Abstract
Free heme binds to heme oxygenase as a prosthetic group and substrate in the conversion of heme to biliverdin, carbon monoxide, and free iron. Current methods for quantifying heme oxygenase-1 (HO-1) involve reconstitution of the enzyme with heme, followed by a hydroxyapatite column to remove the excess heme. As a result of the hydroxyapatite chromatography, there are significant losses of purified protein. We have developed a method which allows accurate quantitation of HO-1 using a heme titration and elimination of the final hydroxyapatite column, increasing the amount of purified protein.
Heme oxygenase-1 (HO-1) is an inducible enzyme responsible for the oxidative cleavage of heme to biliverdin, carbon monoxide and free iron (1). Heme is a heterocyclic porphyrin with an iron center which serves as cofactor or prosthetic group for a number of proteins classified as hemoproteins. The binding of heme to HO-1 is unique because heme serves as both cofactor and substrate, and is degraded upon the addition of reducing equivalents. Recombinant HO-1 proteins from a variety of species have been expressed and purified, with human and rat most commonly studied (2, 3). The deletion of 23 C-terminal amino acids, which serve as the membrane spanning domain (4), allowed for a swift purification of truncated human HO-1 (sHO-1) in large quantities. Standard purification procedures of sHO-1 produce a purified protein that must be reconstituted with heme to calculate the specific HO-1 concentration (3). Excess heme is removed from the HO-1-heme complex via passage through a final hydroxyapatite (HA) column. The HO-1-heme spectrum includes a Soret band at 405 nm, which is commonly utilized to quantify the complex with an extinction coefficient of 140 mM−1 cm−1 (5). The HO-1-heme complex produces a distinct absorption spectrum in comparison to the apoprotein. This report describes an improved method to quantify HO-1 using a heme titration, eliminating the final HA column, and effectively increasing the total purified protein.
For these studies, truncated, human HO-1 was purified by a previously described method, with minor modifications (3). Following the expression of sHO1 in E. coli strain DH5α, the media appeared green in color, due to the accumulation of biliverdin (6). The bacterial cell pellet remained green throughout the purification procedure prior to the ammonium sulfate precipitation. The increase in ionic strength forced the disassociation of the biliverdin from the sHO-1, forming a colorless protein product. Following standard column chromatography, the purified apoprotein was then used to validate the heme titration method in a comparison study with the standard procedure. A protein concentration of 0.763 mg/ml was determined using bicinchoninic acid in a BCA kit purchased from Pierce (7). One aliquot of sHO-1 was quantified using the heme titration and the other using heme reconstitution and subsequent HA column chromatography. The accuracy of both methods was compared via a heme oxygenase activity assay. NADPH-cytochrome P450 reductase (CPR) was purified as described previously (8). Biliverdin reductase was partially purified as described by Kutty (9).
