Abstract
Prolonged exposure to fluid shear stress alters leukocyte functions associated with the immune response. We examined the initial response of freshly isolated human leukocytes to fluid shear stress under high magnification. Adherent leukocytes exhibit a rapid biomechanical response to physiological levels of fluid shear stress. After passive displacement in the direction of a constant fluid shear stress, adherent leukocytes actively recoil back in the opposite direction of the fluid flow. Recoil is observed within seconds of the applied fluid shear stress. Simultaneously, fluid shear stress induces a stiffening of the cell. The immediate cell displacement in response to a step increase in fluid shear stress is greatly attenuated in subsequent steps compared to the initial fluid shear stress step. Recoil is not mediated by actin polymerization-dependent mechanisms, as cytochalasin D had no effect on this early response. However, stiffening was determined in part by an intact actin cytoskeleton. Inhibiting myosin force generation with ML-7 abolished the recoil and stiffening responses, implicating force generation by myosin as an important contributor to the early leukocyte response to fluid shear stress. This initial shear stress response may be particularly important in facilitating leukocyte attachment under sustained fluid shear stress by the flowing blood in the microcirculation.
Introduction
Circulating leukocytes captured by the endothelium experience in seconds an order of magnitude increase in fluid shear stress (FSS) on their surface (1,2). At this instant the leukocytes not only are passively deformed (3) but also exhibit the first indication of a transition from a passive to an active state: surface projections emerge, cytoplasmic processes appear, and the cells start active migration (4).
A variety of active processes on leukocytes are regulated by FSS. An increase in FSS on leukocytes in vitro increases adhesion (5–7), enhances motility (8,9), directs migration (9,10), enhances transmigration of activated lymphocytes (9,11–14), and alters phagocytosis (15,16) compared to cells in a static environment. The FSS response of human leukocytes, therefore, is an important regulator of the immune response.
Here we report that freshly isolated and adherent leukocytes under the influence of FSS recoil into the direction of fluid flow and increase cytoplasmic stiffness. The recoil response occurs early after onset of FSS and requires active force generation by myosin motors. Cytoplasmic stiffening also requires active force generation by myosin motors but not an intact actin cytoskeleton.
Methods
Reagents
All salts (NaCl, KCl, MgCl, and CaCl2) were purchased from Fisher Scientific (Fisher Scientific, Fair Lawn, NJ); glucose was purchased from Analytical Reagent (Mallinckrodt, Paris, Kentucky). HEPES, cytochalasin D, and ML-7 were purchased from Sigma-Aldrich (St. Louis, MO). Water was filtered by the Milli-Q purifying system (Model Biocel A10; Millipore, Billerica, MA) for all experiments. Buffers and solutions were filtered (0.2 μm; Nalgene filtration system; Nalgene, Rochester, NY) before use.
Cell isolation
Human leukocytes were gently isolated from whole blood by 1g sedimentation in microhematocrit capillary tubes (Fisher Scientific) for 30 min. Neutrophil isolation media, erythrocyte lysis buffers, and cell centrifugation were avoided to prevent interference with the FSS response. The neutrophil-containing band was extracted and resuspended in 1 ml of a HEPES buffer solution containing 30 mM HEPES, pH 7.4; 110 mM NaCl; 10 mM KCl; 1 mM MgCl; 10 mM glucose; and 1.5 mM CaCl2.
Cell observation
Isolated leukocytes were plated on clean, uncoated glass coverslips (Fisher Scientific) in HEPES buffer and observed under brightfield illumination on the stage of an inverted microscope. Two microscopy systems were used to observe the cell response to FSS. The first system featured a microscope (Leitz Diavert, Midland, Ontario) with a 50× objective (numerical aperture = 1.00) and 25× projection eyepiece, and cell images were captured by a couple charge device (CCD) camera (Javelin Electronics, Los Angeles, CA) and recorded to compact disk (Terapin, TeraOptix, Singapore). The second system used a microscope (Olympus IX70, Melville, NY) with 100× objective and 2× auxiliary magnification, and cell images were captured using a CCD camera (Olympus U-CMAD3) and recorded to videotape using a video cassette recorder (Panasonic AG 1320, Secaucus, NJ).
