did not produce qualitative differences in the modulation of hippocampal rhythms in these model simulations (results not shown).
3. Results
The interactions of basket and pyramidal cells created a gamma rhythm, which was modulated by theta frequency inhibition from OLM cells (see Fig. 2, right, for temporal interplay of three types of cells at the same spatial position in the network).
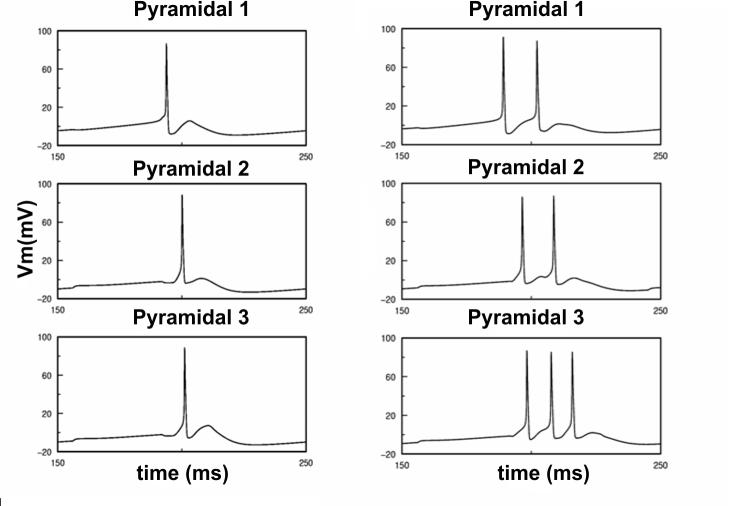
Stimulation of a localized input to the pyramidal cells resulted in the propagation of a wave of activity along the lamellar dimension of the hippocampus, characterized by a monotonic phase shift of the first spike along the chain under theta and theta-gamma conditions (see Fig. 3 for an example of activity propagation along the chain of synaptically connected pyramidal cells at consecutive spatial positions).
Figure 3.
Firing of three pyramidal cells at consecutive spatial positions along the transverse axis in theta (left) and theta-gamma (right) regimes under electric field control. Directional propagation of activity along the synaptic chain is preserved.
All three oscillatory regimes can be reproduced as in Ref. [4]. In agreement with that modeling study, it was possible to switch between regimes by changing excitatory/inhibitory (E/I) balance. This would correspond to the ratio of the efficacy of E/I connections in a transverse vs. longtitudinal slice preparation. In the whole hippocampus, a similar effect can, in principle, be achieved by applying inhibitory/excitatory antagonists.
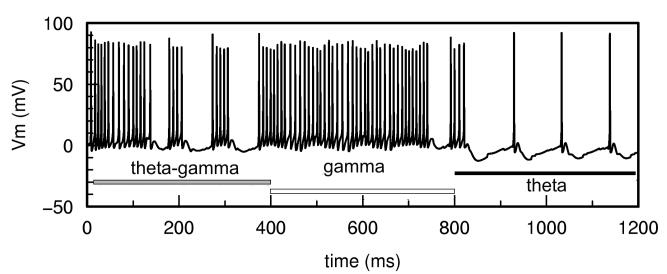
The application of DC electric field at different strengths (estimated ±20 mV/mm, 400ms, Fig.4) was capable of switching regimes from theta/gamma (control) to gamma (negative field) and to pure theta (positive field) without additional pharmacological manipulations. Electric field application did not stop the wave propagation in theta and theta-gamma regimes. It did modify wave characteristics to some extent: hyperpolarizing field tends to decrease the speed of wave propagation and increase the latency of wave initiation (and vice versa for depolarizing field), similar to experimental wave control findings with localized electric fields in the neocortex [14]. The number of gamma spikes per theta cycle in our simulations could also be smoothly modulated by the electric field.
Figure 4.
Switching between theta/gamma, gamma and theta regimes under electric field control (zero field (gray), negative (white), positive (black)). Membrane potential relatively to Vrest is measured from one of the model's pyramidal cells.
4. Discussion
The theta/gamma regime is frequently observed in vivo and has drawn a significant theoretical interest. It has been suggested that different phases of theta rhythm separate encoding and retrieval during hippocampal learning [8], and that nested gamma rhythm in pyramidal cells can serve as a binding mechanism for integration of multiple features [11] or as a substrate for place cell coding [9].
