Abstract
Carbon fiber amperometry is a popular method for measuring single exocytotic events; however, the functional interpretation of the data can prove hazardous. For example, changes to vesicle transmitter levels can appear to cause changes in the timing and rate of the fusion process itself. Use of an analytical technique based on differentiation revealed that an increase in dense-core granule catecholamine content by exogenous application of l-DOPA did not affect initial release rates. Changes to the timing and amplitude of amperometric spikes from l-DOPA-treated cells are, then, likely a reflection of the increased quantal size rather than any direct effect on exocytosis itself. Applying this new analysis to individual fusion events from cells expressing Munc-18-1 with various specific point mutations demonstrated that Munc-18-1 functions at a late stage involved in the determination of the initial rate of fusion. Furthermore, a mutation of the protein that inhibits its biochemical interaction with the t-SNARE syntaxin-1 in a closed conformation caused premature termination of the fusion event. Through these two late-stage functions, Munc-18-1 could act as a key protein involved in the presynaptic control of signaling strength and duration.
Introduction
Regulated exocytosis has been proposed to occur via two functionally related pathways, kiss-and-run and full fusion (1). Both pathways begin with a secretory vesicle or dense-core granule that is delivered to the plasma membrane, undergoes docking and priming, and awaits the final signal to initiate membrane-membrane fusion. A local rise in intracellular calcium is thought to trigger the formation of a transient semistable structural connection between the membranes termed the fusion pore (2). A fusion pore may be a preliminary component for both kiss-and-run and full fusion; however, this has not yet been proven unequivocally. Very little information is known about fusion pores, including what they are made of (lipidic or proteinaceous) and what regulates flux through them (pore geometry, specific proteins) (3). Perhaps more importantly, it is unknown what induces them either to collapse entirely into the plasma membrane and release the complete content of the vesicle (as in full fusion) or to reverse and reseal, thereby permitting only a partial release of vesicle content (as in kiss-and-run exocytosis). Selection of exocytotic mode and/or a regulation in the extent of kiss-and-run may well be a key contributor underlying presynaptic plasticity (4), which itself is a cellular component of learning and memory in the nervous system. It is critical, therefore, to have techniques that monitor individual exocytotic events to investigate their regulation by intracellular mechanisms.
Electrochemical detection via carbon fiber amperometry is currently a popular method for monitoring exocytosis (5,6). It is generally agreed that the technique records single fusion events; however, the functional interpretation of the generated data remains complicated and, in some cases, quite controversial. Amperometry takes advantage of the oxidization properties of chemical transmitters stored in and secreted from some vesicles and granules. Principal work has utilized neuroendocrine model cell types for synaptic release such as adrenal chromaffin cells, PC12 cells, mast cells, and pancreatic β-cells (7–11); however, the technique has also been applied successfully to neuronal cultures (12,13). In amperometry, a recording carbon fiber electrode is placed near to or in direct contact with the secretory cell of interest. The fiber is then held at a constant potential optimal for the oxidation of the released transmitter, and the resulting electrochemical oxidation current is monitored over time. Individual fusion events are recorded as single spikes of current (14), where the integral of the current yields the total charge, proportional to the number of moles of material detected. Specific parameters of the amperometric spike can be altered by numerous variables, including stimulus frequency (15,16), transmitter concentration (17,18), pH (19), osmotic pressure (20), and a number of synaptic proteins (8,21,22). Early amperometric experiments also noted a small pedestal or “foot” preceding the amperometric spike proper (23); however, they only occur before a minority of full spikes (18). The foot has been argued to be the electrochemical signal resulting from the initial leakage of transmitter through the fusion pore. Specific parameters of the foot can also be modified by transmitter concentration (18) and a number of synaptic proteins (21,24,25). As amperometry indirectly measures the fusion event that preceded it, both spatially and temporally, the functional interpretation of alterations to foot/spike parameters remain controversial.
Sec-1/Munc-18-1 (SM) proteins are essential exocytotic vesicle trafficking proteins that have been primarily characterized functionally via a strong interaction with their cognate syntaxins (26,27). Quite a number of different, often conflicting, functions for the protein have been proposed; these include roles as a syntaxin chaperone, in vesicle docking, and in the process of membrane fusion itself. Although either over- or underexpression of wildtype Munc-18-1 has no reported effect on amperometric spike parameters (28,29), expression of the protein with specific point mutations can have dramatic effects (8,30). The functional meaning of these changes, however, is not completely agreed upon. This study, utilizing a new statistical analysis of amperometric spikes, aims to interpret Munc-18-1-induced alterations to spike parameters to a functional role for the protein during the fusion mechanism itself in controlling the initial rate of release.
Materials and methods
Cell Culture and Transfection
Freshly cultured bovine adrenal chromaffin cells were plated onto nontissue culture-treated 10 cm petri dishes and left overnight at 37°C. Nonattached cells were resuspended in growth medium at a density of 1 × 107 cells/ml. Plasmids encoding a protein of interest was mixed with an EGFP plasmid and added to the cell mixture at 20 μg/ml. Cells were then electroporated using a Bio-Rad Gene Pulser II (Bio-Rad, Hercules, CA), subsequently diluted to 1 × 106 cells/ml with fresh growth medium, and maintained in culture for 3–5 d.
