Abstract
The hypothesis that size-selective predation and species-specific prey behaviours facilitate the coexistence between larvae of invasive Aedes albopictus (Skuse) and U.S.A.-native Ochlerotatus triseriatus (Say) was tested experimentally with the predator Corethrella appendiculata (Grabham).
Larval behaviours associated with a higher risk of predation were identified, and prey behavioural responses were tested in either the physical presence of predators or in water containing predation cues. Larvae that thrashed on container bottoms had a higher risk of being captured by fourth instar C. appendiculata than did larvae resting on the water surface. Ochlerotatus triseriatus, but not A. albopictus, adopted low-risk behaviours in response to water-borne cues to predation. Both prey species reduced risky behaviours in the physical presence of the predator, but O. triseriatus showed a stronger response.
The vulnerability of 2nd and 3rd instar prey to predation was compared, and behavioural responses were correlated with prey vulnerability. Second instars of both species were more vulnerable to predation by C. appendiculata than were 3rd instars, and the 3rd instar A. albopictus was more vulnerable than O. triseriatus of the same stage. All instars of O. triseriatus showed a similar reduction of risky behaviours in response to the presence of C. appendiculata despite 4th instar prey being relatively invulnerable to size-selective predation.
Weaker predator avoidance, coupled with superior competitive ability, of invasive A. albopictus is likely to contribute to its coexistence with O. triseriatus in containers of the south-eastern U.S.A., where C. appendiculata can be abundant.
Keywords: Anti-predatory behaviour, Aedes albopictus, Corethrella appendiculata, invasive, native species, Ochlerotatus triseriatus, predation cues and size refuge
Introduction
Behavioural modification in response to predation risk is a major mechanism by which aquatic organisms increase their chances of survival in the presence of predators (Sih, 1980; Werner et al., 1983; Sih, 1986; Lima & Dill, 1990; Van Buskirk & Yurewicz, 1998; Van Buskirk, 2002). For a variety of organisms there is a clear positive relationship between the reduction in activity level and a reduction in predation risk (Lind & Cresswell, 2005), but there are also costs associated with reduced activity, and these costs usually reduce foraging, feeding rate, and growth (Sih, 1980; Sih, 1992; Van Buskirk & Yurewicz, 1998; Van Buskirk, 2000; Relyea, 2002; Lind & Cresswell, 2005).
Predator-induced behavioural change in prey may have important consequences for community composition (Werner & Anholt, 1996; Chase et al., 2002). Trait-mediated indirect effects occur when the traits affecting the interactions between two species (e.g. behaviour, morphology) are influenced by a third species. For example, the presence of predatory species may change prey behaviour (e.g. reduced activity, increased use of refuges), which in turn, may indirectly alter the competitive interactions among prey (Werner, 1991; Werner & Anholt, 1996; Relyea, 2000).
Container-dwelling immature stages of the mosquitoes Ochlerotatus triseriatus (Previously Aedes triseriatus) and Aedes albopictus are preyed upon by the larvae of a corethrellid midge, Corethrella appendiculata, and a mosquito Toxorhynchites rutilus (Griswold & Lounibos, 2006). These prey and predators co-occur commonly in the south-eastern U.S.A. All of these species are native to the U.S.A., except for A. albopictus, which was introduced into North America in the 1980s and has since spread throughout the south-east (Hawley et al., 1987). It is well documented that A. albopictus larvae are competitively superior to O. triseriatus (Livdahl & Willey, 1991; Teng & Apperson, 2000; Aliabadi & Juliano, 2002; Juliano & Lounibos, 2005). Despite the apparent competitive superiority of A. albopictus compared with O. triseriatus under laboratory conditions, the invasion of south Florida by A. albopictus has not resulted in reduced abundances of O. triseriatus in the field (Lounibos et al., 2001).
Aedes albopictus is more vulnerable to predation by C. appendiculata and T. rutilus than by O. triseriatus (Griswold & Lounibos, 2006). Toxorhynchites rutilus usually capture their prey near the bottom of the containers (Steffan & Evenhuis, 1981), where prey are consequently more vulnerable to predation (Juliano & Reminger, 1992). Unlike A. albopictus, O. triseriatus alters its behaviour in the presence of water-borne predation cues from T. rutilus by resting more often at the water surface (Juliano & Gravel, 2002; Kesavaraju & Juliano, 2004). It is not known how prey behaviour is related to the vulnerability to predation by C. appendiculata. Furthermore, it is unclear whether O. triseriatus modify their behaviour in the presence of predation cues from C. appendiculata in a similar way as they do in the presence of cues from T. rutilus.
In addition to the species-specific differences in susceptibility to C. appendiculata predation, the relative sizes of prey and predator may be important for this predator. First instar T. rutilus are comparable in size with 4th instar C. appendiculata (Lounibos, 1983; Lounibos, 1985), whereas 4th instar C. appendiculata (mean ± SE head capsule width, 0.715 ± 0.003 mm) are comparable with the size of the 3rd instars of their mosquito prey, A. albopictus and O. triseriatus (0.671 ± 0.002 and 0.754 ± 0.002 for 3rd instars respectively) (B. W. Alto, unpublished data). Furthermore, for each developmental stage, O. triseriatus are significantly larger than A. albopictus (B. W. Alto, unpublished data). It is not known whether size differences between the predator and prey in this container system result in a prey-size refuge from C. appendiculata predation. In some systems prey that are vulnerable to predation during smaller and younger stages become invulnerable to predation when they grow and become larger than the predator (Lundvall et al., 1999; Nilsson & Bronmark, 2000).
The objective of this research was to test the following.
Whether some behaviours of A. albopictus larvae are associated with a higher risk of predation by C. appendiculata.
Whether A. albopictus and O. triseriatus alter their behaviour in response to water-borne cues from the act of predation by C. appendiculata.
Whether A. albopictus and O. triseriatus alter their behaviour in response to the physical presence of C. appendiculata.
Whether the vulnerability of A. albopictus and O. triseriatus to C. appendiculata predation varies with developmental stage.
If so, whether different stages of these prey show shifts to low-risk behaviours in response to the predation cues from C. appendiculata that are correlated with their stage-specific vulnerability to this predator.