Applying fluid shear stress
Individual adherent leukocytes that exhibited no signs of motility were subjected to FSS from an adjacent pipette (Fig. 1). Pipettes were fabricated from clean borosilicate glass (FHC Frederick Haer, Bowdoinham, ME) using a micropipette puller (David Kopf Instruments, Tujunga, CA) and microforge (Narishige, Tokyo, Japan), filled with HEPES buffer, and mounted on a micromanipulator (Narishige). A precise hydrostatic pressure waveform was delivered to the pipette by a piston pump (Ling Dynamic Systems, Herts, UK) controlled by a waveform generator or a pair of hydrostatic pressure reservoirs. In both cases, hydrostatic pressure immediately produced flow out of the pipette that exerted a maximum shear stress of τMAX = 1, 2, or 4 dyn/cm2 on the cell surface. The leukocyte FSS response was investigated by turning on and off the FSS in a series of steps while observing the cell response. The duration of FSS steps was 1 min. The time between successive steps was 1–3 min. After applying a series of FSS steps, the cell was observed for 1 min in the absence of FSS.
Figure 1.
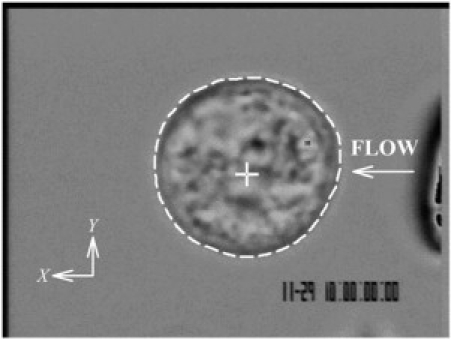
Digital micrograph of a freshly isolated human leukocyte as used in the current experiments. The cell outline is indicated by a white dashed line, and the computed cell centroid is shown as a white cross. The tip of an adjacent pipette used to apply FSS is just visible to the right of the image with the direction of fluid discharge indicated by the arrow.
The flow field around the cell is complex and has not been rigorously analyzed with realistic cell shapes and shear fields originating from an adjacent pipette. Nevertheless, modeling the fluid discharge from a pipette around cells of various geometries showed that FSS will be at a maximum on the cell surface proximal to the pipette and will smoothly diminish toward the opposite side of the cell (17). Parallel plate flow around an adherent cell with hemispherical geometry revealed that highest shear stresses are found apically and changed little for cell geometries, including projections (18). We present results in terms of maximum shear stress on the cell surface, as it may be less sensitive to the simplifying assumptions made in the modeling studies.
Geometric analysis of the cell response
The cell response to FSS was characterized in terms of displacement in cell position, measured by means of the geometric cell centroid and cell shape, and tracked by means of the projected area of the cell. Initially, images of individual cells captured every ∼5 s were outlined manually using image software (Scion Image, Scion, Frederick, MD), as described previously (19). The coordinates of the cell's geometric centroid, xC and yC, were computed from the digitized cell images and normalized by the initial cell diameter, D0, i.e., the average diameter measured during the initial observation without FSS, XC = xC/D0 and YC = yC/D0. To improve the rate of image analysis, cell centroids were computed (Image Pro Plus, Media Cybernetics, Silver Spring, MD). Pseudopod projection was tracked by means of the area, a, enclosed by the cell outline and normalized by the initial cell area, A0, measured during the initial observation without FSS. The normalized area is then A = a/A0.
Cytoskeletal involvement in the fluid shear stress response
To investigate the mechanism underlying the leukocyte response to FSS, the formation of actin filament was blocked in selected cells by incubation with cytochalasin D (1 mM) for at least 10 min before application of FSS. At 1 mM, cytochalasin D reduces the apparent cortical tension of neutrophils by 43% (20) and decreases the apparent cell stiffness by ∼30% (21). Cytochalasin blocks the binding of G-actin to the existing F-actin (22). To investigate the role of myosin force generation in the early FSS response, myosin light chain kinase activity was inhibited in selected cells with ML-7 at 50 μM for at least 10 min before application of FSS. At 50 μM, ML-7 inhibits leukocyte phagocytosis by ∼100% (23) and decreases neutrophil motility ∼100% (24). After treatment with cytochalasin D or ML-7, the cells were exposed to FSS as described.
Statistical analysis
Measurements are presented as mean ± SD. Differences between mean values were evaluated by Student's t-test.