Immunofluorescence
Chromaffin cells were transfected as described above and plated onto glass coverslips. After being washed with PBS twice, cells were fixed in 4% formaldehyde in PBS at room temperature for 30 min. Fixed cells were subsequently probed with either a polyclonal antibody against Munc-18-1 (Cambridge Biosciences, Cambridge, UK) at a 1:100 concentration or a monoclonal syntaxin antibody, HPC-1 clone (Sigma, St. Louis, MO) at a 1:50 dilution. Staining was visualized using a Leica laser scanning confocal microscope with 488-nm excitation and 500-550 nm light collection for EGFP and 543-nm excitation and 600- to 650-nm light collection for Texas Red. For quantification of fluorescence, the cell area was defined, and the Texas Red signal quantified using the Leica confocal software for transfected and nontransfected cells in the same microscope field. At least nine cells were imaged and quantified for each condition.
Amperometric Recording
Electrochemical recording conditions were as previously described (8,30). Briefly, cells were incubated in bath buffer (139 mM potassium glutamate, 0.2 mM EGTA, 20 mM PIPES, 2 mM ATP, and 2 mM MgCl2; pH 6.5), and a 5-μm diameter carbon fiber electrode was positioned in direct contact with a targeted adrenal chromaffin cell. Exocytosis from cells was stimulated with pressure ejection of a permeabilization/stimulation buffer (139 mM potassium glutamate, 20 mM PIPES, 5 mM EGTA, 2 mM ATP, 2 mM MgCl2, 20 μM digitonin, and 10 μM free calcium; pH 6.5) from a glass pipette oriented on the opposite side of the cell. Amperometric responses were monitored with a VA-10 amplifier (NPI Electronic, Tamm, Germany) and saved to computer by Axoscope 8 (Molecular Devices, Sunnyvale, CA). Individual experiments were conducted in parallel on control (untransfected) and transfected cells from the same cell batch in the same cell culture dishes. Transfection was identified by expression of EGFP, as previous studies from our laboratory have established a 95% rate of coexpression (8,31). For l-DOPA experiments, chromaffin cells were incubated in 100 μM l-DOPA at room temperature for 50 min before recordings (17,22).
Amperometric Analysis
Amperometric data were exported from Axoscope and subsequently analyzed using Origin (Microcal Software, Northampton, MA). Spikes were selected for analysis, provided that the spike amplitude was >40 pA and maximal spike rate of rise was >30,000 pA/s to remove any confounding effects of inclusion of those fusion events not occurring directly underneath the carbon fiber end (32). Differentiation was done in Origin by calculating the slopes between two adjacent data points. All of the data are shown as mean ± SE. Significance was tested with nonparametric Mann-Whitney U-tests. Spike parameters were compared by Pearson product moment correlation in SigmaStat.
Results
An investigation of amperometric spikes and their interpretation for the preceding fusion event require a brief description of what the technique actually measures (Fig. 1). Analyses typically measure four distinct, yet interrelated, parameters of single current spikes as the exocytosed catecholamine is oxidized by the carbon fiber adjacent to the cell. Integration of the amperometric oxidation spike (ʃC) measures the total charge (in picocoulombs), from which the number of catecholamine molecules oxidized by the carbon fiber can be calculated. Because single amperometric spikes are equivalent to release events from single granules, the total charge thus represents quantal size. The peak height of the current spike (Ip) measures the maximal rate of catecholamine oxidation (in picoamperes), which may be proportional to the flux of catecholamine from the release site. Finally, the rise time of the spike from onset to peak (RT; in milliseconds) and the fall time from peak back to baseline (FT; in milliseconds) may be related to the timing of the release event itself. An important consideration for interpretation is that both peak current and spike timing will be strongly affected by the concentration gradient of the exocytosed material and by distances between the carbon fiber and the fused vesicle. Concentration gradients, however, are not static over the time course of each event, as the neurotransmitter spreads out from the exocytotic point of origin and as the carbon fiber oxidizes it. This point is illustrated by the relatively longer spike FT in comparison to RT.
Figure 1.

Carbon fiber amperometry measures single release events. (A) Electrochemical detection of exocytosis begins with the placement of a small diameter (∼5–10 μm) carbon fiber electrode adjacent to a cell that is then stimulated to initiate exocytosis. The electrode is kept at a constant potential, optimal for the oxidation of the cell's vesicular transmitter content. In adrenal chromaffin cells, the transmitters released are catecholamines (both epinephrine and norepinephrine). (B) On stimulation of the cell (indicated by the arrow), fusion events release their vesicular contents. When the transmitters encounter the carbon fiber, they are oxidized, creating upward deflections in current. (C) Individual current deflections (spikes) reflect individual fusion events. Amperometric spikes have a common shape but can come either with or without a small preceeding current deflection termed a foot. (D) Amperometric spikes can be analyzed by comparing a variety of parameters, such as the integrated charge (ʃC), the current peak (Ip), the risetime of the spike (RT), and the falltime (FT).
Changing concentration gradients experimentally by manipulating quantal size can have dramatic effects on the size and shape of the amperometric spike (17,18) without necessarily affecting the fusion process itself. l-DOPA is a precursor to epinephrine and norepinephrine and leads to an overaccumulation of total oxidizable catecholamines in dense-core granules in chromaffin cells. In cells treated with 100 μM L-DOPA for 15–20 min, individual amperometric spikes have an increased charge (indicating an increased quantal size), an increased spike height, and a prolongation in both RT and FT in comparison to untreated cells (Table 1 and Fig. 2). This phenotype is not surprising, considering the cellular action of l-DOPA. Increasing total granule content would, by definition, enhance quantal size. Additionally, all other parameters of the spikes were positively correlated with spike charge (p < 0.001); thus, peak height, RT, and FT would all then increase simply as a reflection of increased quantal size. Although increasing catecholamine content has been argued to affect the kinetics of fusion directly (18), caution must be exercised in interpreting the changes in amperometric parameters of spike height and timing back to the preceding fusion event itself. An attempt was therefore made to analyze amperometric spikes differently to look for information unaffected by concentration gradients and thereby gain more insight into the underlying mechanism for exocytosis.
