Abstract
A simple defined medium (neisseria defined medium) was devised that does not require iron extraction to produce iron-limited growth of Neisseria meningitidis (SDIC). Comparison of this medium to Mueller-Hinton broth and agar showed nearly identical growth rates and yields. The defined medium was used in batch cultures to determine the disappearance of iron from the medium and its uptake by cells. To avoid a number of problems inherent in batch culture, continuous culture, in which iron and dissolved oxygen were varied independently, was used. Most of the cellular iron was found to be nonheme and associated with the particulate fraction in sonically disrupted cells. Nonheme and catalase-heme iron were reduced by iron starvation far more than cytochromes b and c and N,N,N',N'-tetramethylphenylenediamine-oxidase. The respiration rate and efficiency also decreased under iron limitation, whereas generation times increased. The iron-starved meningococcus took up iron by an energy-independent system operating in the first minute after an iron pulse and a slower energy-dependent system inhibited by respiratory poisons and an uncoupler. The energy-dependent system showed saturation kinetics and was stimulated nearly fourfold by iron privation. In addition, to determine the availability to the meningococcus of the iron in selected compounds, a sensitive assay was devised in which an iron-limited continuous culture was pulsed with the iron-containing compound.
Full text
PDF


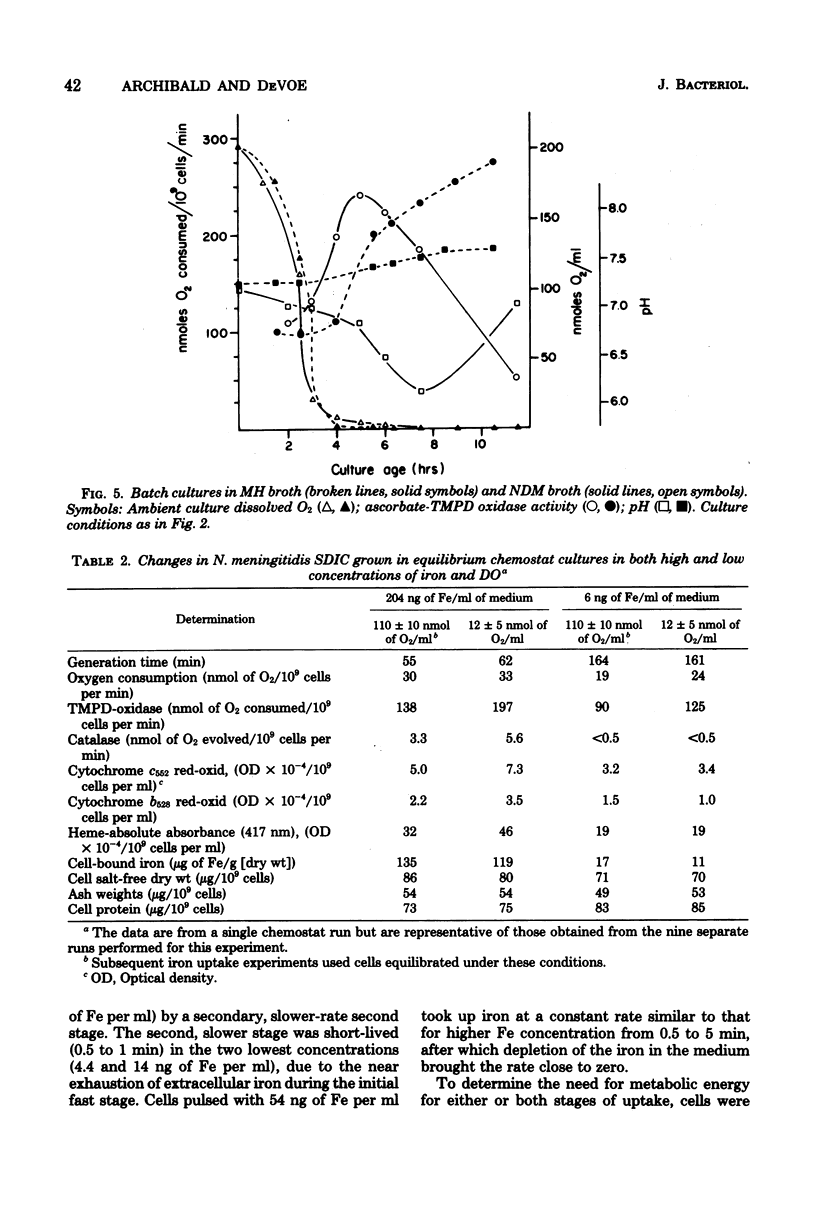
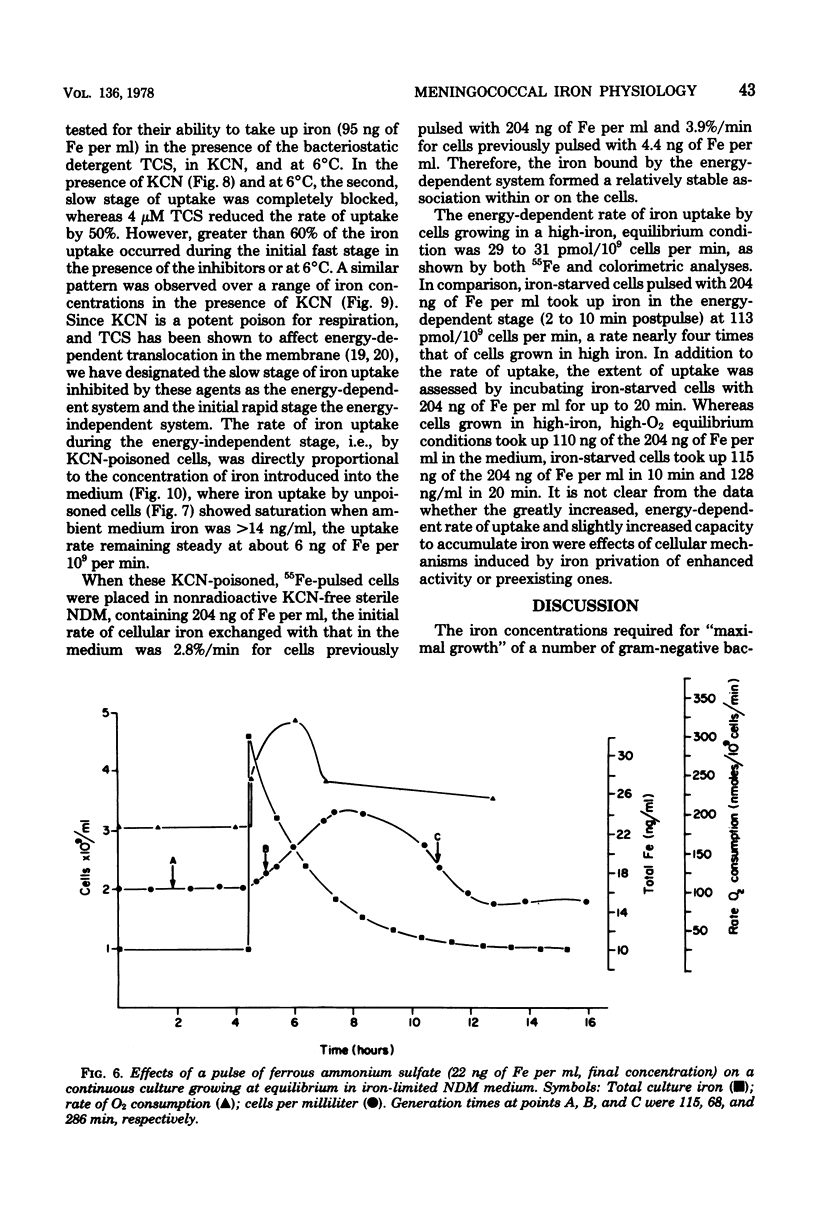
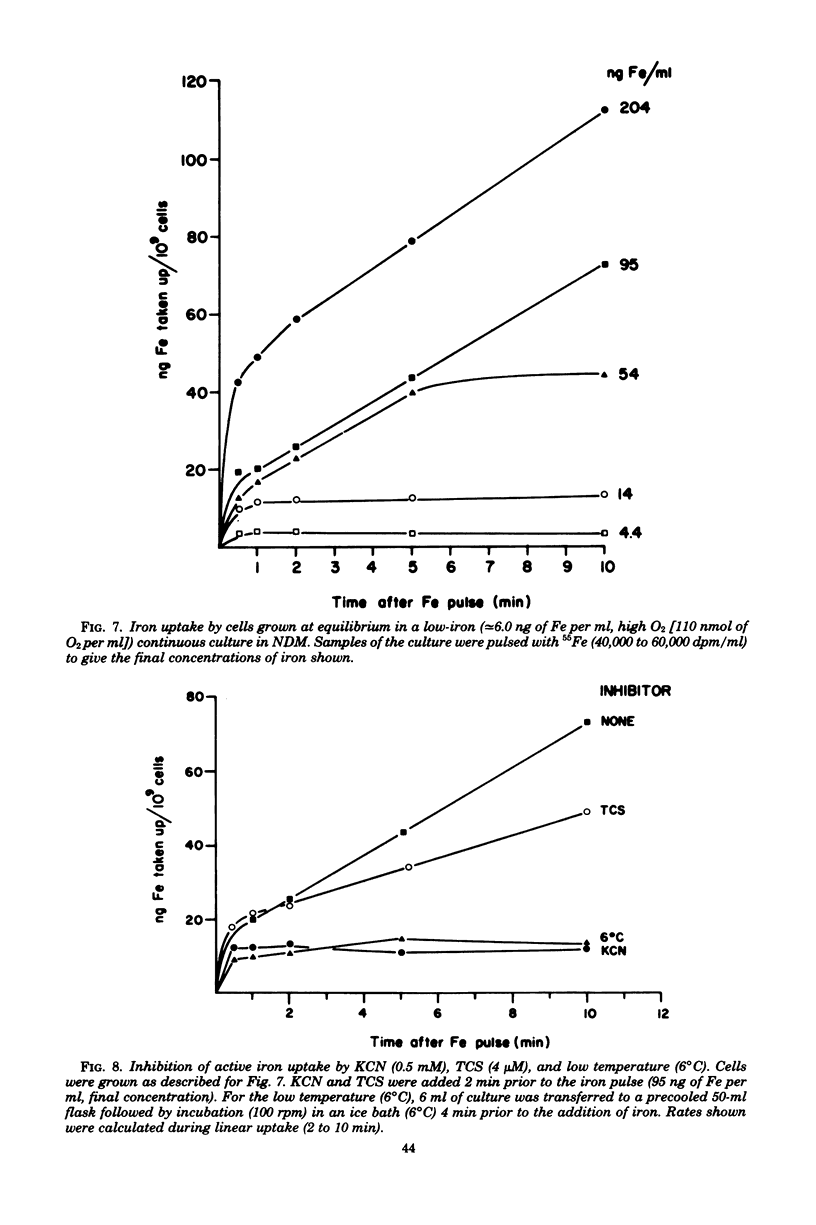
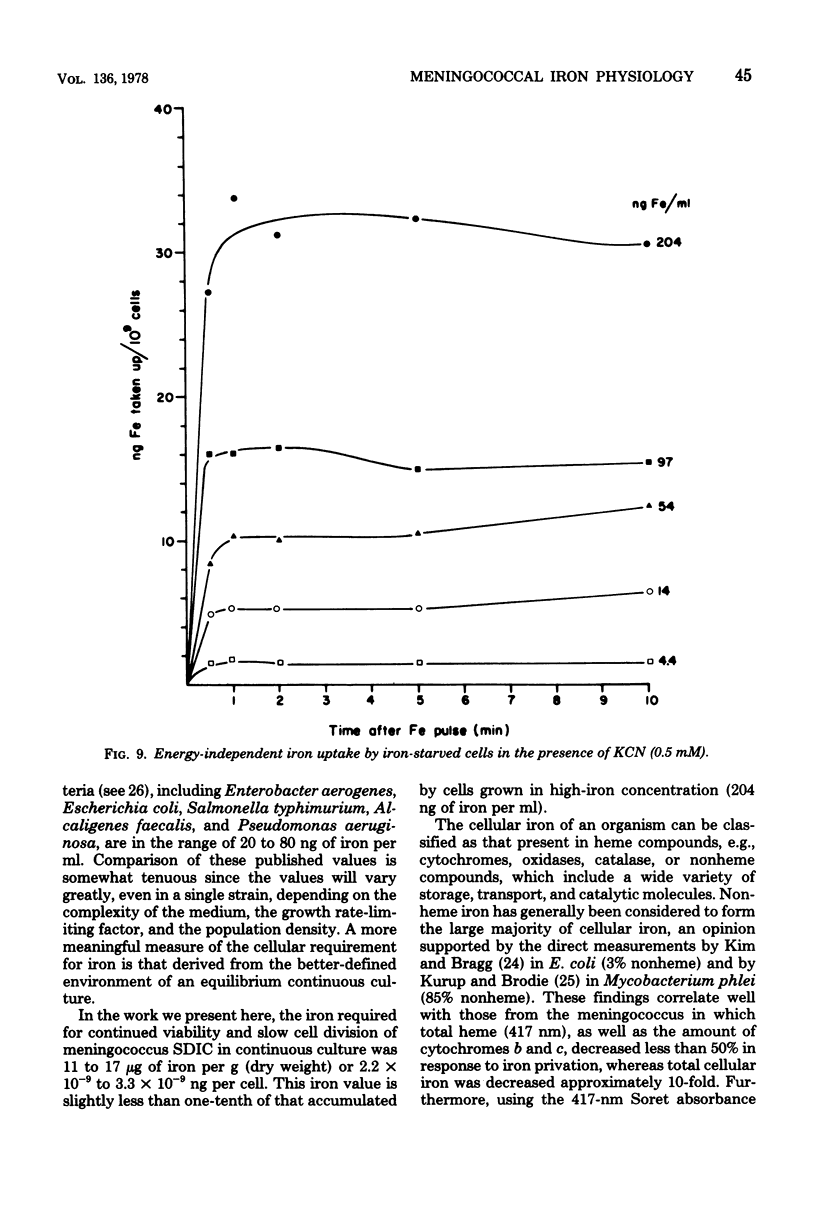


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. E., Byers B. R. Ferric hydroxamate transport without subsequent iron utilization in Bacillus megaterium. J Bacteriol. 1976 Sep;127(3):1324–1330. doi: 10.1128/jb.127.3.1324-1330.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceneaux J. E., Davis W. B., Downer D. N., Haydon A. H., Byers B. R. Fate of labeled hydroxamates during iron transport from hydroxamate-ion chelates. J Bacteriol. 1973 Sep;115(3):919–927. doi: 10.1128/jb.115.3.919-927.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Ratledge C. Iron transport in Mycobacterium smegmatis: ferrimycobactin reductase (nad(p)h:ferrimycobactin oxidoreductase), the enzyme releasing iron from its carrier. FEBS Lett. 1975 May 1;53(2):262–266. doi: 10.1016/0014-5793(75)80033-0. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W., SCHLOER G. M. A defined agar medium for genetic transformation of Neisseria meningitidis. J Bacteriol. 1962 Mar;83:470–474. doi: 10.1128/jb.83.3.470-474.