Abstract
It has previously been observed that expression of chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) and its receptor CCR2 is upregulated by dorsal root ganglion (DRG) neurons in association with rodent models of neuropathic pain. MCP-1 increases the excitability of nociceptive neurons after a peripheral nerve injury, while disruption of MCP-1/CCR2 signaling blocks the development of neuropathic pain, suggesting MCP-1 signaling is responsible for heightened pain sensitivity. In order to define the mechanisms of MCP-1 signaling in DRG, we studied intracellular processing, release, and receptor-mediated signaling of MCP-1 in DRG neurons. We found that in a focal demyelination model of neuropathic pain both MCP-1 and CCR2 were upregulated by the same neurons including TRPV1 expressing nociceptors. MCP-1 expressed by DRG neurons was packaged into large dense-core vesicles (LDCVs) whose release could be induced from the soma by depolarization in a Ca2+-dependent manner. Activation of CCR2 by MCP-1 could sensitize nociceptors via transactivation of transient receptor potential (TRP) channels. Our results suggest that MCP-1 and CCR2, upregulated by sensory neurons following peripheral nerve injury, might participate in neural signal processing which contributes to sustained excitability of primary afferent neurons.
Keywords: Chemokine, Neurotransmitter, DRG, Neuropathic pain, TRPV1, TRPA1
Introduction
Chemokines (CHEMOtaxic cytoKINEs) are a family of small proteins which play a prominent role in the trafficking of immune cells and in the orchestration of inflammatory responses. However, chemokines and their receptors are not only expressed in the immune system, but are also widely expressed in the nervous system during development and in adulthood. Indeed, chemokines appear to play several important roles in neuronal development and in the function of the mature nervous system (Tran and Miller 2003). Chemokine receptors are expressed by diverse populations of neurons and glia. For example, cultured DRG neurons express numerous functional chemokine receptors. Activation of these receptors excites these neurons and produces pain hypersensitivity (Oh et al. 2001). Recent reports have tried to define how such responses may contribute to states of chronic pain. It has been suggested that the chemokine MCP-1/CCL2 and its receptor CCR2 may play a particularly important role in the genesis of neuropathic pain as MCP-1 and CCR2 are not normally expressed in the DRG (White et al. 2005b). However, the expression of both of these molecules increases in DRG neurons in association with nerve injury, and application of MCP-1 to CCR2 expressing neurons strongly depolarizes them (White et al. 2005b; Sun et al. 2006). Furthermore, CCR2 knockout mice show impaired development and maintenance of neuropathic pain (Abbadie et al. 2003). Although these findings suggest that MCP-1/CCR2 signaling may be important in the genesis and/or maintenance of neuropathic pain, the manner in which MCP-1 produces these effects in vivo is unclear.
We now show that MCP-1 expressed in DRG neurons appears to act as a novel neuromodulator, exhibiting localization to neurotransmitter containing secretory vesicles, Ca2+- and voltage-dependent secretion and a potential postsynaptic mechanism of action. Our data support the possibility that MCP-1 signaling in the DRG may contribute to the maintenance of nociceptor hyperexcitability in neuropathic pain.
Materials and methods
Plasmid construction, adenovirus production, and materials
MCP-1-EGFP was made by cloning polymerase chain reaction (PCR) fragment of MCP-1 protein coding sequence into pEGFP-N1 (Clontech, Mountain View, CA). CCR2-expression vectors were made by cloning PCR fragment of CCR2 protein coding sequence into pEGFP-N1 and pIRES2-EGFP (Clontech). Sequence identity was confirmed by dideoxy-sequencing methods. TRPV1- and TRPA1-expression vectors were kindly provided Dr. David Julius (University of California, San Francisco, CA) and by Dr. Jaime García-Añoveros (Northwestern University, Chicago, IL), respectively. To make adenoviruses, the fragments containing promoter, protein coding sequence, and poly-A signal were cloned into pShuttle vector and adenovirus was generated using AdEasy system (He et al. 1998). Chemicals were purchased from Sigma (Grand Island, NY) unless stated otherwise.
Generation of CCR2-EGFP BAC transgenic mice
We utilized bacterial artificial chromosome (BAC) transgenic strategy, which involves transgenesis using a modified BAC clone containing a reporter gene insertion at the end of the gene of interest. In a BAC clone, there is considerable amount of sequence flanking one gene and sufficient cis-elements that regulate endogenous expression should be present. Therefore, reporter gene expression is expected to follow endogenous expression of the gene of interest (Gong et al. 2003). The CCR2 containing BAC clone (MSM-529G05) was obtained from RIKEN DNA Bank (Ibaraki, Japan). We used the protocol described by Lee and colleagues (Lee et al. 2001) with slight modifications. Briefly, to generate the CCR2 BAC reporter vector, EGFP was inserted immediately downstream of the CCR2 coding sequence by λ-Red-mediated recombination. The EGFP-FRT-KAN-FRT targeting cassette was generated by self-ligation of the blunt-ended BglI/SmaI fragment of pIGCN21. The targeting cassette was amplified by PCR using the following chimeric primers, of which the 3’ end was homologous to the targeting cassette and the 5’ end was homologous to the last exon of CCR2: For upstream, 5’-TGAGCTCTACATTCACTCCTTCCACTGGGGAGCAAGAGGTCTCGGTTGGGTTGGATGATAAT ATGGCCACAACC-3’; For downstream: 5’-CTGTCTTTGAGGCTTGTTGCTATGTACAAACTGCT-CCCTCCTTCCCTGCTCTATTCCAGAAGTAGTGAGGA-3’. The primers were designed to target EGFP immediately downstream of the CCR2 coding sequence and upstream of the poly-A site. The stop codon of CCR2 was deleted to generate the CCR2-EGFP fusion construct. Transgenic mice were generated by the Center for Genetic Medicine, Northwestern University.