Materials and methods
Larvae of C. appendiculata were collected from the wild in Florida (from tree holes at the following sites: Florida Medical Entomology Laboratory, Vero Beach, FL, U.S.A.; Myakka River State Park, near Sarasota, FL, U.S.A.; Indrio Road and Sherwood Hammock, Fort Pierce, FL, U.S.A.) just before the experiments were conducted. The C. appendiculata were starved for a period of 4–10 days prior to the experiments. Aedes albopictus were F1 progeny from a colony collected from the wild (Indrio Road, Fort Pierce) as larvae and propagated as adults in cages. Ochlerotatus triseriatus were from a lab colony at the Florida Medical Entomology Laboratory (Vero Beach), which included frequent additions of wild-caught O. triseriatus from Florida (Myakka River State Park, near Sarasota; Indrio Road, Fort Pierce). Both the colonies were blood fed weekly (University of Florida IACUC protocol #VB-17) to obtain eggs.
Experiment 1. High risk activity and position
Experimental units consisted of plastic cups holding 10 ml of distilled water. Hollow glass cylinders were positioned vertically in each cup and one 4th instar C. appendiculata was added to the cylinder, subsequently a 2nd instar A. albopictus was added to each cup, and after a 5-min acclimation period, the glass cylinders were removed releasing the predators. As soon as the glass cylinders were removed the behaviour of the prey, A. albopictus, was videotaped for 30 min. Each video clip showed four cups. The video clips were viewed and the activity and position of each A. albopictus larva was determined every minute by instantaneous scan censuses that lasted for either 30 min or until the prey were captured (Juliano & Reminger, 1992). Following the method described by Juliano and Reminger (1992), four activities: (1) resting – larva neither feeding nor moving; (2) browsing – larva propelled along the surfaces of the cup by the movement of their mouthparts; (3) filtering – larva floating in the water column propelled by the movement of their mouthparts; (4) thrashing – vigorous lateral movements of the body of the larva, propelling it through the water; and four positions: (1) surface – spiracular siphon of the larva in contact with water–air interface; (2) bottom – larva within 1 mm of the bottom of the cup; (3) wall – larva within 1 mm of the cup walls; (4) middle – larva more than 1 mm from any surface of the cup and not in contact with the water–air interface, were identified.
When prey capture occurred, the behaviour of the prey larva immediately before capture by C. appendiculata was determined (capture group) and the behaviour of a prey larva in an adjacent cup that had not been captured by C. appendiculata (no-capture group) was also determined. Differences in the activity and position frequencies between capture and no-capture groups were tested using Fisher’s Exact tests.
Experiment 2. Behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to the presence of Corethrella appendiculata
The behaviour of 2nd instar A. albopictus and O. triseriatus was recorded in predation and control treatments in 10-ml plastic cups with 10 ml of water for the treatments. Predation treatment cups contained one 4th instar C. appendiculata and either one 2nd instar A. albopictus or one 2nd instar O. triseriatus. Controls were similar but had no predators. Test subjects (prey larvae) used were hatched separately, and held in individual vials with 5 ml of distilled water. The larvae were fed 1 ml of liver powder suspension (LPS), which was prepared by adding 0.3 g of liver powder in 1000 ml of water. The LPS was stirred on a stir plate and transferred using a pipette to ensure the transfer of consistent quantities of food (Juliano & Gravel, 2002). The larvae were fed only once, which was sufficient for development to the 2nd instar. Prey larvae used in this experiment were starved for 24 h, to standardize hunger, before transferring them to the treatment cups for behaviour recording. Hollow glass cylinders were used to isolate the predators in the predation treatment. Glass cylinders were empty in the control treatments. After a 5-min acclimation period, cylinders were removed slowly from the cups releasing the predators. Behaviour of the larva was recorded for 30 min, in such a way that each video clip showed four experimental cups (two predation, two controls). The activity and position of the larvae were determined every 15 s for 7.5 min, or until the prey was captured. The short observation period was necessary because a substantial number of larvae were captured within 7.5 min. The activity and position of larvae were determined every minute for 30 min from playback of the video clips. Activities and positions were then converted to proportions and Principal Components Analysis (PCA) was used to obtain uncorrelated descriptors of activity and position, and to reduce the number of variables used in the analyses (SAS Institute Inc., 1990). The principal components (PCs) were analyzed using MANOVA, and standardized canon ical coefficients (SCCs) were used to interpret the relative contribution of PCs to significant effects (Scheiner, 2001).
In some replicates the number of observations was fewer than 10 (out of a maximum of 30) because the prey were captured within a 3-min period. Those replicates were excluded to improve our estimates of the proportions (Juliano & Reminger, 1992). A non-parametric survival analysis (PROC LIFETEST) was also performed to compare the median survival times for larvae of each species in the predation treatment (SAS Institute Inc. (1990).
Experiment 3. Behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to Corethrella appendiculata water-borne cues
The behaviour of 2nd instar A. albopictus and O. triseriatus in control and predation water treatments were recorded. Predation water was prepared by feeding three 4th instar C. appendiculata 10 2nd instar larvae of either A. albopictus or O. triseriatus in 10-ml cups for 5 days. The treatment cups were checked daily and dead or eaten prey larvae and pupating C. appendiculata were replaced. Controls were handled similarly, except that C. appendiculata were absent from the cups. After 5 days prey and predators were removed, leaving behind only water-borne cues from predation (e.g. dissolved compounds, feces, uneaten prey parts) in the treatment cups and similar substances in control cups. Larvae of A. albopictus and O. triseriatus were used as test subjects for recording behaviour, and the species used in preparing the treatment water was matched to the species used as test subjects (i.e. O. triseriatus test subjects were observed in treatment waters that were prepared using O. triseriatus). Test larvae used in this experiment were reared and were starved for 24 h to standardize hunger before transferring them to the treatment cups in the same way as the previous experiment. The behaviour was video recorded, with each video clip 30-min long, and showing four cups (two predation, two control). The values were then converted to proportions of observations in each behavioural category and analyzed using PCA and MANOVA, as in the previous experiment.
Experiment 4. Differences in the vulnerability of 2nd and 3rd instar Aedes albopictus and Ochlerotatus triseriatus to predation by Corethrella appendiculata
This experiment consisted of three parts. For all three parts, experimental units were cups containing 50 ml of water. The predation treatment involved the addition of a 4th instar C. appendiculata to the cup and the controls contained no predators. Treatments were replicated 10 times, and the numbers of surviving larvae were determined at the end of 24 h. All three parts of the experiment were conducted at the same time.
Part 1. Each instar and species combination was tested alone
The survival of 2nd and 3rd instar larvae of A. albopictus and O. triseriatus in control and predation treatments was tested, which resulted in a three-way factorial design with eight treatments that were combinations of instar, species, and predation variables. Twelve larvae of either 2nd or 3rd instar of A. albopictus or O. triseriatus were added, depending on the treatment, to control and predation treatments. The number of survivors in each of the cups was converted to proportions and then arcsine-square-root transformed to meet the assumption of normality. The data was analyzed using ANOVA.