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HERBERT D. The enzymesubstrate compounds of bacterial catalase and peroxides. Biochem J. 1950 Apr;46(4):402–414. doi: 10.1042/bj0460402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLEY P. J., SARKAR B., STITT C. F., SALTMAN P. Chelation of iron by sugars. Biochim Biophys Acta. 1963 Feb 5;69:313–321. doi: 10.1016/0006-3002(63)91264-2. [DOI] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Curran H. R., Brunstetter B. C., Myers A. T. Spectrochemical Analysis of Vegetative Cells and Spores of Bacteria. J Bacteriol. 1943 May;45(5):485–494. doi: 10.1128/jb.45.5.485-494.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W. Egestion of degraded meningococci by polymorphonuclear leukocytes. J Bacteriol. 1976 Jan;125(1):258–266. doi: 10.1128/jb.125.1.258-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A. The mechanism of the bacteriostatic action of tetrachlorosalicylanilide: a Membrane-active antibacterial compound. J Gen Microbiol. 1968 Mar;50(3):441–458. doi: 10.1099/00221287-50-3-441. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- JYSSUM K. Assimilation of nitrogen in meningococci grown with the ammonium ion as sole nitrogen source. Acta Pathol Microbiol Scand. 1959;46:320–332. [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Properties of nonheme iron in a cell envelope fraction from Escherichia coli. J Bacteriol. 