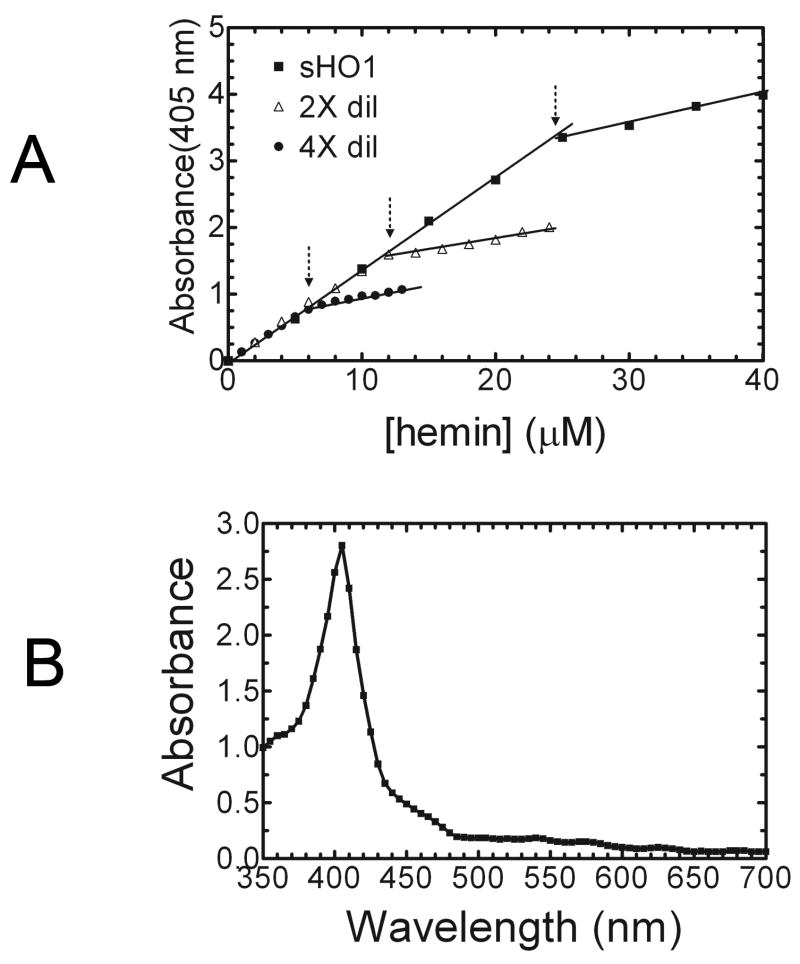
The heme titration was developed utilizing previously described properties of the HO-1-heme complex (10). The fresh heme solution was prepared by adding 0.2 ml 0.1N NaOH to 1.63 mg heme, which was taken up in a final volume of 1 ml of 100 mM KPO4 (pH 7.4), 1 mM EDTA, 0.1% Triton X-100, and 0.1% sodium cholate. The concentration of the heme stock solution was 2.5 mM. At neutral pH, heme exists mainly as a dimer, but in the presence of detergent heme remains in the monomeric form, permitting efficient binding to HO-1 (11). In general, HO-1 has been shown to bind heme at a stoichiometric ratio of 1:1 (10). The addition of known concentrations of heme to 100ul of sHO-1 produced a linear increase of the absorbance at 405 nm. Figure 1A illustrates the addition of 5 μM aliquots of the free heme solution, which associates with the sHO-1 to form the sHO-1-heme complex. The absorbance at 405 nm was recorded after each aliquot and plotted versus the known heme concentration. The protein concentration was determined at the break point at which the increased absorbance at 405 nm deviated from linearity. As indicated by the arrow at the intersection of the two slopes, the concentration of sHO1 was 24 μM. Dilutions of the stock sHO-1 (2 and 4-fold) were also included in the titration experiment to demonstrate the accuracy and range of the titration. For the 2 and 4-fold sHO-1 dilution titrations, the heme stock concentration of 0.2 mM was added in 1μl and 0.5μl increments, respectively. The decreased slope was attributed to the saturation of sHO-1-heme complex formation and the accumulation of free heme, which had a lower extinction coefficient at 405 nm. The extinction coefficient of the ferric heme-sHO-1 complex was 140 mM−1 cm−1, which is consistent with published reports.
Fig. 1.
(A) Quantitation of HO-1 by heme titration – A heme titration was used to quantify purified soluble,human HO-1. Free heme dissolved in 0.1 N NaOH and 100 mM KPO4 containing 0.1% Triton X-100 and 0.1% sodium cholate, pH 7.4 was added to 0.1 ml of sHO-1 in 5 μM aliquots. The absorbance at 405 nm increased linearly with the addition of heme. The sHO-1 concentration is determined by the point at which the absorbance increase deviates from linearity. The concentration, shown here at the point that the two lines intersect, is calculated to be 24 uM. Dilution of the sHO-1 stock (1:4, 1:2) demonstrates the accuracy of the titration. (B) Spectrum of the sHO-1-heme complex – Following the addition of heme to the purified apoprotein, the complex was passed over a hydroxyapatite column to remove excess heme from the sample. The sHO-1-heme complex was quantified using the absorbance at 405 nm divided by the extinction coefficient of 140 mM−1 cm−1 at 405 nm. The absorption spectrum of sHO-1 reconstituted with heme produced a well-defined Soret band at 405 nm. In this sample the complex is calculated to have a concentration of ~ 20 μM.