Results
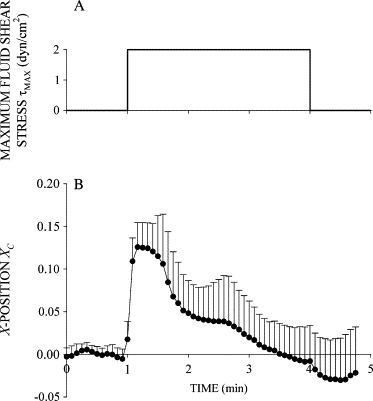
Before application of FSS, XC, YC, and A were constant, indicating that the cell was stationary and quiescent (Fig. 2). A step increase in fluid discharge from the adjacent pipette exposed the cell surface to a step increase in FSS with a peak value of ∼2 dyn/cm2 (Fig. 2 A). FSS immediately increased XC as the adherent cell was passively pushed away in the direction of the fluid flow (Fig. 2 B). The initial cell centroid displacement was proportional to the magnitude of the applied FSS (Fig. 3). In contrast, YC changed little after application of FSS, indicating that the cell moved primarily in the direction of the flow out of the pipette (Fig. 2 C). The normalized cell area A remained close to unity during FSS, indicating that the circular shape of the cell cross section in the focal plane (Fig. 1) was not significantly deformed by the relatively small FSS (Fig. 2 D).
Figure 2.
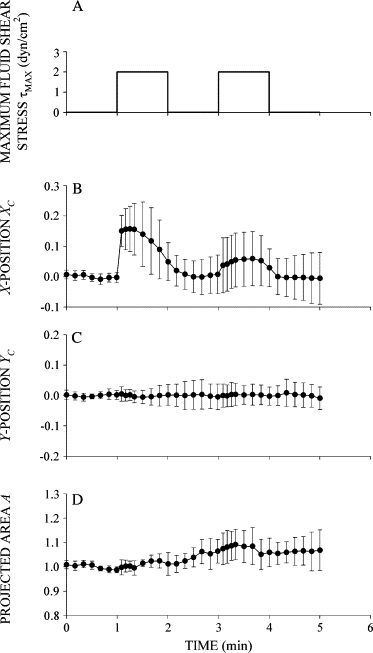
Response of human leukocytes to FSS. (A) Time course of FSS used to examine the FSS response of leukocytes. Cells were exposed to two step increases of FSS with a peak value τMAX = 2 dyn/cm2 and duration of 1 min separated by 1 min. (B) Normalized X-position of the cell centroid XC shown as a function of time during exposure to FSS. The cell moved primarily in the X-direction, showing an immediate displacement in response to FSS followed by recoil back toward its initial position despite the presence of sustained FSS. (C) Normalized Y-position of the cell centroid shown as a function of time during FSS exposure. (D) Normalized projected cell area shown as a function of time during FSS exposure. Data are the mean and standard deviation of 11 observations.
Figure 3.
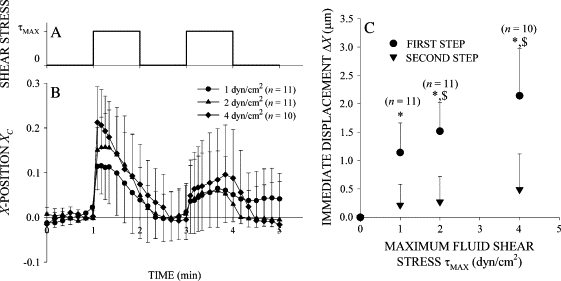
Stress amplitude dependence of the leukocyte response to fluid shear. (A) Time course of FSS exposure. (B) Leukocyte recoil in a fluid shear field. Normalized X-position of the cell centroid of freshly isolated leukocytes exposed to FSS with peak values on the cell surface of τMAX = 1, 2, and 4 dyn/cm2. (C) Leukocyte stiffening response. The immediate displacement in response to a step increase in FSS increased with τMAX but decreased with subsequent shear stress steps. (*) indicates a statistically significant difference (p < 0.05) in immediate displacement between the first and second steps. ($) indicates a statistically significant difference (p < 0.05) in immediate displacement compared to τMAX = 1 dyn/cm2. The number of observations, n, is given in the legend.