Part 2. Single species, 2nd and 3rd instars tested together
This part tested whether C. appendiculata preyed differentially on a particular stage of the prey when they were given a choice. Six 2nd and six 3rd instar larvae of either A. albopictus or O triseriatus were added to each control or predation replicate, depending on the treatment. The number of survivors of 2nd and 3rd instar larvae from each cup was determined and analyzed as two dependent variables in a MANOVA with predation treatments and prey species as factors.
Part 3. Single instar, both species tested together
This part tested whether C. appendiculata preyed differentially on these species when given a choice. Six A. albopictus and six O. triseriatus larvae of either 2nd or 3rd instars were added, depending on the treatment, to each control and predation replicate. The main difference between part 2 and part 3 was that the experimental units in part 2 had treatments with only conspecifics of different instars present, whereas the experimental units in part 3 had A. albopictus and O. triseriatus of the same instar combined. The number of survivors of A. albopictus and O. triseriatus from each cup was determined and analyzed as two dependent variables using a MANOVA with predation treatment and instar as factors.
Experiment 5. Behavioural differences between 2nd and 4th instar Ochlerotatus triseriatus in response to water-borne cues to predation by Corethrella appendiculata
The behaviour of 2nd and 4th instar O. triseriatus in control and predation water was recorded. Predation water was prepared by feeding three 4th instar C. appendiculata with 10 2nd instar O. triseriatus in cups containing 10 ml of water. Cups were checked every day for 5 days and dead or eaten prey and pupated predators were replaced. Controls were maintained similarly, except that C. appendiculata larvae were absent. On the 5th day both predators and prey were removed from the cups leaving behind only the water-borne cues to predation from C. appendiculata. A single either 2nd or 4th instar O. triseriatus, depending on the treatment, was transferred to each of the treatment and control cups. They were given a 5-min acclimation period before their behaviour was video recorded. Each video clip contained four cups representing two predation and two control treatments. Larvae that were used in the behavioural recording were hatched and reared individually in vials with LPS as described in Experiment 2, so that experimental larvae were not exposed to cues to predation until testing. Behaviours were coded as described above and then converted to proportions. The data were then analyzed by PCA, followed by MANOVA on the PCs, as in previous experiments.
Results
Experiment 1. High-risk activity and position
There were highly significant differences in the frequencies of activities (Fisher’s Exact test, χ2 = 25.04, d.f. = 3, P < 0.0001) and positions (Fisher’s Exact test, χ2 = 45.73, d.f. = 3, P < 0.0001) between capture and no-capture groups. Thrashing was more common immediately prior to capture by C. appendiculata (Fig. 1). Resting was more common in the no-capture group. Larvae at the bottom of the cups were most common in the capture group; whereas in the no-capture group, larvae at the surface and in the middle were highly represented (Fig. 1).
Fig. 1.
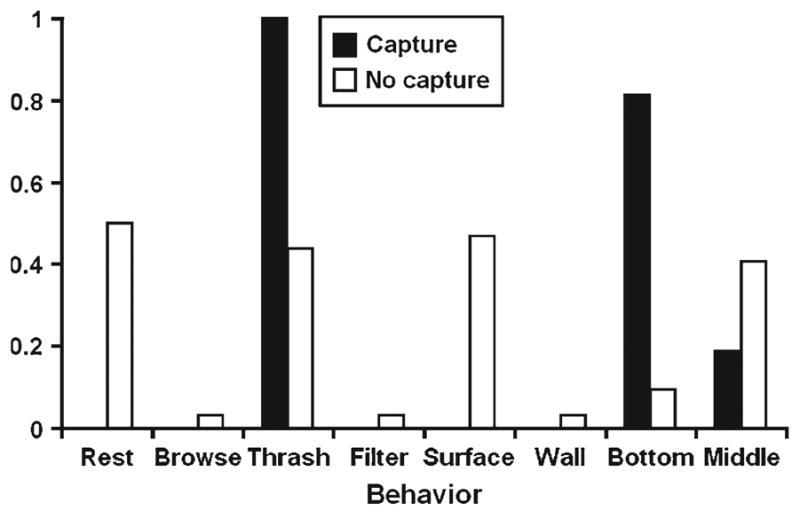
Experiment 1. Average proportions of activities and positions in the two groups (capture and no capture).
Experiment 2. Behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to the presence of Corethrella appendiculata
Two PCs with eigen values > 1 were obtained, accounting for 72.7% of the total variation in these data (Table 1). A large positive score for PC1 indicated that a larva spent more time resting at the surface, and a negative score indicated that it spent more time thrashing in the middle. Similarly, a large positive score on PC2 indicated more time spent filtering in the middle, and a negative score indicated more time spent at the bottom (Table 1).
Table 1.
Experiment 2. Rotated factor patterns testing for the behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to the presence of Corethrella appendiculata. Values greater than 40 are in bold and represent strong factor loading contributions for each principal component (PC).
| Variables | PC1 | PC2 |
|---|---|---|
| Resting | 86 | 1 |
| Browsing | 0 | 0 |
| Thrashing | −88 | −10 |
| Filtering | 14 | 75 |
| Surface | 82 | 33 |
| Wall | 0 | 0 |
| Bottom | −8 | −80 |
| Middle | −70 | 59 |
| Interpretation | resting, surface vs. thrashing, middle | filtering, middle vs. bottom |
There were significant species and treatment effects, but no significant interaction (Table 2). The SCCs indicated that PC1 contributed most to the significant effects of both species and treatment (Table 2). Compared with control, both A. albopictus and O. triseriatus altered their behaviour and reduced their frequency of thrashing in the middle, and increased their frequency of resting at the surface when they were in the predation treatment (Fig. 2). Pairwise differences between control and predation treatments were similar in magnitude for the two species (hence no significant interaction), but were significant only for O. triseriatus (Fig. 2). The species effect was primarily a result of less thrashing in the middle and more resting at the surface for O. triseriatus.
Table 2.
Experiment 2. MANOVA for prey behavioural principal components (PCs) to the presence of Corethrella appendiculata. PCs contributing strongly to significant effects are shown in bold.
| Standardized canonical coefficients
|
||||||
|---|---|---|---|---|---|---|
| Variables | Num. d.f. | Den. d.f. | Pillai’s trace | P | PC1 | PC2 |
| Species | 2 | 72 | 0.168 | 0.0013 | 1.150 | 0.032 |
| Treatment | 2 | 72 | 0.147 | 0.0031 | 1.136 | −0.211 |
| Species–treatment | 2 | 72 | 0.046 | 0.1819 | ||
Fig. 2.