Table 1.
The effect of overexpression of various Munc-18-1 mutant constructs or pharmacological agents on amperometric parameters of single fusion events from adrenal chromaffin cells
Figure 2.

Pharmacological agents or expression of mutated Munc-18-1 protein can affect quantal size. Application of l-DOPA, which acts to increase catecholamine content of adrenal chromaffin cell granules, increased the quantal size of individual fusion events. Expression of Munc-18-1 P242S had a similar effect on quantal size, whereas the PKC phosphomimetic mutation Munc-18-1 dGLU (S306E/S313E) caused a decrease in quantal size. A single glutamate mutation of Munc-18-1 (sGLU; S306E) had no effect on quantal size. Application of the PKC activator PMA duplicated the effect of overexpression of Munc-18-1 dGLU. Note that the percentage change in quantal size by sGLU is too small to be seen on the graph.
The initial release rate of catecholamine is one such exocytotic parameter unaffected by concentration gradients. An increase in the total granule content would not affect initial release because the concentration gradient, at that time point, would be infinitely large and thus unchanged by increases or decreases in catecholamine amounts. It would only be subsequent to the initial release that the altered catecholamine content would begin to exert an effect on the parameters of amperometric spikes. The initial release rate can be determined by a closer statistical examination of the amperometric spike (Fig. 3). Within the risetime of a spike, there is an initial, brief, sharp rise in the spike current that rapidly attains a maximal rising slope (Mmax); the time to reach Mmax comprises approximately the first 60–70% of the total spike RT (MRT; see examples, Figs. 1 and 3). The rate of rise of the spike subsequently slows before attaining the maximal spike current height. It was reasoned that the initial release rate of catecholamine (Ii) from the site of fusion would be represented on an amperometric spike at the point of maximal upward slope (Mmax). Instantaneous measurements of slope can be calculated over the time course of a single spike and subsequently compared by differentiating the amperometric recording (Fig. 3). Additional subtleties in the timing can then be extracted by separating the time from spike onset to the time of maximal slope and the time from the maximal slope to the peak height, literally subdividing the amperometric spike RT into two sections, one of which is unaffected by changes in quantal size.
Figure 3.

Differentiation of amperometric traces. (A) The entire amperometric recording (top) can be differentiated to produce a record of the rate of change in amperometric current (bottom). (B) The differentiated traces can provide a new analysis. The maximum slope of the rising amperometric spike (Mmax) can be calculated by the peaks of the differential trace. This point in time should represent the initial release rate of catecholamine from the site of fusion. The initial release rate (Ii) is then calculated from the amperometric current at the time of the differential maximum (MRT).
If the spike current at Mmax indeed represents the initial release rate that is unaffected by concentration gradients, this creates two testable predictions. The first is that because the distance to diffuse between cell and carbon fiber is, on average, the same: the time to Mmax will be constant in all experiments. The second prediction is that overloading the granules with catecholamine will not affect the spike current at Mmax or the slope itself. To test these predictions, the amperometric recordings from l-DOPA-treated cells, where the total amounts of catecholamine are increased per granule, were differentiated and analyzed. It was found that both predictions were indeed true (Fig. 4). Although the quantal size was increased, both the maximal slope and the oxidative current at Mmax were statistically unaffected by prior application of l-DOPA. Time to reach the maximal spike slope was also unaffected; only subsequent to this time point did the temporal parameters of the spike broaden. Indeed, the time until the slope of a spike reaches its maximum was on average 3 ms in these experiments (see examples, Figs. 1 and 3; but also compare to Figs. 4–7), and this timing has not been observed to differ under any condition yet examined (J. Barclay, unpublished observations). Therefore, the increase in the RT of amperometric spikes from l-DOPA-treated cells was completely caused by an increase in the time from the maximal slope of the spike to the peak height, despite this only contributing 30–40% of the total spike RT. After the spike's peak height was attained, the rest of the spike was lengthened as well in comparison to spikes from untreated control cells. Thus, in cells treated with l-DOPA, despite there being an increased total extent of release, the initial release rate was statistically unaffected.
Figure 4.

Increasing granule content by l-DOPA application does not change initial exocytotic release rates. (A) The maximal slope of the amperometric spikes did not differ between controls and cells exposed to l-DOPA despite l-DOPA increasing granule catecholamine content. (B) The current of the spike at the time of the maximal slope was also unaffected by application of l-DOPA. (C) The time to attain the maximal slope of amperometric spikes was unaffected by application of l-DOPA. (D) The time from maximal slope to the peak of the spike was significantly increased in cells exposed to l-DOPA in comparison to controls. (E) The time from the peak of the amperometric spike until return to baseline was increased in l-DOPA-treated cells.
Figure 5.