After the immediate displacement, XC decreased toward zero as the cell moved backward to its initial position (Figs. 2 B and 3 B). Importantly, leukocyte recoil into the fluid flow persisted while FSS was maintained. Again, YC remained close to zero, indicating that the cell centroid displacement was primarily against the fluid flow direction (Fig. 2 C). The normalized cell area remained constant at A = 1 during the application of FSS, indicating that the circular shape of the cell cross section remained not significantly changed during recoil (Fig. 2 D). The displacement of the cell centroid position during active recoil, ΔXR, during the first shear step increased almost linearly with τMAX (Fig. 4), indicating that an increasing FSS elicits a stronger active recoil response. Recoil during the second shear step, ΔXR, was constant and reduced by more than half compared to the first.
Figure 4.
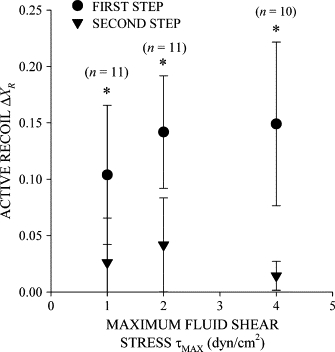
The extent of active recoil in sustained fluid shear. The active recoil ΔXC in the X-position of the cell centroid increased with the maximum FSS on the cell surface in the first shear step. The magnitude of active recoil decreased in subsequent fluid shear steps. (*) indicates statistical significance (p < 0.05) in active recoil between the first and second steps. The number of observations, n, at each maximum shear stress is given above the symbol.
After application of FSS for 1 min, the cell remained displaced relative to its initial position (Figs. 2 B and 3 B), indicating that active recoil during shear was incomplete. About 15 s after reduction of the FSS to zero, XC steadily decreased and remained close to zero for the next 45 s. To determine if complete recoil was possible, the duration of the FSS step was extended to 3 min. The recoil response brought the cell back to its initial position after ∼2.5 min of FSS (Fig. 5).
Figure 5.
(A) Time course of FSS application for a single step increase in fluid discharge from the pipette of 3 min duration. (B) Normalized X-position of the cell centroid XC shown as a function of time during 3 min exposure to FSS. With longer FSS application, cells eventually recoil back to their initial position and even move toward the pipette. Data are the mean and standard deviation of 10 observations.
The immediate cell displacement in response to a second step increase in FSS was reduced by more than half compared to the displacement after the first step (Fig. 3 B). Using a series of FSS steps of 1 min duration in 2-min intervals showed that the initial centroid displacement decreased with each successive step (Fig. 6), indicating that each FSS application caused stiffening of the cell attachment. Evidence of cell activation with pseudopod projection emerged between the first and second FSS steps (1 < t < 2) as the normalized area A/A0 increased significantly above the values measured before FSS application (Fig. 2 D).
Figure 6.
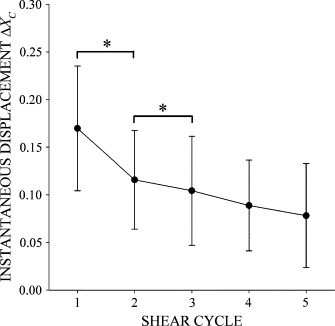
The instantaneous displacement of the cell centroid, ΔXC, in response to a step increase in FSS with a peak value of τMAX = 2 dyn/cm2. (*) indicates statistical significance (p < 0.05). Data are mean and standard deviation of 17 observations.
The onset of the recoil response in the second FSS step was delayed (Fig. 3 B). After the initial displacement, XC slowly increased for 50–55 s before decreasing toward zero (Fig. 3 B). In addition to the delayed onset of the recoil response in the second FSS step, the magnitude of the recoil response was attenuated relative to the first shear step. The change in centroid position during recoil in the second step was reduced compared to that of the first (Figs. 1 B and 2 A).
To determine if force generation by myosin within the cortical actin network underlies the recoil and stiffening responses, cells were subjected to FSS whose actin filament network and myosin activity were selectively inhibited. The initial recoil response to FSS was unaltered in cells whose actin cytoskeleton was disrupted by cytochalasin D compared to control cells (Fig. 7 A). However, cell stiffening required an intact actin network since the initial displacement in response to a second shear step was the same as the first in cytochalasin D treated cells. Inhibiting myosin force generation with ML-7 completely blocked the recoil and stiffening responses to FSS (Fig. 7 B). After blockade of myosin light chain kinase activity, the cells were passively displaced like an elastic body: XC increased immediately with the onset of shear stress, remained constant during shear, and instantly returned to zero upon cessation of FSS.
Figure 7.