Sciatic nerve demyelination
Animals were anesthetized with 4% isoflurane and maintained on 2% isoflurane (Halocarbon, River Edge, NJ) in O2. For all demyelination experiments, lysophosphatidylcholine (LPC), (type V, 99% pure; Sigma-Aldrich, St. Louis, MO) was dissolved in buffered sterile saline (pH 7.2) to give a final concentration of 10 mg/ml. The right sciatic nerve of the mouse was exposed at the mid-thigh level under sterile conditions. A sterile polyvinyl acetal (PVAc) sponge (Ivalon, San Diego, CA), 2-mm × 2-mm soaked in 7 μl of LPC was placed adjacent to the sciatic nerve. The dermal incision site was closed with 4.0 suture thread. Sham control animals were prepared as described above, but buffered sterile saline was used in place of LPC. Animals were allowed to survive for fourteen days. All experiments complied with protocols approved by the Northwestern University and Loyola University Chicago Institutional Animal Care and Use Committee.
Cell culture and transfection
F11 (DRG neuronal cell line) and HEK293 (tSA201 subclone) cells were maintained in Dulbecco’s modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.5% penicillin–streptomycin (P/S) at 37°C under 5% CO2. One microgram of plasmid DNA was transfected using Mirus-LT1 (Mirus, Madison, WI) according to the manufacturer’s instructions. To induce differentiation of F11 cells, medium was changed to DMEM supplemented with 0.5% FBS, 0.5% P/S, and 0.5 mM dibutyryl-cAMP 24 hr after the transfection. Cells were incubated for 48–72 hr to induce differentiation. Materials for cell culture were purchased from Invitrogen (Carlsbad, CA) unless stated otherwise.
DRG culture and adenoviral infection
Mouse DRG neurons were prepared as described elsewhere with slight modifications (Oh et al. 2001). Cells were plated on poly-L-lysine (BD Biosciences)- and laminin (BD Biosciences)-coated coverslips and incubated in F12 supplemented with 0.5% FBS, 1% N2, 50 ng/ml NGF and 0.5% P/S. On the next day, 10 μM cytosine arabinoside was added to eliminate mitotic cells including ganglionic fibroblasts. Medium was replaced every 2–3 days. Cultures were maintained at 37°C with 5% CO2 for up to 2 weeks. After 3–5 days in culture, DRG neurons were infected with adenovirus expressing MCP-1-EGFP with 50–100 multiplicity of infection (MOI) and used in 3–5 days.
Immunofluorescence labeling
For tissue sections, lumbar ganglia associated with the sciatic nerve ipsilateral to focal nerve demyelination injury (n=5) or sham treatment (n=3) were immediately removed from mice transcardially perfused with 4% paraformaldehyde (PFA) and postfixed for 4 hr. DRGs were sectioned at 14 μm and stored at −20°C until use. Cultured cells on coverslips were fixed with 4% PFA for 15 min. Antibodies used are as follows: anti-TGN38 mouse monoclonal antibody (1:300; Affinity Bioreagents, Golden, CO); anti-Synaptophysin I rabbit polyclonal antibody (1:300; Santa Cruz Biotechnology, Santa Cruz, CA); anti-CGRP goat polyclonal antibody (1:300, Santa Cruz); anti-MCP-1 rabbit polyclonal antibody (1:1,000; Chemicon, Temecula, CA); anti-TRPV1 rabbit polyclonal antibody (1:1000, Neuromics, Edina, MN); Texas Red-conjugated secondary antibodies (1:500; Jackson Immunoresearch Laboratories, West Grove, PA). Images were taken in a laser-scanning confocal microscope (Olympus, Melville, NY) equipped with a 60X oil immersion objective lens (NA, 1.40) or in a Zeiss Axioplan II epifluorescence microscope. Percentages of immunopositive neurons were acquired using a previously described methodology (White et al. 2005b).
Fluorescence release assay
Cells plated on to a 35 mm culture dish were mounted on the stage of upright fluorescence microscope (Zeiss Axioplan II, 40X water immersion objective lens) and perfused with a balanced salt solution (BSS containing in mM: 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose) at a rate of 1.5 ml/min by a gravity-fed system. To induce depolarization, high potassium solution (50K) was prepared by adjusting concentration of KCl from 5 to 50, and of NaCl from 145 to 100. Calcium free solutions were prepared by replacing CaCl2 with equimolar MgCl2 and adding 0.5 mM EDTA. EGFP signal was recorded every 10 to 40 sec for 20 min and signal intensity was calculated using MetaMorph software (Molecular Devices, Sunnyvale, California).
Calcium imaging
The AM form of fura-2 (Invitrogen) was used as the fluorescent Ca2+ indicator. All measurements were made at RT as described previously (Bhangoo et al. 2007). The DRG cells loaded with fura-2 AM (3 μM) were mounted onto the chamber (500 μl total volume), which then was placed onto the inverted microscope, and perfused continuously by BSS. Ratiometric images were monitored every 3 sec and Ca2+ concentration was calculated by a standard curve generated using calcium calibration buffer kit (C-3721, Invitrogen). For MCP-1 (R & D Systems, Minneapolis, MN), capsaicin, and allyl isothiocyanate, we applied 1 ml of solution directly to the bath chamber after stopping the flow.
Statistics
Data for ELISA and some Ca2+ imaging experiments were presented as mean ± SEM. ELISA experiment to measure the regulated release of MCP-1-EGFP and the inhibitor experiment of TRPV1 sensitization were analyzed by one-way ANOVA followed by post hoc Bonferroni’s multiple comparison test. TRPV1 sensitization experiment with varying concentration of capsaicin was analyzed by unpaired Student’s t-test. Statistical significance was set at p<0.05.