Experiment 2. Behaviour of Aedes albopictus and Ochlerotatus triseriatus in response to the physical presence of Corethrella appendiculata. Principal component 1 describes resting at the surface vs. thrashing in the middle of the container. Means with similar letters are not significantly different from each other (Tukey–Kramer multiple comparisons test).
Survival analysis indicated that the survivorship curves of A. albopictus and O. triseriatus differed significantly (Log-Rank test, χ2 = 5.65, d.f. = 1, P < 0.0174). Median ± SE survival times were 24.5 ± 2.44 and 8.0 ± 2.35 min for O. triseriatus and A. albopictus, respectively (Fig. 3).
Fig. 3.

Experiment 2. Survival analysis describing the survival distribution functions of Aedes albopictus and Ochlerotatus triseriatus. Standard error bars are included but are too small to be visible.
Experiment 3. Behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to Corethrella appendiculata water-borne cues
Three PCs with eigen values > 1 were obtained, accounting for 88.5% of the total variance in these data (Table 3). Based on rotated factor patterns (Table 3), a large positive score on PC1 could be interpreted as more time spent resting at the surface and a large negative score as more time spent either browsing or thrashing at the bottom. For PC2 large positive scores indicated frequent browsing at the wall, whereas negative scores indicated thrashing in the middle. For PC3 large positive scores indicated frequent filtering in the middle, whereas large negative scores indicated more time spent at the bottom (Table 3).
Table 3.
Experiment 3. Rotated factor patterns testing behavioural responses of Aedes albopictus and Ochlerotatus triseriatus to water-borne predation cues. Values greater than 40 are in bold and represent strong factor loading contributions for each principal component (PC).
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Resting | 99 | −13 | 2 |
| Browsing | −48 | 77 | −36 |
| Thrashing | −41 | −77 | −30 |
| Filtering | −1 | 1 | 92 |
| Surface | 99 | −9 | 3 |
| Wall | −34 | 82 | −6 |
| Bottom | −45 | −13 | −70 |
| Middle | −24 | −54 | 77 |
| Interpretation | resting, surface vs. browsing, thrashing, bottom | browsing, wall vs. thrashing, middle | filtering middle vs. bottom |
There were significant effects of species and treatment, and a marginally significant interaction (Table 4), which was included when interpreting these results. SCCs indicated that PC1 contributed more to producing the interaction than did the two other PCs (Table 4). Multiple comparisons for PC1 showed that O. triseriatus reduced their time spent either browsing or thrashing at the bottom and increased their time spent resting at the surface when they were in predation water, compared with control (Fig. 4). In contrast to O. triseriatus, A. albopictus did not significantly change its behaviour between control and predation water (Fig. 4).
Table 4.
Experiment 3. MANOVA for prey behavioural principal compinents (PCs) to Corethrella appendiculata water-borne cues. PCs contributing strongly to significant effects are shown in bold.
| Standardized canonical coefficients
|
|||||||
|---|---|---|---|---|---|---|---|
| Variables | Num. d.f. | Den. d.f. | Pillai’s trace | P | PC1 | PC2 | PC3 |
| Species | 3 | 70 | 0.340 | <0.0001 | 0.801 | 0.348 | 0.921 |
| Treatment | 3 | 70 | 0.277 | <0.0001 | 1.115 | −0.426 | 0.364 |
| Species–treatment | 3 | 70 | 0.095 | 0.0702 | 1.221 | 0.081 | 0.171 |
Fig. 4.

Experiment 3. Behaviour of Aedes albopictus and Ochlerotatus triseriatus in response to water-borne Corethrella appendiculata predation cues. Principal component 1 describes resting at the surface vs. browsing and thrashing at the bottom of the container. Means with similar letters are not significantly different from one another (Tukey–Kramer multiple comparisons test).
Experiment 4. Differences in the vulnerability of 2nd and 3rd instar Aedes albopictus and Ochlerotatus triseriatus to predation by Corethrella appendiculata
Part 1. Each instar and species combination was tested alone
The three-way interaction was not significant, but all two-way interactions were significant (Table 5). The reduction in survival as a result of the presence of C. appendiculata was greater for A. albopictus than for O. triseriatus (Fig. 5a). Across both predator treatments, O. triseriatus had a greater survival rate than did A. albopictus as 3rd instars, but A. albopictus had greater survival rate than did O. triseriatus as 2nd instars (Fig. 5b). Although 2nd instar larvae had lower survival rates than did 3rd instar larvae in control and predation treatments, the reduction in survival as a result of the presence of C. appendiculata was greater for 2nd instar larvae than for 3rd instar larvae (Fig. 5c).
Table 5.
Experiment 4 (part 1). ANOVA for Corethrella appendiculata affects on survival of prey at each instar (2nd and 3rd) and species combination tested alone.
| Variables | d.f. | F | P |
|---|---|---|---|
| Species (S) | 1 | 0.01 | 0.9418 |
| Treatment (T) | 1 | 142.01 | <0.0001 |
| Instar (I) | 1 | 102.09 | <0.0001 |
| S–T | 1 | 16.05 | 0.0001 |
| S–I | 1 | 19.56 | <0.0001 |
| T–I | 1 | 9.79 | 0.0025 |
| S–T–I | 1 | 0.93 | 0.3393 |
Fig. 5.