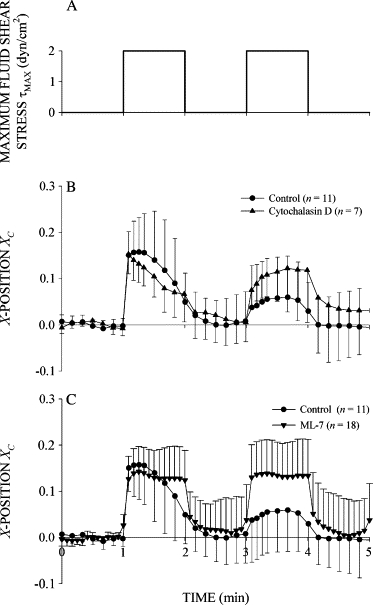
Cytoskeletal protein involvement in the active recoil and stiffening responses. (A) Time course of shear stress. (B) Normalized X-position of control cells and cells pretreated with the actin filament disrupting drug cytochalasin D at 1 mM. (C) Normalized X-position of control cells and cells pretreated with the myosin light chain kinase inhibitor ML-7 at 50 μM. The number of observations, n, is given in the legend.
Discussion
The results here indicate that freshly isolated human leukocytes respond to FSS by active recoil against the shear flow and increasing cytoplasmic stiffness. These active responses occur rapidly, within seconds, and precede typical indicators of leukocyte activity on the endothelium, including pseudopod projection, shape change, and cell spreading.
Previous studies of the FSS response in leukocytes focused on longer responses that emerge after minutes (8–10,12–14,17,19) and hours (15,16,25) of FSS exposure. Although experimental procedures vary from study to study, common behaviors are emerging. Slower FSS responses enhance processes associated with the immune response: leukocyte adhesion (5–7), motility (8–10), phagocytosis (15), and transmigration (9,11–14) are enhanced by FSS. Recoil and stiffening may not have been observed previously because cells were typically observed at low magnification (where small changes in cell position could not be resolved) or after several minutes (when the early FSS response was completed). Our studies that focused on changes in cell shape, not position, of freshly isolated, passive human leukocytes (19) lead to the current experimental approach to examine the FSS response in more detail at high magnification and during early time periods.
The immediate cell displacement, ΔXC, due to FSS appears to be a passive response determined by extension of microvilli and deformation of cytoplasm. It increased in direct proportion to the maximum shear stress on the cell surface, suggesting an elastic mechanism of internal energy storage. In the initial spherical configuration, the cell has excess membrane area stored as microvilli. Adhesive interactions are likely mediated through L- and P-selectin concentrated at microvilli tips. The fluid stresses generated by fluid discharge from the pipette pulls out the excess membrane area and stretches the microvilli. Pulling slack from microvilli requires no significant force, whereas stretching microvilli requires force (26). Microvilli stretching forces balance the resulting shear forces and torques generated by the fluid flow. Pulling and stretching microvilli account for ∼0.3 and 0.75 μm, respectively, of the immediate cell displacement in response to FSS (26), which is close to the measured values (Fig. 3 C). Cytoplasmic deformation contributes an additional small displacement of the cell centroid. Integrins have been implicated in mediating adhesion of leukocytes to uncoated glass substrates (27). This opens the possibility that a portion of the cell recoil is due to cytoskeletal strengthening of sites of integrin attachment (28). A better understanding of the manner by which the cells adhere to the glass substrate would provide some insight into the roles of these mechanisms in the early FSS response. Microvilli and integrin receptors are supported intracellularly by actin filaments. Yet, a central role for actin filaments or myosin molecules in resisting this immediate displacement is not supported by our current evidence since pretreatment of leukocytes with cytochalasin D or ML-7 had little effect compared to untreated cells (Fig. 7).
The subsequent recoil and stiffening under FSS cannot be explained by passive physical properties of leukocytes. Irrespective of whether leukocytes are regarded as a linear viscoelastic (29) or fluid-like material with cortical tension (30–32), XC would remain constant or increase in sustained FSS. But in fact, XC decreased within 5 s of the onset of FSS. Moreover, the response to a second step increase in FSS would be virtually identical to the first. The mechanism underlying these active responses remains elusive. Both active recoil and stiffening required myosin activation and possible growth of F-actin fibers. Membrane stretch may lead to an influx of calcium ions or force-dependent kinase activation at focal adhesion sites, which may initiate signaling cascades that activate myosin force generation. Without the well-developed cytoskeleton typical of adherent cells, myosin force generation within leukocytes is likely to be limited to the cell periphery, perhaps exerting force directly on adhesion receptors rather than on actin. Surprisingly, an intact actin cytoskeleton is not required for the recoil response. However, intact actin filaments are required for the stiffening response. This suggests that the active recoil response is independent of the actin polymerization that accompanies leukocyte activation and likely underlies the pseudopod projection, cell spreading, and cell motility associated with leukocyte activation.