Results
Mouse DRG neurons express both MCP-1 and CCR2 in association with cutaneous hyperalgesia
The expression of MCP-1 and CCR2 is upregulated in the DRG in several rodent models of neuropathic pain (White et al. 2005a; White et al. 2005b; Bhangoo et al. 2006; Sun et al. 2006; Xie et al. 2006). However, the relative location of MCP-1 and CCR2 expressing neurons has not been determined. In order to address this question, we induced a state of cutaneous hyperalgesia in CCR2-EGFP BAC reporter mice (see Materials and Methods) using lysophosphatidylcholine (LPC)-mediated focal demyelination of sciatic nerve (Wallace et al. 2003; Bhangoo et al. 2006). Fourteen days after focal demyelination, the associated lumbar DRG were isolated, cryosectioned, and subjected to immunohistochemistry using a polyclonal antibody against MCP-1. In accordance with previous results in rats, both MCP-1 and CCR2 were upregulated in comparison to sham-operated control animals where neither molecule was expressed by DRG neurons (Bhangoo et al. 2006) (Fig 1A-F). Interestingly, following LPC treatment, neurons in close apposition co-expressed both MCP-1 and CCR2 in their cell bodies, suggesting some type of autocrine regulatory role for MCP-1/CCR2 signaling within the DRG.
Fig. 1. DRG neurons express MCP-1 and CCR2 in association with peripheral neuropathy.
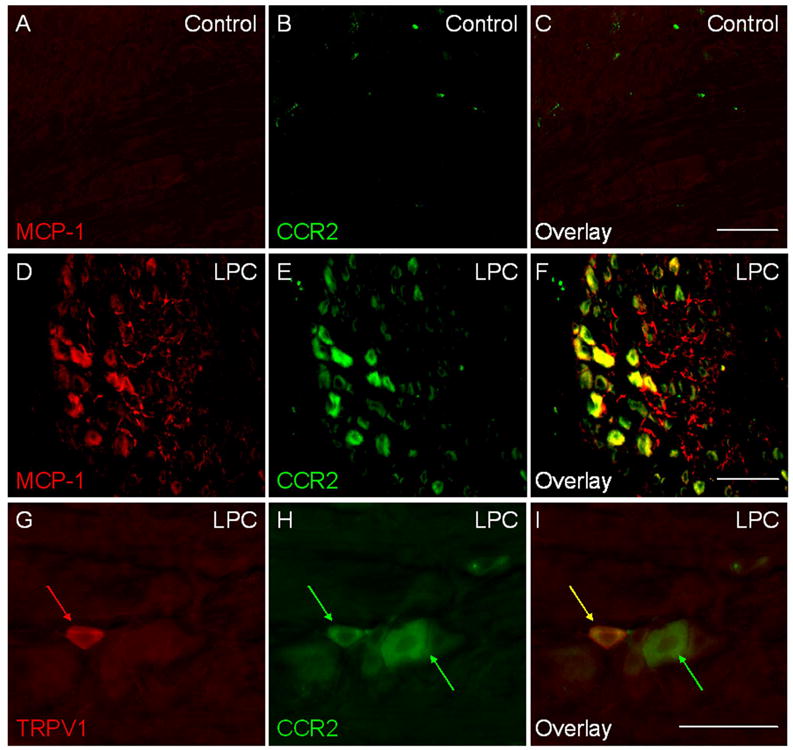
Sciatic nerve demyelination was induced by lysophosphatidylcholine (LPC) in CCR2-EGFP BAC transgenic mice. DRG were isolated at post operation day (POD) 14, cryosectioned, and subjected to immunohistochemistry using a polyclonal anti-MCP-1 antibody. A-C) Sham-operated control. D-F) LPC-treated group. Note that many neuronal cell bodies express both MCP-1 and CCR2. In addition, MCP-1 is also observed in numerous axon processes throughout the ganglion. G-I) TRPV1 expressing nociceptors (red arrows) upregulated CCR2 expression (yellow arrow). Some of larger neurons that do not express TRPV1 also expressed CCR2 (green arrow). Scale bars, 100 μm.
MCP-1-EGFP is localized in secretory vesicles
MCP-1, like other chemokines, is a small secreted protein. In order to examine how it is processed once it is expressed by DRG neurons, we utilized an MCP-1-EGFP fusion protein expressed by cultured DRG neurons and sensory neuron-derived F11 cells (Francel et al. 1987). In contrast to EGFP, which was diffusively localized throughout cells including the nucleus (supporting figure 1A), MCP-1-EGFP exhibited a distinct punctate cytoplasmic localization with a concentrated perinuclear pattern (Fig 2A and supporting figure 1B). The perinuclear signal co-localized with TGN38, a marker for trans-Golgi network (supporting figure 2A-C), indicating the MCP-1-EGFP fusion protein was sorted into the secretory pathway in sensory neurons. Punctate signals in the cytoplasm of cell bodies and axonal processes co-localized with the neuropeptide calcitonin gene-related peptide (CGRP), suggesting that MCP-1-EGFP was packaged into large dense-core vesicles (LDCVs) (Fig 2B and supporting figure 2D-F). Accordingly, MCP-1-EGFP did not generally co-localize with Synaptophysin I (SynI), a marker for small synaptic vesicles (SSVs) (Fig 2C and supporting figure 2G-I). The number and distribution of CGRP-positive and SynI-positive vesicles were indistinguishable between untransfected and transfected neurons, indicating that the adenovirus-mediated expression of MCP-1-EGFP did not significantly alter the secretory pathways in DRG neurons (data not shown). In addition, immunofluorescence labeling of endogenous MCP-1 upregulated in DRG isolated from LPC-treated animals exhibited a similar punctate intracellular localization, suggesting that MCP-1 is also processed and stored in vesicles in DRG neurons in vivo (supporting figure 1C).