Expression of Munc-18-1 P242S increases initial exocytotic release rates. (A) The maximal slope of amperometric spikes from Munc-18-1 P242S-expressing cells was significantly increased in comparison to control cells. (B) The current of the spike at the time of maximal slope was also increased for cells expressing P242S. (C) The time to attain the maximal rate of rise was not affected for P242S; however, the time from the maximal slope to the spike peak (D) and the time from the spike peak to the end (E) were both increased in P242S-expressing cells.
Figure 6.
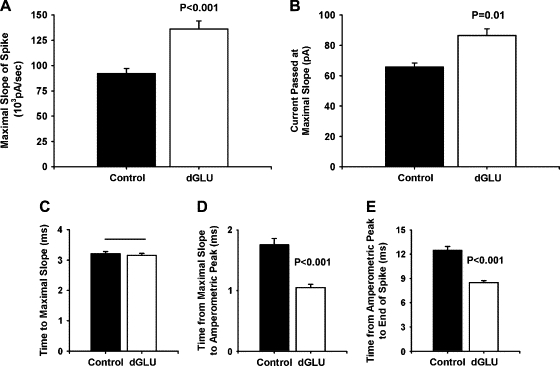
Expression of Munc-18-1 dGLU, a mutation that reduces quantal size, increases initial exocytotic release rates. (A) The maximal slope of amperometric spikes from Munc-18-1 dGLU (S306E/S313E)-expressing cells was increased in comparison to controls despite dGLU-expressing cells decreasing quantal size. (B) The current of the spike at the time of maximal slope was also increased for dGLU-expressing cells. (C) The time to reach the maximal slope was not affected by expression of dGLU. (D) Although initial release rates were increased, the time from the maximal slope to the spike peak was actually significantly decreased in dGLU cells in comparison to controls. (E) The timing of the remainder of the spike was also decreased by expression of the mutation.
Figure 7.

Application of the phorbol ester PMA restores the dGLU phenotype. (A) Expression of a Munc-18-1 construct (sGLU; S306E) that has no general effect on amperometric spikes or quantal size does not alter the maximal slope of spikes. Addition of the PKC agonist, PMA, duplicates the dGLU phenotype of increased maximal slope. (B) The spike current at maximal slope was also not affected by expression of sGLU, but PMA application restored the dGLU phenotype of an increased current. (C) The time to reach the maximal slope was not affected by either sGLU expression or PMA application. The times from maximal slope to peak (D) and peak to baseline (E) were unaffected by expression of Munc-18-1 sGLU. Application of PMA again restored the dGLU phenotype of a reduction in these times.
Overexpression of wild-type Munc-18-1 has no effect on amperometric spikes from chromaffin cells (28,29). We have previously examined the effects of overexpression of a number of Munc-18-1 mutant proteins on the measured parameters of amperometric spikes (Table 1). Because these mutations also affect quantal size (Fig. 2), this could potentially confound the functional interpretation of the effects of the protein. Therefore, the new differential analysis was applied to spikes from cells expressing the specific Munc-18-1 mutations to determine the protein's function during the fusion process. Two characterized mutations were of particular interest, Munc-18-1 P242S and dGLU (S306E/S313E).
P242S is a mutation originally isolated in Drosophila causing an increase in EJP amplitude at the larval neuromuscular junction (33). Biochemically, the mutation has no obvious effect on the binding of Munc-18-1 to syntaxin but appears to reduce binding to Mint (Munc-18-interacting) proteins by ∼50% (30). Overexpression in chromaffin cells causes increases in spike charge, height, RT, and FT (Table 1 and Fig. 2) identical to the addition of l-DOPA; yet, there is no evidence to support a role for Munc-18-1 in catecholamine synthesis or granule loading. Comparison of the differential traces shows that, unlike for l-DOPA-treated chromaffin cells, spikes from cells overexpressing the P242S mutation had an increased maximal slope of the amperometric spike (Fig. 5). The spike current released at the time of maximal slope was also increased in comparison to controls, but the time to the maximal slope was unchanged by expression of the mutation. It was only after this time that the spike duration broadened. The time from maximal slope to peak height was significantly increased in P242S cells, and this increase underlies the increase seen in the amperometric RT. The timing of the remainder of the spike was also increased in comparison to controls. Thus, in cells overexpressing Munc-18-1 P242S, there is a larger initial release rate of catecholamine, and that release subsequently lasts for a longer duration.
The mutation Munc-18-1 dGLU is a phosphomimetic double mutation (S306E/S313E) (8). Mutation of the two protein kinase C (PKC) phosphorylation serines of the Munc-18-1 protein to glutamate is thought to act electrostatically to repulse syntaxin and reduce the binding affinity for the SNARE protein. Overexpression of dGLU in chromaffin cells has the polar opposite phenotype to P242S; amperometric spikes from dGLU-expressing cells have decreased spike charge, RT, and FT (Table 1 and Fig. 2). Examination of the differential spike shows that for cells overexpressing dGLU, despite the fact that the total charge is greatly reduced and that spike height is unaffected, the maximal slope of the spike current was actually increased, and the total current at the time of the maximal slope was increased as well (Fig. 6). The time to reach the maximal slope was the same as that in control cells. Thus, up to the time of the maximal spike slope, the P242S and dGLU mutations were phenotypically identical. It was only after the maximal slope had been attained that a phenotypic difference between the two mutations became evident, although this was not readily apparent without use of the differential analytical approach. In P242S, the timing of the spike was lengthened in comparison to control untransfected cells after the initial larger catecholamine flux. In cells overexpressing dGLU, however, the time between the maximal slope and the peak height of the spike was decreased in comparison to controls, and this decrease was responsible for the significant decrease in amperometric RT seen for this mutation. For the rest of the spike, there was a similar reduction in the timing in spikes from dGLU cells, which would underlie the decrease in quantal size seen for this mutation. Thus, for cells overexpressing Munc-18-1 dGLU, there is a larger initial release rate of catecholamine, which then abruptly terminates in comparison to controls.