One might speculate that propagation of the wave of activity, after localized input activation, could represent a flow of information in two dimensions: a processing of a single feature or a place field through the pyramidal cell collaterals within the lamella; and integration between different features, place fields or spatial scales across lamellae in a septotemporal direction. While interactions between basket and pyramidal cells are responsible for the production of the gamma rhythms and possibly pattern learning within each lamellae, the OLM cells may provide theta rhythm synchronization across lamellae, thus establishing a common “frame rate”. The computational demonstration of both global and spatially localized effects of electric fields on hippocampal rhythmicity provides a framework for using these techniques to experimentally probe hippocampal cognitive functions.
Here, we developed a two-dimensional model of the hippocampus implementing experimentally observed orthogonal arrangements of theta and gamma generation circuits. In this reduced model, the application of an electric field allowed us to switch between oscillatory regimes without additional pharmacological manipulations. Applying positive DC fields shifted oscillations from theta-gamma to theta; applying negative DC fields shifted them to gamma. This paradigm can be used both in vitro to study the transition between different oscillatory regimes, and in vivo during learning and spatial navigation tasks to clarify the role of these different rhythms. In addition, electric field manipulations preserved (within certain limits) synaptic wave propagation, which might permit modulation of theta rhythm with preservation of cognitive information processing.
Further improvements of this model would include a more complete representation of different neuronal subtypes (the number of distinct interneuronal types is greater than 10 in CA3, [16]), more complete inclusion of membrane currents, and more realistic morphology and connectivity patterns. On the experimental side, we have an incomplete understanding regarding how electric field effects on cellular polarization profiles depend upon an individual neuron's morphology and ionic channel composition. Furthermore, embedding this model within an appropriately resistive network reflective of tissue impedance [12] is important to more realistically model electric field application and the field interaction between neurons.
While this computational study employed an intentionally simple protocol, an adaptive subthreshold electric field modulation technique represents a flexible and powerful tool for both research and therapeutic applications.
Acknowledgements.
We thank E. Barretto, B. Gluckman, P.So and N. Chernyy for helpful discussions and for the assistance with the circuit design for electric field control experiments.
Supported by NIH grants R01MH50006 (JB, SJS) and K02MH01493 (SJS)
References
- 1.Berzhanskaya JB, et al. Mechanisms of electric field suppression of neuronal activity in a hippocampal slice model of epilepsy. Epilepsia. 2005;46(Suppl 8):329–329. [Google Scholar]
- 2.Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–90. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 4.Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, Whittington MA, Kopell N. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(37):13295–300. doi: 10.1073/pnas.0506259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–47. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gluckman BJ, Nguyen H, Weinstein SL, Schiff SJ. Adaptive electric field control of epileptic seizures. J Neurosci. 2001;21(2):590–600. doi: 10.1523/JNEUROSCI.21-02-00590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorchetcnikov A, Versace M, Hasselmo ME. KInNeSS: A new software environment for simulations of neuronal activity; 9th International conference on Cognitive and Neural Systems; Boston, MA. 2004. [Google Scholar]
- 8.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14(4):793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3(3):317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 10.Kunec S, Hasselmo ME, Kopell N. Encoding and retrieval in the CA3 region of the hippocampus: a model of theta-phase separation. J Neurophysiol. 2005;94(1):70–82. doi: 10.1152/jn.00731.2004. [DOI] [PubMed] [Google Scholar]
- 11.Lisman JE. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15(7):913–22. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 12.Park EY, Barreto E, Gluckman BJ, Schiff SJ, So P. A model of the effects of applied electric fields on neuronal synchronization. J. Comp. Neurosci. 2005;19:53–70. doi: 10.1007/s10827-005-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radman T, Parra L, Bikson M. Amplification of small electric fields by neurons; implications for spike timing; Annual International Conference of the IEEE Engineering in Medicine and Biology Society; New York. August 2006; [DOI] [PubMed] [Google Scholar]
- 14.Richardson KA, Schiff SJ, Gluckman BJ. Control of traveling waves in the Mammalian cortex. Phys Rev Lett. 2005;94(2):028103. doi: 10.1103/PhysRevLett.94.028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotstein HG, Pervouchine DD, Acker CD, Gillies MJ, White JA, Buhl EH, Whittington MA, Kopell N. Slow and fast Inhibition and H-current interact to create a theta rhythm in a model of CA1 interneuron network. J. Neurophysiol. 2005;94:1509–1518. doi: 10.1152/jn.00957.2004. [DOI] [PubMed] [Google Scholar]
- 16.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50(6):1139–56. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zugaro MB, Monconduit L, Buzsaki G. Spike phase precession persists after transient intrahippocampal perturbation. Nat Neurosci. 2005;8(1):67–71. doi: 10.1038/nn1369. [DOI] [PMC free article] [PubMed] [Google Scholar]