Fig. 2. MCP-1-EGFP localizes to large dense-core vesicles (LDCVs) in DRG neurons.
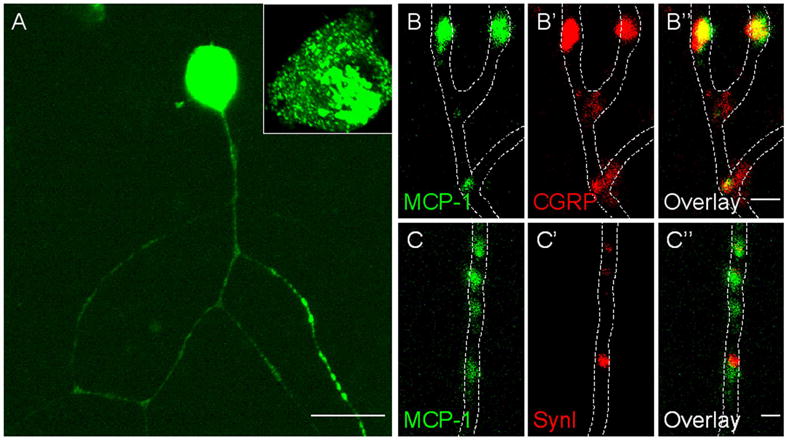
Cultured DRG neurons were infected with an MCP-1-EGFP expressing adenovirus and stained for CGRP and SynI. A) MCP-1-EGFP was concentrated in the perinuclear area, and localized in a punctate pattern in the soma and along axons. Signals in the soma were saturated to allow appreciation of the punctate localization along axons. An unsaturated image of a soma is magnified in the inset. B-C) Magnified view of axonal localization of MCP-1-EGFP. B-B’’) MCP-1-EGFP co-localized with CGRP. C-C’’) MCP-1-EGFP did not co-localize with SynI. Scale bars: A, 20 μm; B’’, C’’, 1 μm.
MCP-1-EGFP is released upon depolarization
Neuropeptides expressed by DRG neurons such as Substance P and CGRP can be secreted from cell bodies by Ca2+-dependent exocytosis (Huang and Neher 1996; Ouyang et al. 2005). We therefore investigated if the secretion of MCP-1 from DRG neurons could also be induced in the same manner. To monitor the release, we used a fluorescence release assay. The MCP-1-EGFP fusion protein expressed in DRG neurons was imaged in real-time. After the recording, the changes in fluorescence signal intensity were analyzed using MetaMorph software. In order to selectively record release from cell bodies, neurons were re-plated prior to the recording to eliminate axonal processes (Fig 3A). We regarded any decrease in fluorescence intensity as release, since we found that photobleaching of EGFP during the recording was negligible (dashed trace in Fig 3B). The fluorescence intensity of the soma declined steeply following depolarization by a high concentration of extracellular potassium (50K), indicating that MCP-1-EGFP release from soma could be induced by depolarization (Fig 3B: red trace). However, release was not induced in the absence of extracellular Ca2+, indicating the involvement of voltage-dependent Ca2+ influx in this process (Fig 3B: blue trace). The Ca2+-dependency of MCP-1-EGFP release was also confirmed by an enzyme-linked immunoabsorbent assay (ELISA), where release of MCP-1-EGFP was observed only when cells were depolarized in the presence of extracellular Ca2+ (supporting figure 3A). These results suggest that MCP-1, upregulated in DRG neurons and packaged into vesicles, can be released from soma when DRG neurons are excited by stimuli that increase [Ca2+]i.
Fig. 3. MCP-1-EGFP is released from the somata of DRG neurons.
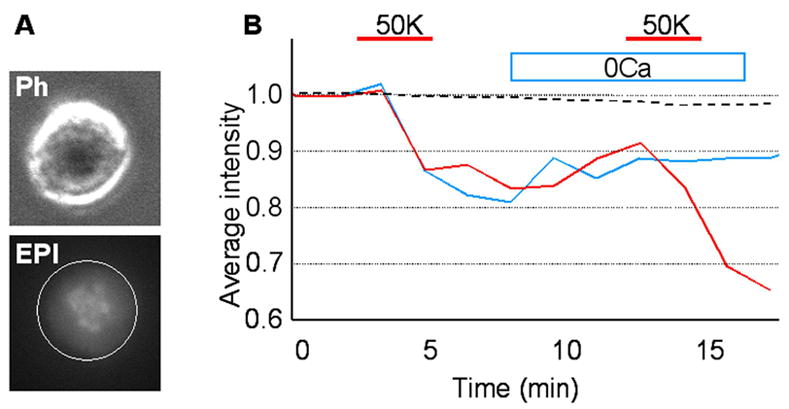
DRG neurons infected with an MCP-1-EGFP expressing adenovirus were analyzed using a fluorescence release assay. A) Representative phase contrast (ph) and fluorescence (EPI) images of a DRG neuronal soma are shown. DRG neurons were identified by their round and phase-bright somata. The entire soma was selected as a region of interest (ROI: white circle). B) Depolarization by high potassium (50K) induced the release of MCP-1-EGFP (red trace) (n=8). When neurons were not stimulated, fluorescence intensity was not significantly changed for the duration of recording (dashed trace). No release was detected when extracellular Ca2+ was absent (0Ca) (blue trace) (n=4).