The effects on the parameters of the spike from the differential analysis were not seen for mutations that do not have a general effect on spikes. If cells are transfected with a single glutamate mutation of one of the PKC phosphorylation sites (S306E; sGLU) that has no effect on syntaxin binding (J. Barclay, unpublished data) and has no general effect on amperometric spikes or measured quantal size (Table 1 and Fig. 2), there was no effect on the maximal spike slope (Fig. 7). The total current by the time of the maximal slope was also unaffected, and there were no differences in any of the timing parameters. Addition of the phorbol ester, PMA, which pharmacologically activates PKC to phosphorylate Munc-18-1 (34), has an identical effect on quantal size as that seen with the dGLU mutation (Table 1 and Fig. 2). The differential analysis demonstrated that PMA, like expression of dGLU, also increased the maximal slope and the current at that time point of the spike and decreased the subsequent timing of the spike in comparison to controls (Fig. 7). Therefore, application of a PKC activator increases the initial release rate of catecholamines and causes a premature termination of the fusion process.
Previous experiments have demonstrated overexpression of Munc-18-1 in cells cotransfected with a plasmid encoding EGFP (8,30); however, any differences in the phenotypic effects of the mutations could reflect differences in expression or an altered cellular localization of the protein. Assessed by immunofluorescence, neither the extent of overexpression (p > 0.10) nor the localization of Munc-18-1 protein was different in transfected cells for all three expressed mutations (Fig. 8). Because syntaxin trafficking has been suggested to be dependent on Munc-18-1 (35), the localization of endogenous syntaxin was also compared. Syntaxin protein was similarly localized to the plasma membrane in both untransfected and transfected cells for all three Munc-18-1 mutations (Fig. 8).
Figure 8.

Expression and localization of Munc-18-1 mutants and endogenous syntaxin. (A) Indicated Munc-18-1 mutant proteins were cotransfected with an EGFP reporter plasmid into bovine adrenal chromaffin cells. Expression of Munc-18-1 was detected by immunofluorescence using a polyclonal antibody, whereas EGFP expression was indicated by endogenous fluorescence. Confocal images indicated that expression level and localization of Munc-18-1 in transfected cells were not dependent on the Munc-18-1 mutation. (B) Expression of endogenous syntaxin in chromaffin cells transfected with the indicated mutant Munc-18-1 was detected using a monoclonal antibody. Localization of syntaxin in transfected cells was not altered by any of the Munc-18-1 mutations.
Discussion
Previous investigations of exocytosis using carbon fiber amperometry drew interpretations about the fusion process by examining changes in spike heights and timing; however, these parameters are partially determined by concentration gradients and can be modified by altering catecholamine content without necessarily altering the kinetics of exocytosis. Because specific Munc-18-1 mutations also alter recorded quantal size (8,30), interpretations of the protein's function during the preceding fusion event could be misleading. This article demonstrates that altering concentration gradients by increasing granule catecholamine content does not alter the spike current at the point of maximal rate of rise. The time to this point is also unaffected by any conditions investigated thus far, which indicates that this point of the spike likely represents the initial release of catecholamine from the spatial origin of exocytosis. The oxidation rate at this point would then equal the initial release rate of transmitter. This new analysis suggests that both Munc-18-1 P242S and dGLU (S306E/S313E) mutations cause an increase in the initial rate of catecholamine release despite their having opposite effects on quantal size. This interpretation may resolve a discrepancy between amperometry results and previous findings from synaptic electrophysiology. At a synapse, an increase in initial release rates will result in a larger initial transmitter concentration in the synaptic cleft, leading to an enhanced postsynaptic response (36). This is indeed true at the Drosophila neuromuscular junction, where expression of the P242S mutation causes an increase in neuromuscular EJC amplitude (33). An R39C mutation of Munc-18-1 also increases Drosophila EJC amplitude; however, the R39C amperometric phenotype is opposite to P242S and similar to dGLU (Table 1 and Fig. 2). Results here demonstrate that the P242S and R39C mutations may in fact have one common exocytotic phenotype between them that could account for their similar synaptic phenotypes.