1971 Sep;107(3):664–670. doi: 10.1128/jb.107.3.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXVII. The nature of nonheme iron in Mycobacterium phlei. J Biol Chem. 1967 Jun 25;242(12):2909–2916. [PubMed] [Google Scholar]
- LENHOFF H. M., NICHOLAS D. J., KAPLAN N. O. Effects of oxygen, iron, and molybdenum on routes of electron transfer in Pseudomonas fluorescens. J Biol Chem. 1956 Jun;220(2):983–995. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS F. Adaptation of the cytochromes of Aerobacter aerogenes in response to environmental oxygen tension. Aust J Exp Biol Med Sci. 1956 Oct;34(5):395–405. doi: 10.1038/icb.1956.48. [DOI] [PubMed] [Google Scholar]
- MOSS F. The influence of oxygen tension on respiration and cytochrome a2 formation of Escherichia coli. Aust J Exp Biol Med Sci. 1952 Dec;30(6):531–540. doi: 10.1038/icb.1952.51. [DOI] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C., Nocton J. C. Extracellular iron acquisition by mycobacteria: role of the exochelins and evidence against the participation of mycobactin. Infect Immun. 1975 Dec;12(6):1242–1251. doi: 10.1128/iai.12.6.1242-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. P. EXPERIMENTAL MENINGOCOCCAL INFECTION IN MICE. Science. 1933 Oct 13;78(2024):340–341. doi: 10.1126/science.78.2024.340. [DOI] [PubMed] [Google Scholar]