CCR2 activation hypersensitizes nociceptors
The expression of MCP-1 and CCR2 is upregulated in DRG neurons under pathological conditions, while DRG neurons from normal animals do not express MCP-1 and CCR2 in vivo (Fig 1A-F). This population of neurons included TRPV1-expressing nociceptors, although CCR2 upregulation was not limited to these cells (Fig 1G-I). The majority of TRPV1-positive neurons co-expressed CCR2 (82 ± 9% co-localization; n=4; expressed as mean ± SEM). TRPV1 is a major TRP channel involved in temperature sensation and nociception (Tominaga and Caterina 2004). It is activated by painful stimuli such as heat, capsaicin, protons, and a number of inflammatory mediators including prostaglandins, adenosine, serotonin, nerve growth factor (NGF) and bradykinin. NGF and bradykinin sensitize TRPV1 via the activation of phospholipase C (PLC), PI3 kinase, and protein kinase C ε (PKCε) (Chuang et al. 2001; Bhave et al. 2003; Zhang et al. 2005). TRPA1, another TRP channel that is critical in nociception, contributes to cold, mechanical, and chemical pain. TRPA1 is a receptor-operated channel that can also be gated by the activation of the PLC pathway, and is expressed by a subset of TRPV1-positive neurons in the DRG (Bautista et al. 2006; Kwan et al. 2006). Sensitization of TRP channels is believed to contribute to pain hypersensitivity (Huang et al. 2006). CCR2 is a G protein-coupled receptor (GPCR) which can couple to Gαi or Gαq. In either case, MCP-1 binding leads to the activation of PLCβ (Kuang et al. 1996). Therefore, we reasoned one of the possible effects of CCR2 activation in the DRG might be sensitization of TRP channels.
In order to examine this possibility, CCR2 and TRPV1 were co-expressed in HEK293 cells which were twice treated with capsaicin (CAP) separated by a 20 min interval, during which MCP-1 or buffer solution was applied to the bath. The amplitudes of the two CAP responses were examined by Ca2+ imaging experiments, and compared for each cell. While the two responses were not different in the buffer-treated controls (Fig 4A: blue trace), MCP-1 treatment significantly enhanced the second response indicating CCR2 activation had sensitized TRPV1 (Fig 4A: red trace). The enhancement was more prominent at lower concentrations of CAP, because the CAP responses saturated at higher concentrations (Fig 4B and data not shown). MCP-1 induced sensitization was almost completely blocked by staurosporine, a pan-PKC inhibitor, indicating that PKC activation is crucial in this process (Fig 4C). Moreover, the inhibition of PLC by U73122 even decreased the second CAP response (Fig 4C). Because PIP2 inhibits TRPV1 (Chuang et al. 2001), the sustained inhibition of PLC might have resulted in accumulation of PIP2 thereby desensitizing TRPV1. Next, we examined whether CCR2 activation had any effect on TRPA1 function. When only CCR2 receptors were expressed in HEK293 cells, MCP-1 treatment induced a transient increase in [Ca2+]i which returned to baseline levels (Fig 5A). However, when TRPA1 was co-expressed with CCR2, the initial rise in [Ca2+]i reached a plateau level above the baseline which persisted for the duration of the recording (Fig 5B: red trace). This effect was completely blocked by the TRPA1 antagonist ruthenium red (RR), indicating that the activation of CCR2 had transactivated TRPA1 channels (Fig 5B: blue trace). Expression of TRPA1 by these cells was confirmed by their responses to TRPA1 agonist allyl isothiocyanate (AITC) at the end of the recording.
Fig. 4. CCR2 activation sensitizes TRPV1.
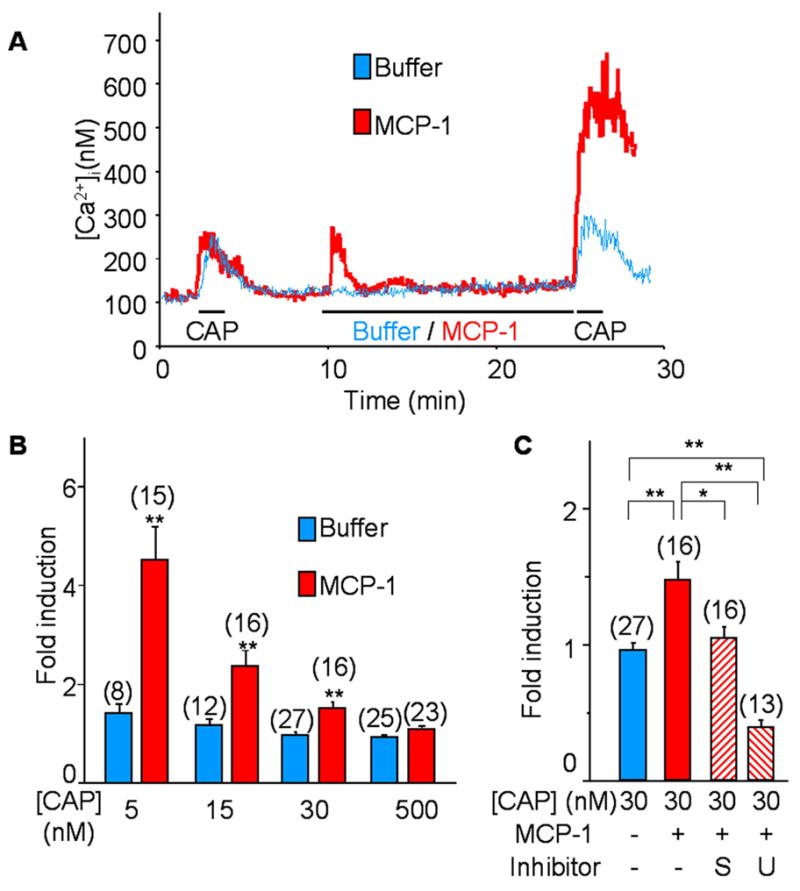
HEK293 cells were transfected with CCR2 and TRPV1 expressing vectors and the responses of TRPV1 to capsaicin (CAP) were measured by Ca2+ imaging. Cells were treated with CAP for 1 min at the beginning and end of the recording (30 min). Between two CAP treatments, cells were incubated with either 100 ng/ml MCP-1 or buffer solution for 15 min. CAP responses were compared before and after the MCP-1 treatment in each cell. Sensitization of TRPV1 was represented by the enhancement of the second CAP response compared to the first one. A) Without MCP-1, the amplitude of two CAP responses was similar (blue trace), whereas the second CAP responses increased after incubation with MCP-1 (red trace). B) The result of TRPV1 sensitization by MCP-1 is summarized. Sensitization was measured with varying concentration of CAP, and represented by a fold induction of the second CAP responses to the first responses in each cell. Sensitization is prominent in lower concentrations of CAP (**p<0.01 vs. mock application control of each concentration). C) The mechanism of TRPV1 sensitization was examined with inhibitors of PLC (U; 10 μM of U73122) and PKC (S; 10 nM of staurosporine). The inhibitors were added 5 min before the recording. Sensitization was completely blocked by PKC and PLC inhibitors. Moreover, PLC inhibitors desensitized TRPV1 (*p<0.05 and **p<0.01). Numbers of recorded cells are noted in parentheses.