Previous studies using pharmacology and capacitance measurements have shown that PKC activation can increase the fusion pore expansion rate (37,38). Data here demonstrate that either phorbol ester treatment or expression of a phosphomimetic PKC target, Munc-18-1, also increases initial release rates of exocytosis. The greater initial release rate of catecholamine from cells expressing either P242S or dGLU Munc-18-1 demonstrates that these mutations increase either the initial size of the fusion pore or the rate of its expansion. It is difficult to distinguish between these two options because at time t = 0 they are essentially synonymous. Such a conclusion would indicate that the fusion pore in chromaffin cells initially limits release by restricting either transmitter extrusion or water entry via the pore geometry. Changes to fusion pore dimensions and/or expansion rate by Munc-18-1 could be achieved either via a modified protein structure or an altered interaction with syntaxin. Syntaxin, as a t-SNARE, is a core component of the fusion machinery and has recently been suggested to line the fusion pore (25). Any alteration in the syntaxin/Munc-18-1 interaction could thereby act to open the pore further or accelerate its expansion. In this manner, Munc-18-1 could function as a rate determinant of the fusion process. The P242S mutant, however, also showed an increase in the initial release rate but with apparently wild-type syntaxin binding affinity. Although this study does not address the issue experimentally, it has recently been reported that Munc-18-1 also binds to syntaxin while in the SNARE complex and to the SNARE complex directly (39–41). These alternative modes of binding occur via different interactions than those for the binding of closed-conformation syntaxin (42), which has been previously investigated for the P242S and dGLU mutations (8,30). One such novel form of binding strongly accelerates the fusion reaction between reconstituted liposomes expressing SNAREpins (39), corroborating the findings demonstrated here that Munc-18-1 functions in determining the rate of fusion. It is presently unknown whether the specific point mutations discussed here would affect the alternative modes of binding to syntaxin or to the SNARE complex. Although introduction of either the R39C or P242S point mutation does not alter the acceleration of lipsosome fusion (39), such fusion assays do not always correlate well with electrophysiological data (43) and thus do not preclude a functional effect of the mutations presented here. Because the yeast Munc-18-1 homolog Sec-1 also binds to its assembled SNARE complex (44), regulation of fusion speed may well be a universal role for the SM proteins in fusion reactions.
A reduction in Munc-18-1 binding to syntaxin via the classically described closed conformation mode, evident for dGLU, is additionally correlated with a decrease in the timing of the amperometric spike after the initial increased rate of release of catecholamine. This timing change indicates another role for the protein in determining the duration of the fusion event, putatively via this mode of syntaxin interaction. Control of fusion duration could be achieved physiologically by regulating whether fusion events are kiss-and-run or develop into full fusion. If this form of syntaxin binding is specifically associated with a premature closure of the fusion pore, however, then the effects of dGLU/R39C on amperometric spikes are not associated with a more ready donation of syntaxin into the SNARE complex as has been previously suggested (8,28). Instead, this could implicate a different role for this form of interaction in regulating the switch from subquantal to quantal release or sequestration of syntaxin post-SNARE-complex formation. Such a role could possibly explain why incomplete fusion events only occur for steps in which the closed conformation mode of syntaxin/Munc-18-1 binding occurs (neuronal and neuroendocrine exocytosis) and may indicate an evolutionary specialization for neuron/neuroendocrine subquantal release.
To what extent would these effects of Munc-18-1 mutation during neuroendocrine release be applicable to neuronal exocytosis? Alterations in the initial rate of release would change postsynaptic response strength, as has been demonstrated for mutations in Munc-18-1 at the Drosophila neuromuscular junction (33). Subquantal release, or kiss-and-run exocytosis, has been definitively demonstrated for neuroendocrine models of exocytosis (1); however, the existence of kiss-and-run at a synapse remains controversial despite several recent studies in support (45–48). A study at the Drosophila neuromuscular junction also demonstrated that the decay times of excitatory postsynaptic currents were protracted to a variable extent suggestive of a kiss-and-run mode of exocytosis (49). Interestingly, the decay time was increased on application of the PKC inhibitor staurosporine, which would fit logically with a PKC-induced reduction in fusion duration.
Amperometric interpretation has argued over whether full amperometric spikes represent full fusion events only or whether kiss-and-run events can be accounted for by full spikes as well (6). Within either model there are only limited ways in which parameters of spikes can be modified: changing transmitter content, altering the kinetics of release from the intragranular matrix (in dense-core granules), and, most importantly, altering the kinetics/extent of exocytosis itself. Several factors preclude the simplistic answer that the Munc-18-1-dependent effects demonstrated here are caused by a role external to the fusion process itself. First, both over- and underexpression of Munc-18-1 do not affect quantal size, and quantal size at the Drosophila neuromuscular junction is unaffected for either of the P242S and R39C (equivalent to the dGLU) mutations. Second, an increase in quantal size by l-DOPA application does not change initial release rates as specific Munc-18-1 mutations do. Third, specific to the dGLU mutant, increased initial release rates do not fit logically with the evident reduction in quantal size, as greater amounts of released catecholamine should cause the opposite result. Finally, it is unlikely that the conserved function of Munc-18-1 is to affect intravesicular matrices because they are not a feature of all vesicle-trafficking events within the cell, nor do they feature in neuronal exocytosis, physiological processes in which SM proteins play intrinsic roles. This leaves the remaining conclusion that changes to the amperometric spike indicate an effect of Munc-18-1 on the fusion process itself. This conclusion lends support to the interpretation of amperometric spikes in which full spikes can represent both full fusion and also transmitter release through fusion pores. It is proposed that the new analysis presented here represents an improvement for future interpretation of effects of individual proteins on the exocytotic process.
A general model for Munc-18-1, then, is as a multifunctional component integral to the exocytotic process. Evidence from the mouse and C. elegans knockouts (29,50), as well as amperometric (30) and genetic (51) data, support a role for the protein in vesicle translocation/docking. The data presented here, however, support the well-established role for Munc-18-1 as a positive promoter of vesicle fusion (52–56) and are in agreement with recent evidence that it does so via regulation of fusion rate (39). Additionally, there appears to be a time point at which fusion events can be prematurely terminated, and Munc-18-1 appears to be involved in this process, at least for the one mutation that reduces a specific, closed-conformation mode of syntaxin binding. Control of initial release rate makes Munc-18-1 an ideal presynaptic target for the regulation of synaptic strength. The control of the subsequent duration would alternatively regulate the length of action of that synaptic signal by the activation of postsynaptic targets further away or simply as a long-term strengthening of the synapse. Such effects would not readily be made apparent by examination of individual postsynaptic potentials or amperometric spikes. Thus, Munc-18-1 could be an ideal cellular target for short-term presynaptic plasticity, a speculation recently confirmed at glutamatergic, GABAergic, and neuromuscular synapses (57).