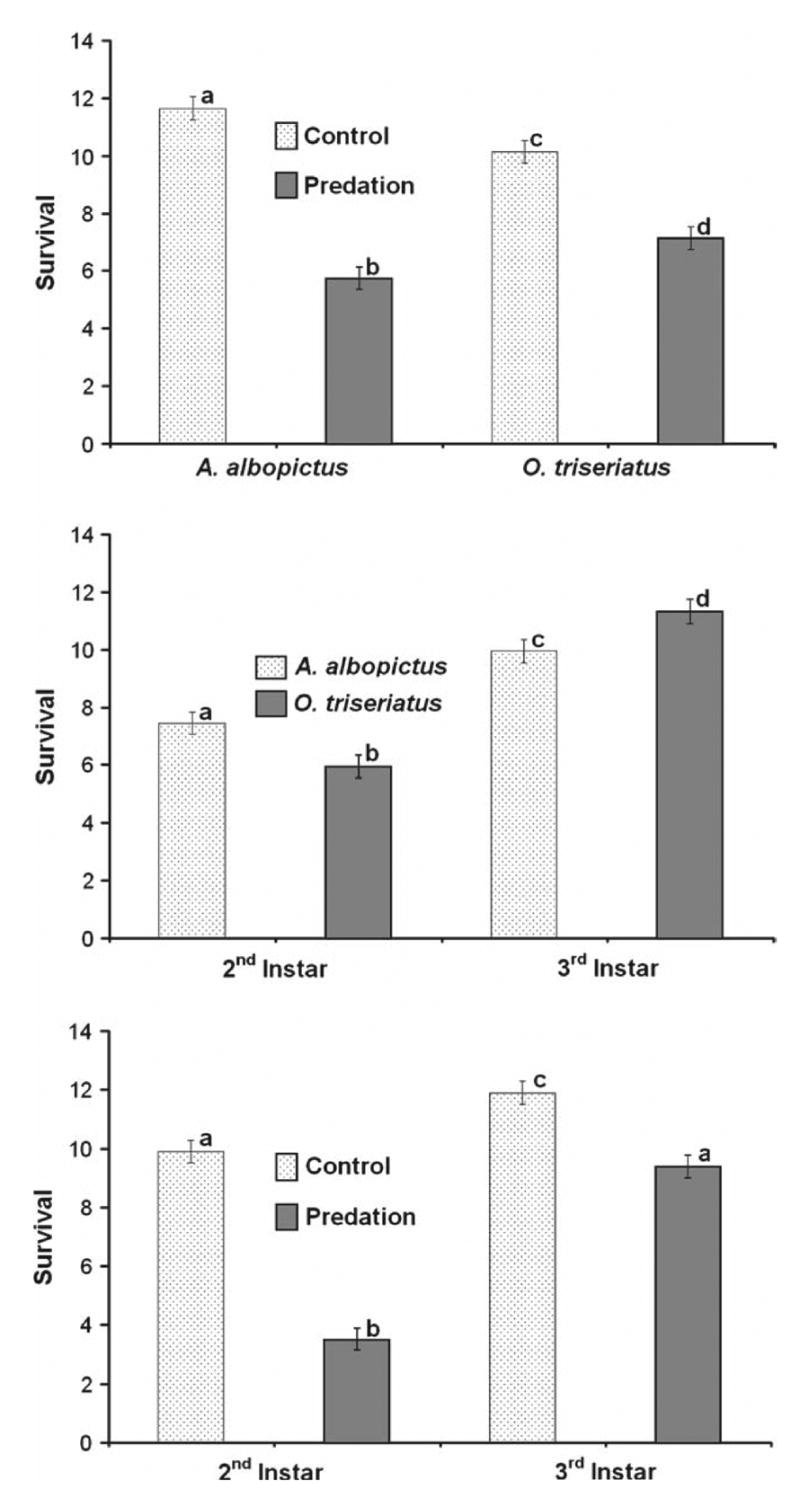
Experiment 4 (part 1). The effects of Corethrella appendiculata on the survival of Aedes albopictus and Ochlerotatus triseriatus prey in the 2nd and 3rd instars, with each stage and species tested alone: (a) significant treatment–species interaction (pooled across instars); (b) significant stage–treatment interaction (pooled across species); (c) significant stage–species interaction (pooled across predation treatments). Means with similar letters are not significantly different from one another (Tukey–Kramer multiple comparisons test).
Part 2. Single species, 2nd and 3rd instars tested together
The treatment by species interaction was marginally significant, and the main effects of treatment and species were both significant (Table 6). As before, this marginally significant interaction was included when the data were interpreted. SCCs indicated that only numbers of surviving 2nd instars contributed to significant main effects and the marginally significant interaction (Table 6). For both species, the effects of treatments on the survival of 3rd instar larvae were negligible (Fig. 6; note the small scatter in the mean values along the x-axis). The survival rate of 2nd instar larvae was reduced significantly by predation treatment for both prey species, but the reduction as a result of predation was somewhat greater for O. triseriatus than for A. albopictus (Fig. 6). This difference was likely to be the source of the marginally significant interaction.
Table 6.
Experiment 4 (part 2). MANOVA for Corethrella appendiculata effects on survival of 2nd and 3rd instars tested together for each species. Stage survival variables contributing strongly to significant effects are shown in bold.
| Standardized canonical coefficients
|
||||||
|---|---|---|---|---|---|---|
| Variables | Num. d.f. | Den. d.f. | Pillai’s trace | P | 2nd Instar | 3rd Instar |
| Species | 2 | 33 | 0.257 | 0.0074 | 2.504 | −0.094 |
| Treatment | 2 | 33 | 0.837 | <0.0001 | 2.475 | 0.003 |
| Species–treatment | 2 | 33 | 0.148 | 0.0706 | 2.498 | −0.068 |
Fig. 6.

Experiment 4 (part 2). The effects of Corethrella appendiculata on the survival of Aedes albopictus and Ochlerotatus triseriatus prey. Survival of 2nd and 3rd instars for the treatment and species effect on 2nd and 3rd instar prey tested together, single species.
Part 3. Single instar, both species tested together
Interaction between instar and treatment was significant (Table 7). SCCs indicated that both species contributed substantially to the instar–treatment interaction (Table 7). In the 2nd instar group, the effect of predation was a large reduction of survival rates for both species, although the survival rate of A. albopictus was reduced to a slightly greater extent (Fig. 7). In contrast, in the 3rd instar group, the effect of predation was a large reduction in the survival rate of A. albopictus only (Fig. 7; note the scatter in the mean values along the y-axis). For both species, the survival rate of 3rd instar larvae was greater than that of 2nd instar larvae (Fig. 7).
Table 7.
Experiment 4 (part 3). MANOVA for Corethrella appendiculata effects on survival of Aedes albopictus and Ochlerotatus triseriatus tested together for each instar (either both species 2nd or both species 3rd). Species survival variables contributing strongly to significant effects are shown in bold.
| Standardized canonical coefficients
|
||||||
|---|---|---|---|---|---|---|
| Variables | Num. d.f. | Den. D.f. | Pillai’s trace | P | A. albopictus | O. triseriatus |
| Instar | 2 | 34 | 0.753 | < 0.0001 | 1.757 | 1.698 |
| Treatment | 2 | 34 | 0.870 | < 0.0001 | 2.446 | 1.030 |
| Instar–Treatment | 2 | 34 | 0.604 | < 0.0001 | 1.847 | 1.643 |
Fig. 7.