Fig. 5. CCR2 activation sensitizes TRPA1.
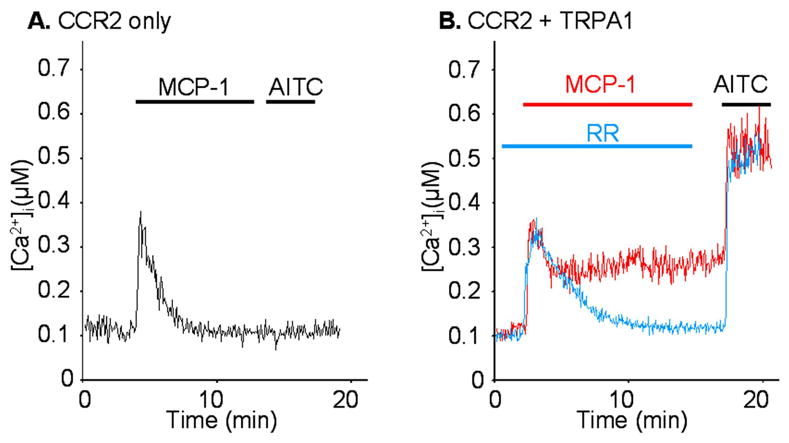
HEK293 cells were transfected with CCR2 and/or TRPA1 expressing vectors, and the responses to MCP-1 (100 ng/ml) and allyl isothiocyanate (AITC) (10 μM), a TRPA1 agonist, were measured by Ca2+ imaging experiments. Ruthenium red (RR; 30 μM), a TRP channel blocker, was added to block TRPA1 channels. A) The response to MCP-1 consisted of a transient component when it was expressed alone. B) An additional sustained component appeared when it was co-expressed with TRPA1 (red trace). The sustained component was abolished by the TRP channel blocker ruthenium red (blue trace). At the end of recording, cells were treated with AITC after washing out of RR to confirm the expression of TRPA1.
These results suggest that DRG neurons that release MCP-1 from the soma by depolarization might activate themselves or neighboring TRPV1/A1-expressing neurons. In order to determine whether this was potentially the case, we used cultured DRG neurons overexpressing CCR2. Cultured neurons were treated with MCP-1 or buffer solutions for 10 min, and then twice with increasing dosage of CAP. At the end of recording, 50K was added to identify neurons. In the control group, where CCR2 expressing neurons were not treated with MCP-1, roughly 40% of neurons expressed TRPV1 (identified by responsiveness to any concentration of CAP) and these neurons only responded to the higher concentration of CAP used (Fig 6 A and B). When neurons were pretreated with MCP-1, the overall number of TRPV1 expressing neurons was unchanged (Fig 6B). However, most of these neurons now also responded to the lower concentration of CAP indicating that MCP-1 had sensitized TRPV1 (Fig 6A and B).
Fig. 6. MCP-1 sensitizes the capsaicin-responsiveness of cultured DRG neurons.
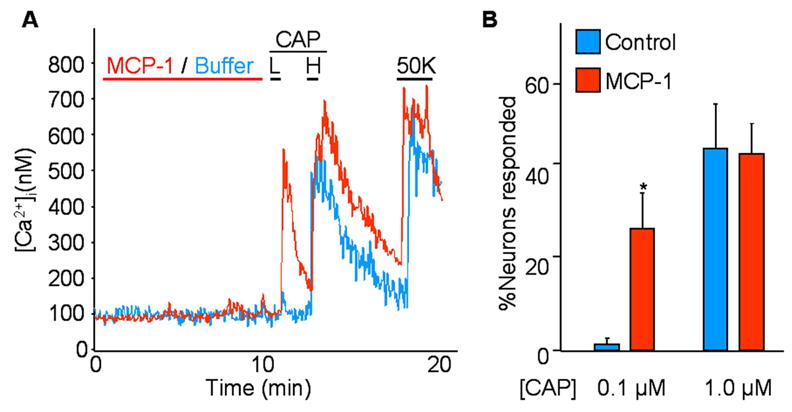
Cultured DRG neurons transduced with a CCR2 expressing adenovirus were pre-treated with MCP-1 or buffer solution for 10 min. Subsequently, neurons were treated with a low concentration (0.1 μM) of capsaicin (CAP) (L), a high concentration (1.0 μM) of CAP (H), and high potassium (50K). Neurons were identified by 50K-responsiveness. A) In the control group, most neurons did not respond to 0.1 μM CAP (blue trace). In the MCP-1-treated group, most neurons that responded to 1.0 μM CAP also responded to 0.1 μM CAP (red). B) Results are summarized. There was no difference in the total number of CAP-responsive neurons. However, MCP-1 pre-treatment increased neurons that were responsive to 0.1 μM CAP (pooled result of four different experiments. In the control group 27 out of 66 neurons responded to 1.0 μM CAP, of which only one responded to 0.1 μM CAP. In MCP-1-treated group, 44 out of 89 responded to 1.0 μM CAP, of which 28 responded to 0.1 μM CAP).