- Negrin R. S., Neilands J. B. Ferrichrome transport in inner membrane vesicles of Escherichia coli K12. J Biol Chem. 1978 Apr 10;253(7):2339–2342. [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Imferon agar: improved medium for isolation of pathogenic Neisseria. J Clin Microbiol. 1977 Sep;6(3):293–297. doi: 10.1128/jcm.6.3.293-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. Phenolic acids and iron transport in Bacillus subtilis. Biochim Biophys Acta. 1968 Sep 3;165(2):225–232. doi: 10.1016/0304-4165(68)90050-0. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., KOGUT M. The influence of growth rate and aeration on the respiratory and cytochrome system of fluorescent pseudomonad grown in continuous culture. J Gen Microbiol. 1958 Oct;19(2):228–243. doi: 10.1099/00221287-19-2-228. [DOI] [PubMed] [Google Scholar]
- ROUF M. A. SPECTROCHEMICAL ANALYSIS OF INORGANIC ELEMENTS IN BACTERIA. J Bacteriol. 1964 Dec;88:1545–1549. doi: 10.1128/jb.88.6.1545-1549.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIMPENNY J. W., RANLETT M., GRAY C. T. Repression and derepression of cytochrome c biosynthesis in Escherichia coli. Biochim Biophys Acta. 1963 May 7;73:170–172. doi: 10.1016/0006-3002(63)90436-0. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