Experiment 4 (part 3). The effects of Corethrella appendiculata on the survival of Aedes albopictus and Ochlerotatus triseriatus prey. Survival of 2nd and 3rd instar for the instar and treatment effects on species tested together at the same instar.
Experiment 5. Behavioural differences between 2nd and 4th instar Ochlerotatus triseriatus in response to water-borne cues to predation by Corethrella appendiculata
Three PCs with eigen values > 1 were obtained, accounting for 88.9% of the variation in behaviour (Table 8). A high positive score on PC1 indicated that larvae spent more time browsing at the wall, and a negative score indicated more time spent resting at the surface. A high positive score on PC2 indicated more time spent filtering in the middle, as opposed to other behaviours. A high positive score on PC3 indicated greater time spent thrashing at the bottom, as opposed to more time spent at the surface (Table 8). There was a significant treatment effect but neither instar nor interaction effects were observed (Table 9). SCCs indicated that both PC1 and PC2 contributed substantially to the treatment effect. Both 2nd instar and 4th instar O. triseriatus altered their behaviour, increasing time spent resting at the surface, when in predation water (Fig. 8). Despite being invulnerable to predation by C. appendiculata, 4th instar O. triseriatus altered their behaviour in response to water-borne cues to predation in the same way as did highly vulnerable 2nd instars (Fig. 8).
Table 8.
Experiment 5. Rotated factor patterns testing for behavioural differences among 2nd vs. 4th instar of Ochlerotatus triseriatus. Values greater than 40 are in bold and represent strong factor loading contributions for each principal component (PC).
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Resting | −83 | −38 | −34 |
| Browsing | 96 | 1 | 22 |
| Thrashing | 1 | 12 | 85 |
| Filtering | 12 | 96 | −14 |
| Surface | −78 | −38 | −44 |
| Wall | 94 | −1 | −14 |
| Bottom | 28 | 0 | 80 |
| Middle | 10 | 90 | 35 |
| Interpretation | browsing, wall vs. resting, surface | filtering, middle vs. other | thrashing, bottom vs. surface |
Table 9.
Experiment 5. MANOVA table for the behavioural principal components (PCs) of Ochlerotatus triseriatus (2nd and 4th instar) in response to water-borne predation cues. PCs contributing strongly to significant effects are shown in bold.
| Standardized canonical coefficients
|
|||||||
|---|---|---|---|---|---|---|---|
| Variables | Num. d.f. | Den. d.f. | Pillai’s trace | P | PC1 | PC2 | PC3 |
| Instar | 3 | 65 | 0.019 | 0.7291 | |||
| Treatment | 3 | 65 | 0.361 | < 0.0001 | 0.906 | 0.821 | 0.110 |
| Instar–treatment | 3 | 65 | 0.114 | 0.8605 | |||
Fig. 8.
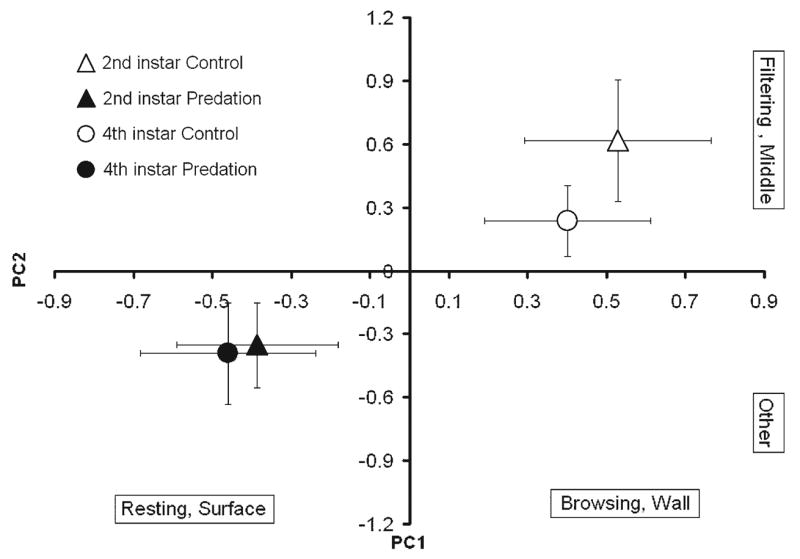
Experiment 5. Behaviour of 2nd and 4th instar Ochlerotatus triseriatus in response to water-borne Corethrella appendiculata predation cues. Plot between principal component 1 (PC1) and PC2.
Discussion
Mosquito larvae thrashing at the bottom of the containers were more vulnerable to predation from C. appendiculata than were larvae resting at the surface and in the middle of the containers. Because most prey were captured at the bottom of the containers (Fig. 1), C. appendiculata may predominantly hunt at the bottom. Thrashing involves vigorous movement of the mosquito larva and is the behaviour that involves maximum movement compared with other behaviours (Juliano & Reminger, 1992). Because prey that were thrashing were captured more often (Fig. 1) it seemed likely that C. appendiculata were using mechanoreceptors to detect their prey, as do predatory Toxorhynhites spp. (Steffan & Evenhuis, 1981). Results for A. albopictus behavioural (position and activity) vulnerability to C. appendiculata predation were similar to findings for O. triseriatus behavioural vulnerability to the ambush predator T. rutilus (Juliano & Reminger, 1992). Together with earlier studies (Juliano & Reminger, 1992), the results identify important behaviours contributing to the vulnerability of container-dwelling mosquito larvae to the two dominant predators in North-American container systems.
Second instars of O. triseriatus reduced their activity at the bottom of the container and rested more frequently at the surface in the presence of water-borne predation cues from C. appendiculata. In contrast, A. albopictus did not change their behaviour significantly in the presence of water-borne predation cues, and continued to thrash in the bottom of the containers (Fig. 4). Because resting at the surface reduced the risk of predation from C. appendiculata (Fig. 1), this response of O. triseriatus to water-borne predation cues constituted an anti-predatory behaviour relative to C. appendiculata. In the physical presence of the predator, both O. triseriatus and A. albopictus changed their behaviour and reduced their activity at the bottom of the containers. However, the species differed in their responses: O. triseriatus rested more often at the surface of the container than did A. albopictus (Fig. 2). Combinations of cues from predators and cues from the actual predation event have been shown to elicit a stronger anti-predatory response from the prey (Schoeppner & Relyea, 2005). In the experiments described here the behavioural responses of O. triseriatus and A. albopictus were tested separately to assess responses to water-borne cues from the act of predation, without the predator present, and to chemical and physical cues emanating from the predator itself in the absence of cues from the act of predation. Ochlerotatus triseriatus showed a stronger response to water-borne cues from predation than to cues from the presence of the predator (compare Figs 2 and 4). Studies have also shown that 4th instar O. triseriatus shows a stronger response to water-borne cues from T. rutilus (Juliano & Gravel, 2002; Kesavaraju & Juliano, 2004) than to the presence of the predator (Hechtel & Juliano, 1997). Although A. albopictus did not show significant behavioural changes in response to water-borne cues from predation, they did reduce movement at the bottom of the container when they were in the physical presence of the predator (Table 2; Fig. 2).