The observations in this work complement our previous results showing that freshly isolated passive leukocytes exposed to FSS exhibit pseudopod projection and cell spreading (19). Of particular interest is the observation that FSS exposure as brief as 1 min is sufficient to evoke the slower shear stress responses (i.e., cell spreading and pseudopod projection), but spreading and projecting are not immediately observed after FSS exposure. Rather, spreading and projecting emerge after a quiescent period of several minutes. It is during the shear stress exposure and quiescent period that recoil and stiffening occur. Our notion is that the recoil and stiffening are the initial steps in the leukocyte FSS response that prepare the cell to perform functions necessary for the immune response under sustained FSS of the flowing blood. In the microcirculation, the time between the sudden increase in FSS associated with leukocytes in free suspension and capture on the endothelium and a reduction in FSS associated with spreading and extravasation may be of the order of minutes (4). FSS responses that occur rapidly might play a key role in strengthening the membrane attachment during the initial capture, forming firm adhesion and thereby facilitating leukocyte spreading and eventual extravasation across the endothelium.
Acknowledgments
The authors thank Frank A. DeLano for excellent technical assistance.
This work was supported by U.S. Public Health Service Program project grant HL43026.
Footnotes
Editor: David W. Piston.
References
- 1.Gaver D.P., Kute S.M. A theoretical model study of the influence of fluid stresses on a cell adhering to a microchannel wall. Biophys. J. 1998;75:721–733. doi: 10.1016/S0006-3495(98)77562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugihara-Seki M., Schmid-Schönbein G.W. The fluid shear stress distribution on the membrane of leukocytes in the microcirculation. J. Biomech. Eng. 2003;125:628–638. doi: 10.1115/1.1611515. [DOI] [PubMed] [Google Scholar]
- 3.Shen Z., Lipowsky H.H. Image enhancement of the in vivo leukocyte-endothelium contact zone using optical sectioning microscopy. Ann. Biomed. Eng. 1997;25:521–535. doi: 10.1007/BF02684192. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi K.L., Tung D.K.-L., Wilson J., Zweifach B.W., Schmid-Schönbein G.W. Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation. 1996;3:199–210. doi: 10.3109/10739689609148289. [DOI] [PubMed] [Google Scholar]
- 5.McIntire L.V., Dewitz T.S., Martin R.R. Mechanical trauma effects on leukocytes. Trans. Am. Soc. Artif. Intern. Organs. 1976;22:444–449. [PubMed] [Google Scholar]
- 6.Dewitz T.S., Hung T.C., Martin R.R., McIntire L.V. Mechanical trauma in leukocytes. J. Lab. Clin. Med. 1977;90:728–736. [PubMed] [Google Scholar]
- 7.Okuyama M., Kambayashi J.-i., Sakon M., Monden M. LFA-1/ICAM-3 mediates neutrophil homotypic aggregation under fluid shear stress. J. Cell. Biochem. 1996;60:550–559. doi: 10.1002/(SICI)1097-4644(19960315)60:4%3C550::AID-JCB11%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Tomczok J., Sliwa-Tomczok W., Klein C.L., van Kooten T.G., Kirkpatrick C.J. Biomaterial-induced alterations of human neutrophils under fluid shear stress: scanning electron microscopical study in vitro. Biomaterials. 1996;17:1359–1367. doi: 10.1016/0142-9612(96)87275-9. [DOI] [PubMed] [Google Scholar]
- 9.Kitayama J., Hidemura A., Saito H., Nagawa H. Shear stress affects migration behavior of polymorphonuclear cells arrested on endothelium. Cell. Immunol. 2000;203:39–46. doi: 10.1006/cimm.2000.1671. [DOI] [PubMed] [Google Scholar]
- 10.Rainger G.E., Buckley C.D., Simmons D.L., Nash G.B. Neutrophils sense flow-generated stress and direct their migration through aVb3-integrin. Am. J. Physiol. 1999;276:H858–H864. doi: 10.1152/ajpheart.1999.276.3.H858. [DOI] [PubMed] [Google Scholar]