In order to validate the heme titration method, the purified sHO-1 was reconstituted with heme followed by chromatography using a hydroxyapatite column to remove the excess heme and the eluate quantified by standard spectral methods (3). A fresh heme solution (2.5 mM), prepared as described above, was added to 1 ml (0.763 mg) of sHO-1 to a final concentration of 0.06 mM, or approximately a 2:1 heme/sHO-1 ratio. The complex was incubated on ice for ten minutes and applied to a 0.5 ml hydroxyapatite column, pre-equilibrated with 10 mM KPO4, pH 7.4. The column was washed with 10-20 column volumes of the 10 mM KPO4 to remove all excess heme and eluted with 0.6 ml 100 mM KPO4, pH 7.4. The fraction containing the sHO-1-heme complex was red in color due to the incorporation of the heme. The sHO-1-heme displayed typical spectral characteristics, as shown in Figure 1B. The Soret band at 405 nm is consistent with previous reports. The complex concentration was calculated to be 20 μM using the absorbance and the known extinction coefficient 140 mM−1 cm−1 at 405 nm. The total protein was determined by BCA as described above to be 0.62 mg/ml, or 0.37 mg. The amount of sHO-1 applied to the HA column was 0.76 mg, so approximately 50% of the total sHO-1 was lost in the removal of excess heme.
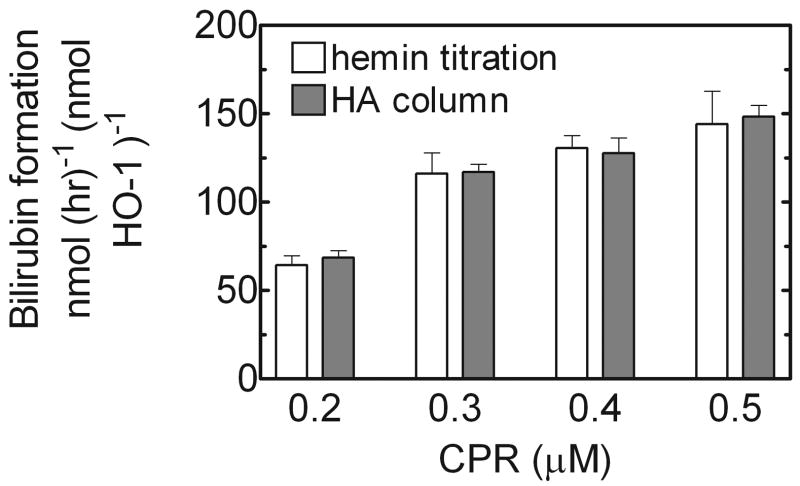
A standard HO assay (2, 5), which monitored the formation of bilirubin, was used to compare the quantitation methods. Although the two procedures generated different sHO-1-heme complex concentrations, the specific activity of 0.01 nmole from both samples was analyzed. The reaction mixture consisted of 0.1 μM HO-1, 15 μM heme, saturating levels of NADPH-cytochrome P450 reductase (CPR), and an excess (50 U/ml) of partially purified biliverdin reductase from rat liver cytosol (9). Increasing amounts of CPR were used ranging from 0.2 μM to 0.5 μM. This mixture was brought to a final volume of 100 μl with 100 mM KPO4 supplemented with bovine serum albumin (12.5 mg/ml), pH 7.4. The reaction mixture was preincubated for 2 minutes at 37º C in the dark prior to the addition of 100 mM NADPH to initiate the reaction. The rate of bilirubin formation was monitored by the increase in absorbance at 468 nm for ten minutes in the dark. The rate of bilirubin formation was calculated from the linear increase at 468 nm, which was divided by the extinction coefficient for bilirubin (43.5 mM−1 cm−1). As seen in Figure 2, the reaction rates for both sHO-1 samples were almost identical at each CPR concentration. As the CPR concentration was increased, both rates increased accordingly. The specific activity of both sHO-1 samples was the best way to contrast the two quantitation methods because it allowed for direct comparison of a calculated protein concentration. Regardless of the quantitation method, the specific activity of 0.01 nmole sHO1 was equal, illustrating the accuracy of the heme titration method.