Second instars of both A. albopictus and O. triseriatus were more vulnerable to predation than were 3rd instars, which is a result consistent with general patterns for size-limited predators (Persson et al., 1996; Wellborn, 2002). Aedes albopictus 3rd instars were relatively more vulnerable to predation than were O. triseriatus 3rd instars, which were largely invulnerable. Ochlerotatus triseriatus 2nd instars suffered greater mortality when they were present with 3rd instar conspecifics than did 2nd instar A. albopictus (Fig. 6), suggesting that mortality of more-vulnerable stages may be partially dependent on the presence and vulnerability (or lack thereof) of other, less vulnerable stages. Greater predation on O. triseriatus 2nd instars may thus be an indirect result of the relatively low vulnerability of 3rd instar O. triseriatus. Species-specific differences in anti-predatory behaviour might account for this interspecific difference, but species-specific differences between A. albopictus and O. triseriatus in relative instar sizes might also have contributed to the greater vulnerability of A. albopictus 2nd instars to predation when 3rd instars were present. For 1st, 2nd, and 3rd instars, O. triseriatus were significantly larger than A. albopictus (head-capsule width, body length; B.W. Alto, unpublished data). Consistent with the observations of prey vulnerability in the behaviour experiments, O. triseriatus survived longer in the presence of C. appendiculata than did A. albopictus (Fig. 3).
Competitive interactions between prey species can be altered by differential prey responses to predators (Werner, 1991; Werner & Anholt, 1996; Relyea, 2000). Classic studies (Morin, 1981) and more recent work (Ciros-Perez et al., 2004) show that the coexistence of a competitively inferior species with a superior species is aided by selective predation on the competitively superior species. Competition studies on A. albopictus and O. triseriatus in the presence of C. appendiculata showed that A. albopictus survivorship is considerably diminished and that they were preferentially preyed upon by C appendiculata (Griswold & Lounibos, 2005), and this could influence the species composition of mosquito assemblages (Griswold & Lounibos, 2006). In the absence of predation, O. triseriatus competed poorly with A. albopictus (Teng & Apperson, 2000; Aliabadi & Juliano, 2002; Alto et al., 2005; Griswold & Lounibos, 2005). Thus, the data reported here suggest that differential anti-predatory responses by O. triseriatus and A. albopictus might be the main behavioural mechanism that produced the observed pattern of coexistence of these two competitors in the field (Lounibos et al., 2001). This container system provided the latest example of how behavioural properties of individuals can contribute to population and community patterns; in this case, probably via selective predation on the superior competitor promoting coexistence among competitors (Paine, 1966; Morin, 1981; Ciros-Perez et al., 2004). Thus, C. appendiculata might be playing the roles of both a barrier to invasion by A. albopictus and facilitating the coexistence with O. triseriatus in the systems in which they co-occur.
Third instar O. triseriatus attained a size refuge and were less vulnerable to predation than were 2nd instars. Fourth instar O. triseriatus are expected to be even less vulnerable to predation than 3rd instars. Because reduced prey activity in response to water-borne predation cues translated to reduced foraging activity, and is therefore costly (Relyea & Werner, 1999; Relyea, 2003), selection should favor discrimination between high- and low-risk predators. Putlitz et al. 1999 showed that larger tadpoles were both less vulnerable to salamander predation and exhibited more limited anti-predatory responses to those predators compared with smaller, more vulnerable tadpoles. Although 4th instar O. triseriatus were less vulnerable to predation by C. appendiculata, they modified their behaviour and increased the frequency of resting at the surface of containers, just as 2nd instars did in the presence of water-borne predation cues from C. appendiculata. In the south-eastern U.S.A., especially in Florida, O. triseriatus are most often are found with the predators T. rutilus or C. appendiculata, and these two predators are the most common predators in tree-hole systems. Toxorhynchites rutilus are voracious predators that prey on all life-cycle stages of O. triseriatus. As with C. appendiculata, prey that are active in the bottom of the container were more vulnerable to predation from T. rutilus (Juliano & Reminger, 1992). Fourth instar O. triseriatus reduced their activity at the bottom of the containers in response to water-borne predation cues from T. rutilus (Juliano & Reminger, 1992; Juliano & Gravel, 2002; Kesavaraju & Juliano, 2004). In a study on variation in the plasticity of tadpoles response to different predators, Richardson (2001) found that tadpoles reduced their activity in a general response to any predator, despite having no history of encountering some of the test predators in their native habitats. Richardson (2001) argued that tadpoles might use a general cue for all predators, and hence would not discriminate among threatening vs. non-threatening predators. O. triseriatus, likewise, could be responding to a general cue from the act of predation, regardless of whether that cue comes from C. appendiculata, T. rutilus, or indeed a predator it has not previously encountered. Thus, the observed response of 4th instar O. triseriatus to a small predator that presents little or no threat might be a response to a general cue from the act of predation, even though O. triseriatus were relatively invulnerable to C. appendiculata predation at that stage.
The results showed a striking parallel to those of Sih (1986), who found that larval Culex pipiens showed more precise and pronounced behavioural responses to water-borne cues to predation by Notonecta. He argued that this difference arose because of the greater evolutionary history of C. pipiens with that predator (Sih, 1986). Results of this research were similar because O. triseriatus has a history of co-occurrence with C. appendiculata, whereas non-native A. albopictus does not. An important new aspect of the work described here is that since the invasion of North America by A. albopictus, these three species now co-occur (Lounibos et al., 2001). As a result, these behavioural effects arising from responses to predation cues may have community level consequences, and the data indicated that behaviour of invasive and resident prey could be important determinants of the outcomes of biological invasions.