Discussion
It has become clear that many mature neurons express chemokines and their receptors under a variety of circumstances (Horuk et al. 1997; Coughlan et al. 2000; van der Meer et al. 2000; Banisadr et al. 2002a; Banisadr et al. 2002b; Gillard et al. 2002) suggesting that chemokines may have functions in the adult nervous system, in addition to regulating the influx of leukocytes during neuroinflammatory responses. Cultured neonatal DRG neurons express multiple types of chemokine receptors (Oh et al. 2001). However, many of these receptors are not expressed constitutively by adult neurons in vivo, although their expression can be upregulated together with their chemokine ligands in association with peripheral nerve injury-induced neuropathic pain (Tanaka et al. 2004; White et al. 2005a; White et al. 2005b; Bhangoo et al. 2006; Sun et al. 2006; Zhang and De Koninck 2006; Bhangoo et al. 2007). Several chemokines and their receptors have also been shown to be expressed by peripheral non-neuronal cells in the DRG and spinal cord during states of pain hypersensitivity (Abbadie et al. 2003; Milligan et al. 2004; Verge et al. 2004; White et al. 2005a; Bhangoo et al. 2007). MCP-1 and CCR2 upregulation was observed in peripheral sensory neurons in two different pain models (White et al. 2005b; Bhangoo et al. 2006). Significantly, DRG neurons isolated from animals exhibiting neuropathic pain behavior are strongly depolarized by MCP-1, whereas neurons from naïve animals are not (White et al. 2005b; Sun et al. 2006). Moreover, CCR2 knockout mice did not develop neuropathic pain in a nerve injury model (Abbadie et al. 2003), suggesting that CCR2 signaling is involved in the generation of states of chronic pain hypersensitivity.
It is not clear, however, how MCP-1 normally signals in the DRG. What is the status of MCP-1 when it is expressed by DRG neurons? Is it secreted from these cells and if so, how is this process regulated? What are the targets of secreted MCP-1 and how is MCP-1 signaling transduced? Indeed, does MCP-1 function as a neuromodulator in the DRG? The data presented in this paper suggests that this is certainly the case. We have observed that in common with other peptide neurotransmitters, MCP-1 expressed in DRG neurons is processed into the secretory pathway through LDCVs, and can be released by neuronal depolarization. It appears that MCP-1 can be stored in the same vesicles as CGRP, an established neurotransmitter. Calcium influx via voltage-dependent Ca2+ channels was required to release MCP-1 from the somata of cultured neurons. Substance P and CGRP have also been shown to be released from the somata of DRG neurons by Ca2+-dependent regulated exocytosis (Huang and Neher 1996; Ouyang et al. 2005). Since MCP-1 and CCR2 seem to be co-expressed in a population of closely juxtaposed neurons (Fig 1F), MCP-1/CCR2 signaling may participate in somatosomatic communication between DRG neurons. Furthermore, because chemokines such as MCP-1 can also induce the release of CGRP (Qin et al. 2005), and CGRP has also been suggested as mediating somatosomatic signaling in the DRG (Knopp and Oxford 2006), upregulated MCP-1 expression and release could help to drive a coordinated state of hyperexcitability throughout the entire DRG.
Two other recent reports in the literature suggest that chemokines may be released from neurons in an activity-dependent manner. The chemokine CCL21 is induced by glutamate treatment in dissociated hippocampus and cortical neurons as well as in neurons in hippocampal slice culture (de Jong et al. 2005). CCL21 containing vesicles were observed in trans-Golgi network, axons, and presynaptic structures of cultured neurons. It is likely that CCL21 could also be released by neurons, since supernatant of cultured neurons treated with glutamate attracted microglia in vitro. Furthermore, Fryer et al. demonstrated the expression of eotaxin/CCL3 by parasympathetic neurons in a model of asthma and suggested release of this chemokine from neurons played a role in the attraction of eosinophils (Fryer et al. 2006).
It is now clear that chemokines can be synthesized and released by many different types of cells. In most instances chemokines are upregulated in the face of some prevailing pathology. In leukocytes and endothelial cells, where chemokine function has been most widely studied, chemokines are also stored in secretory granules and their release can be regulated by different stimuli (Oynebraten et al. 2004; Oynebraten et al. 2005). Interestingly, single endothelial cells can express multiple types of chemokines and these are targeted to different populations of secretory vesicles that can be selectively released by different stimuli. In the present case it is also clear that DRG neurons can upregulate and store different chemokines (White et al. 2005a; White et al. 2005b; Bhangoo et al. 2006; Bhangoo et al. 2007) and that these may also be targeted to diverse populations of secretory vesicles (Jung and Miller, unpublished data). Thus, the ultimate role of chemokine release in the context of neuropathic pain may be complex with different chemokines serving different roles. At any rate, when chemokines are synthesized in sensory neurons they seem to be stored in secretory vesicles as is the case in leukocytes and endothelial cells.