Acknowledgments
This work was supported by a Research Councils UK Academic Fellowship. I thank Robert D. Burgoyne and Alan Morgan (University of Liverpool) for critical comments on earlier drafts of this manuscript, R. D. Burgoyne for laboratory space and materials, and Mark Sherwood for assistance with the confocal microscopy.
Footnotes
Editor: Barry R. Lentz.
References
- 1.Burgoyne R.D., Morgan A. Secretory granule exocytosis. Physiol. Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R., Lang T., Sudhof T.C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 3.Jackson M.B., Chapman E.R. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu. Rev. Biophys. Biomol. Struct. 2005;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 4.Choi S., Klingauf J., Tsien R.W. Postfusional regulation of cleft glutamate concentration during LTP at “silent synapses.”. Nat. Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- 5.Travis E.R., Wightman R.M. Spatio-temporal resolution of exocytosis from individual cells. Annu. Rev. Biophys. Biomol. Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Mosharov E.V., Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat. Methods. 2005;2:651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 7.Finnegan J.M., Pihel K., Cahill P.S., Huang L., Zerby S.E., Ewing A.G., Kennedy R.T., Wightman R.M. Vesicular quantal size measured by amperometry at chromaffin, mast, pheochromocytoma, and pancreatic β-cells. J. Neurochem. 1996;66:1914–1923. doi: 10.1046/j.1471-4159.1996.66051914.x. [DOI] [PubMed] [Google Scholar]
- 8.Barclay J.W., Craig T.J., Fisher R.J., Ciufo L.F., Evans G.J., Morgan A., Burgoyne R.D. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J. Biol. Chem. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- 9.Braun M., Wendt A., Karanauskaite J., Galvanovskis J., Clark A., Macdonald P.E., Rorsman P. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic {beta} cells. J. Gen. Physiol. 2007;129:221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sombers L.A., Maxson M.M., Ewing A.G. Loaded dopamine is preferentially stored in the halo portion of PC12 cell dense core vesicles. J. Neurochem. 2005;93:1122–1131. doi: 10.1111/j.1471-4159.2005.03087.x. [DOI] [PubMed] [Google Scholar]
- 11.Travis E.R., Wang Y.-M., Michael D.J., Caron M.G., Wightman R.M. Differential quantal release of histamine and 5-hydroxytyptamine from mast cells of vesicular monoamine transporter 2 knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:162–167. doi: 10.1073/pnas.97.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruns D., Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- 13.Koh D.-S., Hille B. Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc. Natl. Acad. Sci. USA. 1997;94:1506–1511. doi: 10.1073/pnas.94.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wightman R.M., Jankowski J.A., Kennedy R.T., Kawagoe K.T., Schroeder T.J., Leszczyszyn D.J., Near J.A., Diliberto E.J., Viveros O.H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhamdani A., Palfrey H.C., Artalejo C.R. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 16.Fulop T., Radabaugh S., Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J. Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pothos E.N., Mosharov E., Liu K.-P., Setlik W., Haburcak M., Baldini G., Gershon M.D., Tamir H., Sulzer D. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J. Physiol. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sombers L.A., Hanchar H.J., Colliver T.L., Wittenberg N., Cans A., Arbault S., Amatore C., Ewing A.G. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J. Neurosci. 2004;24:303–309. doi: 10.1523/JNEUROSCI.1119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowski J.A., Finnegan J.M., Wightman R.M. Extracellular ionic composition alters kinetics of vesicular release of catecholamines and quantal size duriing exocytosis at adrenal medullary cells. J. Neurochem. 1994;63:1739–1747. doi: 10.1046/j.1471-4159.1994.63051739.x. [DOI] [PubMed] [Google Scholar]
- 20.Borges R., Travis E.R., Hochstetler S.E., Wightman R.M. Effects of external osmotic pressure on vesicular secretion from bovine adrenal medullary cells. J. Biol. Chem. 1997;272:8325–8331. doi: 10.1074/jbc.272.13.8325. [DOI] [PubMed] [Google Scholar]
- 21.Wang C.-T., Grishanin R., Earles C.A., Chang P.Y., Martin T.F.J., Chapman E.R., Jackson M.B. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- 22.Graham M.E., Barclay J.W., Burgoyne R.D. Syntaxin/Munc18 interactions in the late events during vesicle fusion and release in exocytosis. J. Biol. Chem. 2004;279:32751–32760. doi: 10.1074/jbc.M400827200. [DOI] [PubMed] [Google Scholar]
- 23.Chow R.H., von Ruden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 24.Barclay J.W., Aldea M., Craig T.J., Morgan A., Burgoyne R.D. Regulation of the fusion pore conductance during exocytosis by cyclin-dependent kinase 5. J. Biol. Chem. 2004;279:41495–41503. doi: 10.1074/jbc.M406670200. [DOI] [PubMed] [Google Scholar]