Acknowledgments
We thank R.L. Escher for help with providing us with C. appendiculata larvae and O. triseriatus eggs, N. Kinal and C.L. Villanueva for help with the experiment involving behavioural differences between O. triseriatus 2nd and 4th instars in response to predation cues, the Florida Department of Environmental Protection for permission to collect mosquitoes from Myakka River State Park and Highland Hammock State Park, and two anonymous referees for their helpful comments. This research was supported by the grants from the National Institute of Allergy and Infectious Disease (R01-AI-44793 and R15-AI-051374), National Science Foundation grant (DEB 0507015) and a Phi-Sigma Biological Honor Society, Beta Lambda chapter, grant to Banugopan Kesavaraju.
References
- Aliabadi BW, Juliano SA. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biological Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Griswold M, Lounibos LP. Habitat complexity and sex-dependent predation of mosquito larvae in containers. Oecologia. 2005;146:300–310. doi: 10.1007/s00442-005-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ. The interaction between predation and competition: a review and synthesis [Review] Ecology Letters. 2002;5:302–315. [Google Scholar]
- Ciros-Perez J, Carmona MJ, Lapesa S, Serra M. Predation as a factor mediating resource competition among rotifer sibling species. Limnology and Oceanography. 2004;49:40–50. [Google Scholar]
- Griswold M, Lounibos LP. Competitive outcomes of aquatic container Diptera depend on Predation and resource levels. Annals of the Entomological Society of America. 2005;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus. North America: Probable introduction in used tires from Northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hechtel LJ, Juliano SA. Effects of a Predator on Prey Metamorphosis: Plastic Responses by Prey or Selective Mortality? Ecology. 1997;78:838–851. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behaviour: an experiment with tree hole mosquitoes. Behavioural Ecology. 2002;13:301–311. [Google Scholar]
- Juliano SA, Philip Lounibos L. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecology Letters. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behaviour of larval tree-hole mosquitoes: Geographic and ontogenetic differences. Oikos. 1992;63:465–467. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioural responses to water-borne cues to predation in two container-dwelling mosquitoes. Annals of the Entomological Society of America. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SL, Dill LM. Behavioural decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology. 1990;68:619–640. [Google Scholar]
- Lind J, Cresswell W. Determining the fitness consequences of antipredation behaviour. Behavioural Ecology. 2005;16:945–956. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: Terrestrial Plants as Hosts for Aquatic Insect Communities. Plexus, Medford; New Jersey, U.S.A.: 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions infl uencing production of treehole mosquitoes in south Florida. Ecology of Mosquitoes. In: Lounibos LP, Rey JR, Frank JH, editors. Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA. 1985. pp. 65–77. [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger Mosquito Aedes albopictus. Florida, U.S.A. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Lundvall D, Svanback R, Persson L, Bystrom P. Size-dependent predation in piscivores: interactions between predator foraging and prey avoidance abilities. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:1285–1292. [Google Scholar]
- Morin PJ. Predatory salamanders reverse the outcome of competition among 3 species of anuran tadpoles. Science. 1981;212:1284–1286. doi: 10.1126/science.212.4500.1284. [DOI] [PubMed] [Google Scholar]
- Nilsson PA, Bronmark C. Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos. 2000;88:539–546. [Google Scholar]
- Paine RT. Food web complexity and species diversity. American Naturalist. 1966;100:65–75. [Google Scholar]
- Persson L, Andersson J, Wahlstrom E, Eklov P. Size–specific interactions in lake systems: Predator gape limitation and prey growth rate and mortality. Ecology. 1996;77:900–911. [Google Scholar]
- Putlitz MH, Chivers DP, Kiesecker JM, Blaustein AR. Threat-sensitive predator avoidance by larval pacific treefrogs (Amphibia, Hylidae) Ethology. 1999;105:449–456. [Google Scholar]
- Relyea RA. Trait-mediated indirect effects in larval anurans: Reversing competition with the threat of predation. Ecology. 2000;81:2278–2289. [Google Scholar]
- Relyea RA. Costs of phenotypic plasticity. American Naturalist. 2002;159:272–282. doi: 10.1086/338540. [DOI] [PubMed] [Google Scholar]
- Relyea RA. How prey respond to combined predators: a review and an empirical test. Ecology. 2003;84:1827–1839. [Google Scholar]
- Relyea RA, Werner EE. Quantifying the relation between predator-Induced behavior and growth performance in larval anurans. Ecology. 1999;80:2117–2124. [Google Scholar]
- Richardson JML. A comparative study of activity levels in larval anurans and response to the presence of different predators. Behavioural Ecology. 2001;12:51–58. [Google Scholar]
- SAS Institute Inc. SAS/STAT Users Guide, Version 6. 4. SAS Institute Inc; Cary, NC: 1990. [Google Scholar]
- Scheiner SM. MANOVA: Multiple response variables and multi-species interaction. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. Oxford University Press; 2001. pp. 99–115. [Google Scholar]
- Schoeppner NM, Relyea RA. Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defenses. Ecology Letters. 2005;8:505–512. doi: 10.1111/j.1461-0248.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- Sih A. Optimal behavior: can foragers balance two confl icting demands? Science. 1980;210:1041–1043. doi: 10.1126/science.210.4473.1041. [DOI] [PubMed] [Google Scholar]
- Sih A. Antipredator responses and the perception of danger by mosquito larvae. Ecology. 1986;67:434–441. [Google Scholar]
- Sih A. Prey Uncertainty and the Balancing of Antipredator and Feeding Needs. American Naturalist. 1992;139:1052–1069. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Annual Review of Entomology. 1981;26:159–181. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: Effects of density, food, and competition on response to temperature. Journal of Medical Entomology. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J. The costs of an inducible defense in anuran larvae. Ecology. 2000;81:2813–2821. [Google Scholar]
- Van Buskirk J. Phenotypic lability and the evolution of predator-induced plasticity in tadpoles. Evolution. 2002;56:361–370. doi: 10.1111/j.0014-3820.2002.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Yurewicz KL. Effects of predators on prey growth rate – relative contributions of thinning and reduced activity. Oikos. 1998;82:20–28. [Google Scholar]
- Wellborn GA. Trade-off between competitive ability and antipredator adaptation in a freshwater amphipod species complex. Ecology. 2002;83:129–136. [Google Scholar]
- Werner EE. Nonlethal effects of a predator on competitive interactions between two anuran larvae. Ecology. 1991;72:1709–1720. [Google Scholar]
- Werner EE, Anholt BR. Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology. 1996;77:157–169. [Google Scholar]
- Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. An experimental test of the effects of predation risk on habitat use in fish. Ecology. 1983;64:1540–1548. [Google Scholar]