- 11.Cinamon G., Grabovsky V., Winter E., Franitza S., Feigelson S., Shamri R., Dwir O., Alon R. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J. Leukoc. Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- 12.Cinamon G., Shinder V., Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 13.Cuvelier S.L., Patel K.D. Shear-dependent eosinophil transmigration on interlukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3. J. Exp. Med. 2001;194:1699–1709. doi: 10.1084/jem.194.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber K.S.C., von Hundelshausen P., Clark-Lewis I., Weber P.C., Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 1999;29:700–712. doi: 10.1002/(SICI)1521-4141(199902)29:02<700::AID-IMMU700>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Rosenson-Schloss R.S., Vitolo J.L., Moghe P.V. Flow-mediated cell stress induction in adherent leukocytes is accompanied by modulation of morphology and phagocytic function. Med. Biol. Eng. Comput. 1999;37:257–263. doi: 10.1007/BF02513296. [DOI] [PubMed] [Google Scholar]
- 16.Shive M.S., Salloum M.L., Anderson J.M. Shear stress-induced apoptosis of adherent neutrophils: a mechanism for persistence of cardiovascular device infections. Proc. Natl. Acad. Sci. USA. 2000;97:6710–6715. doi: 10.1073/pnas.110463197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moazzam F., DeLano F.A., Zweifach B.W., Schmid-Schönbein G.W. The leukocyte response to shear stress. Proc. Natl. Acad. Sci. USA. 1997;94:5338–5343. doi: 10.1073/pnas.94.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su, S. S. 2007. Fluid stress on the surface of a migrating leukocyte in a flow field and the involvement of formyl peptide receptor in its mechanotransduction. PhD thesis. University of California, San Diego, La Jolla, CA.
- 19.Coughlin M.F., Schmid-Schönbein G.W. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys. J. 2004;87:2035–2042. doi: 10.1529/biophysj.104.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting-Beal H.P., Lee A.S., Hochmuth R.M. Effect of cytochalasin D on the passive mechanical properties and morphology of passive human neutrophils. Ann. Biomed. Eng. 1995;23:666–671. doi: 10.1007/BF02584463. [DOI] [PubMed] [Google Scholar]
- 21.Worthen G.S., Schwab B., Elson E.L., Downey G.P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 22.Goddette D.W., Frieden C. Actin polymerization. The mechanism of action of cytochalasin D. J. Biol. Chem. 1986;261:15974–15980. [PubMed] [Google Scholar]
- 23.Mansfield P.J., Shayman J.A., Boxer L.A. Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood. 2000;95:2407–2412. [PubMed] [Google Scholar]
- 24.Eddy R.J., Pierini L.M., Matsumura F., Maxfield F.R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 2000;113:1287–1298. doi: 10.1242/jcs.113.7.1287. [DOI] [PubMed] [Google Scholar]
- 25.Shive M.S., Hasan S.M., Anderson J.M. Shear stress effects on bacterial adhesion, leukocyte adhesion, and leukocyte oxadative capacity on a polyetherurethane. J. Biomed. Mater. Res. 1999;46:511–519. doi: 10.1002/(sici)1097-4636(19990915)46:4<511::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Shao J.-Y., Ting-Beall H.P., Hochmuth R.M. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marschel P., Schmid-Schönbein G.W. Control of fluid shear response in circulating leukocytes by integrins. Ann. Biomed. Eng. 2002;30:333–343. doi: 10.1114/1.1475342. [DOI] [PubMed] [Google Scholar]
- 28.Choquet D., Felsenfeld D.P., Sheetz M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 29.Schmid-Schönbein G.W., Sung K.-L.P., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys. J. 1981;36:243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984;64:1028–1035. [PubMed] [Google Scholar]
- 31.Dong C., Skalak R., Sung K.-L.P., Schmid-Schönbein G.W., Chen S. Passive deformation analysis of human leukocytes. J. Biomech. Eng. 1988;110:27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- 32.Drury J.L., Dembo M. Hydrodynamics of micropipette aspiration. Biophys. J. 1999;76:110–128. doi: 10.1016/S0006-3495(99)77183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