- 25.Han X., Wang C.-T., Bai J., Chapman E.R., Jackson M.B. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- 26.Rizo J., Sudhof T.C. Snares and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 27.Toonen R.F.G., Verhage M. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 2003;13:177–186. doi: 10.1016/s0962-8924(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 28.Fisher R.J., Pevsner J., Burgoyne R.D. Control of fusion pore dynamics during exocytosis by Munc18. Science. 2001;291:875–878. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- 29.Voets T., Toonen R., Brian E.C., de Wit H., Moser T., Rettig J., Sudhof T.C., Neher E., Verhage M. Munc-18 promotes large dense-core vesicle docking. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 30.Ciufo L.F., Barclay J.W., Burgoyne R.D., Morgan A. Munc18–1 regulates early and late stages of exocytosis via syntaxin-independent protein interactions. Mol. Biol. Cell. 2005;16:470–482. doi: 10.1091/mbc.E04-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham M.E., O’Callaghan D.W., McMahon H.T., Burgoyne R.D. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. USA. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafez I., Kisler K., Berberian K., Dernick G., Valero V., Yong M.G., Craighead H.G., Lindau M. Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc. Natl. Acad. Sci. USA. 2005;102:13879–13884. doi: 10.1073/pnas.0504098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M.N., Littleton J.T., Bhat M.A., Prokop A., Bellen H.J. ROP, the Drosophila sec1 homolog, interacts with syntaxin and regulates neurotransmitter release in a dosage-dependent manner. EMBO J. 1998;17:127–139. doi: 10.1093/emboj/17.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig T.J., Evans G.J.O., Morgan A. Physiological regulation of Munc18/nSec1 phosphorylation on serine-313. J. Neurochem. 2003;86:1450–1457. doi: 10.1046/j.1471-4159.2003.01955.x. [DOI] [PubMed] [Google Scholar]
- 35.Rowe J., Calegari F., Taverna E., Longhi R., Rosa P. Syntaxin 1A is delivered to the apical and basolateral domains of epithelial cells: the role of munc-18 proteins. J. Cell Sci. 2001;114:3323–3332. doi: 10.1242/jcs.114.18.3323. [DOI] [PubMed] [Google Scholar]
- 36.Clements J.D. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- 37.Fulop T., Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem. J. 2006;399:111–119. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scepek S., Coorssen J.R., Lindau M. Fusion pore expansion in horse eosinophils is modulated by Ca2+ and protein kinase C via distinct mechanisms. EMBO J. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen J., Tareste D.C., Paumet F., Rothman J.E., Melia T.J. Selective activation of cognate SNAREpins by Sec1/Munc18 (SM) proteins. Cell. 2007;128:1–13. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Dulubova I., Khvotchev M., Liu S., Huryeva I., Sudhof T.C., Rizo J. Munc18–1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. USA. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickman C., Medine C.N., Bergmann A., Duncan R.R. Functionally and spatially distinct modes of Munc18-syntaxin 1 interaction. J. Biol. Chem. 2007;282:12097–12103. doi: 10.1074/jbc.M700227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Sudhof T.C., Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizo J., Chen X., Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Togneri J., Cheng Y.-S., Munson M., Hughson F.M., Carr C.M. Specific SNARE complex binding mode of the Sec1/Munc-18 protein, Sec1p. Proc. Natl. Acad. Sci. USA. 2006;103:17730–17735. doi: 10.1073/pnas.0605448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L., Wu X.S., Mohan R., Wu L.G. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 46.Klyachko V.A., Jackson M.B. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- 47.Aravanis A., Pyle J.L., Tsien R.W. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 48.Gandhi S.P., Stevens C.F. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 49.Pawlu C., DiAntonio A., Heckmann M. Postfusional control of quantal current shape. Neuron. 2004;42:607–618. doi: 10.1016/s0896-6273(04)00269-7. [DOI] [PubMed] [Google Scholar]
- 50.Weimer R.M., Richmond J.E., Davis W.S., Hadwinger G., Nonet M.L., Jorgensen E.M. Defects in synaptix vesicle docking in unc-18 mutants. Nat. Neurosci. 2003;6:1023–1030. doi: 10.1038/nn1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dascher C., Ossig R., Gallwitz D., Schmitt H.D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the ras superfamily. Mol. Cell. Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison S.D., Broadie K., van de Goor J., Rubin G.M. Mutations in the drosophila rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 54.Hosono R., Hekimi S., Kamiya Y., Sassa T., Murakami S., Nishiwaki K., Miwa J., Taketo A., Kodaira K.I. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J. Neurochem. 1992;58:1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 55.Verhage M., Maia A.S., Plomp J.J., Brussaard A.B., Heeroma J.H., Vermeer H., Toonen R.F., Hammer R.E., van den Berg T.K., Missler M., Geuze H.J., Sudhof T.C. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 56.Scott B.L., Van Komen J.S., Irshad H., Liu S., Wilson K.A., McNew J.A. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J. Cell Biol. 2004;167:75–85. doi: 10.1083/jcb.200405018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toonen R.F.G., Wierda K., Sons M.S., de Wit H., Cornellisse L.N., Brussaard A., Plomp J.J., Verhage M. Munc18–1 expression levels control synapse recovery by regulating readily releasable pool size. Proc. Natl. Acad. Sci. USA. 2006;103:18332–18337. doi: 10.1073/pnas.0608507103. [DOI] [PMC free article] [PubMed] [Google Scholar]