Considering that many chemokines and their receptors are expressed in the nervous system, it is likely that chemokines participate widely in neural communication (Adler and Rogers 2005). The expression of chemokines and their receptors in brain are often not uniform but region-specific (Horuk et al. 1997; van der Meer et al. 2000; Banisadr et al. 2002a; Banisadr et al. 2002b). In this regard, it will be interesting to see if individual chemokines have specific functions in different regions of the nervous system. As chemokines are not generally expressed by neurons at high concentrations (SDF-1/CXCL12 being an important exception to this), they may be viewed as generally inducible neuromodulators that are particularly important under pathological circumstances. Thus, neuronally expressed chemokines may be thought of as a component of the innate immune response in the nervous system.
It is interesting to note that Gosselin et al. reported that spinal cord neurons constitutively express CCR2 under normal circumstances and MCP-1 inhibits GABAergic transmission in these cells (Gosselin et al. 2005). Although we did not investigate whether MCP-1 is transported to the terminals of spinal afferent in vivo, we observed that MCP-1 containing vesicles were transported all along the axons in cultured DRG neurons (Fig 2A and supporting figure 1B), and that MCP-1 release could also be induced from the neurite terminals of cultured sensory neurons (supporting figure 3B). Furthermore, immunohistochemical localization of MCP-1 in DRG sections suggested its axonal localization in vivo (Fig 1B). Since different neuropeptides may be co-stored and co-released, MCP-1 may also function together with other neuropeptides such as Substance P and CGRP in modulating synaptic transmission in the dorsal horn of spinal cord. In addition, CCR2 expression can also be upregulated in microglial cells in the spinal cord providing yet another potentially important target for MCP-1 released centrally (Abbadie et al. 2003). If MCP-1 acts as a DRG neuromodulator, how does it act once it is released? As we have now demonstrated, MCP-1 and CCR2 are frequently expressed by the same neurons suggesting that MCP-1 release may act in an autoregulatory manner. The two molecules were upregulated by the majority of TRPV1 expressing nociceptors. We also demonstrated that the activation of CCR2 induced the sensitization of TRPV1 and TRPA1 cation channels expressed by nociceptive DRG neurons. The sensitization of TRPV1 by the CCR1 chemokine receptor and its ligand MIP-1α was also recently reported by Zhang et al. (2005). Thus, chemokines are part of a ever growing number of potential mediators of pain hypersensitivity that transactivate TRP receptors expressed by DRG neurons. The circumstances in which this activation occurs, however, seem to be ligand specific. In the case of chemokines, their expression is specifically associated with states of neuropathic pain, suggesting that their role in generating DRG neuron excitability is particularly associated with this phenomenon.
In summary, we can conclude that MCP-1 acts as a neuromodulator in DRG based on the following findings: 1) It is expressed by DRG neurons; 2) It is packaged into secretory vesicles together with molecules such as CGRP that are known to act as neurotransmitters in the DRG; 3) It is released by neuronal excitation in a Ca2+-dependent manner; 4) It can modulate the excitability of DRG neurons. Because MCP-1/CCR2 signaling in the DRG is not present under normal circumstances but is induced under pathological conditions, the antagonism of MCP-1/CCR2 signaling may be a novel therapeutic approach in treating neuropathic pain.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DA013141 (R.J.M.), NS043095 (R.J.M.), MH040165 (R.J.M.), NS049136 (F.A.W.) an Illinois Excellence in Medicine Grant (F.A.W.), and the National Multiple Sclerosis Society (F.A.W.).
Abbreviation
- MCP-1
monocyte chemoattractant protein-1
- DRG
dorsal root ganglion
- TRP
transient receptor potential
- LDCV
large dense-core vesicle
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005;78:1204–1209. doi: 10.1189/jlb.0405222. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002a;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Melik Parsadaniantz S. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. Journal of Neurochemistry. 2002b;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bhangoo SK, Jung H, Chan DM, Ripsch M, Miller RJ, White FA. Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Peripheral demyelination injury induces upregulation of chemokine/receptor expression and neuronal signaling in a model of neuropathic pain. 2006, Online 250, 3. [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, et al. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Boddeke HW, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francel PC, Harris K, Smith M, Fishman MC, Dawson G, Miller RJ. Neurochemical characteristics of a novel dorsal root ganglion X neuroblastoma hybrid cell line, F-11. J Neurochem. 1987;48:1624–1631. doi: 10.1111/j.1471-4159.1987.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Fryer AD, Stein LH, Nie Z, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard SE, Lu M, Mastracci RM, Miller RJ. Expression of functional chemokine receptors by rat cerebellar neurons. J Neuroimmunol. 2002;124:16–28. doi: 10.1016/s0165-5728(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R, Martin A, Wang Z, et al. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- Huang J, Zhang X, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol. 2006;17:638–645. doi: 10.1016/j.semcdb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Knopp K, Oxford G. Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Is enhanced intraganglion excitability a paracrine effect of CGRP release from DRG somata? 2006, Online 441.16. [Google Scholar]
- Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang K, Zheng H, Qin X, Zhang C, Yang D, Wang X, Wu C, Zhou Z, Cheng H. Ca2+ sparks and secretion in dorsal root ganglion neurons. Proc Natl Acad Sci U S A. 2005;102:12259–12264. doi: 10.1073/pnas.0408494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oynebraten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Barois N, Hagelsteen K, Johansen FE, Bakke O, Haraldsen G. Characterization of a novel chemokine-containing storage granule in endothelial cells: evidence for preferential exocytosis mediated by protein kinase A and diacylglycerol. J Immunol. 2005;175:5358–5369. doi: 10.4049/jimmunol.175.8.5358. [DOI] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, Lamotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical Analysis of CCR2, CCR3, CCR5, and CXCR4 in the Human Brain: Potential Mechanisms for HIV Dementia. Experimental and Molecular Pathology. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005a;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005b;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