Fig. 2.
Comparison of HO-1 activities from the heme titration and column chromatography methods – sHO-1 was quantified by two methods in order to validate the heme titration assay. The specific activities of sHO-1 from both methods were compared using a standard heme oxygenase assay, which uses the rate of bilirubin formation to determine activitHO-1 was added to a final concentration of 0.1 μM, with saturating levels of NADPH- cytochrome P450 reductase ranging from 0.2 μM to 0.5 μM. The specific activities of both samples were very similar, indicating that the heme titration can be used as an effective and accurate method of HO-1 quantitation.
The heme titration method had multiple advantages over the standard technique of HO-1 quantitation. First, the titration provided a rapid, precise procedure for determining protein concentration using a very small amount of the total preparation. The linear increase in absorbance at 405 nm was visualized and plotted versus the known heme concentration in each aliquot. The protein concentration was calculated from the point where the increase in absorbance began to deviate from linearity. Since free heme has a lower extinction coefficient than does the sHO-1-heme complex at 405 nm, additional heme resulted in a linear increase, but with a decreased slope. A second advantage to the heme titration allowed for the elimination of the final HA column. Because the heme titration did not require the removal of excess heme from the solution, the HA column was unnecessary. As shown above, the use of HA resin to remove excess heme resulted in the loss of ~ 50% of the total protein applied to the column. Interestingly, the heme titration only used a fraction of the preparation to determine protein concentration and reduced the amount of protein lost during the purification procedure. A third benefit of the heme titration involved the enzyme remaining in the apo form, excluding the small fraction used for HO-1 quantitation. Because previous methods are limited to quantifying only HO-1 reconstituted with heme, this required the addition of heme to the entire sample. The heme titration allows for a majority of the HO-1 to be stored as apoprotein, which is important for various experiments comparing catalytic characteristics of HO-1 in the presence or absence of the substrate, heme.
The heme titration is a valuable assay that was developed to quantify HO-1 and eliminate additional purification steps. The precision of the heme titration relies on the absorbance of the HO-1-heme complex at 405 nm. Most of the recombinant sHO-1 was purified as an apoprotein, hence it had very little absorbance at 405 nm prior to the addition of heme. The heme titration can be used to quantify HO-1 from various species and sources. The procedure described here is important not only in the ease and speed of quantitation, but also in the increased final yield of purified protein by as much as 50%.
Acknowledgments
We are grateful to Dr. Paul Ortiz de Montellano for supplying the truncated human HO-1 expression vector. This work is supported by a U.S. Public Health Services research grant from the National Institute of Environmental Health Sciences, R01-ES004344.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. Journal of Biological Chemistry. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 2.Wilks A, Ortiz de Montellano PR. Rat liver heme oxygenase. High level expression of a truncated soluble form and nature of the meso-hydroxylating species. Journal of Biological Chemistry. 1993;268:22357–22362. [PubMed] [Google Scholar]
- 3.Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry. 1995;34:4421–4427. doi: 10.1021/bi00013a034. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Sato M. Posttranslational and direct integration of heme oxygenase into microsomes. Biochemical and Biophysical Research Communications. 1989;163:1086–1092. doi: 10.1016/0006-291x(89)92332-2. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Kikuchi G. Purification and properties of heme oxygenase from pig spleen microsomes. Journal of Biological Chemistry. 1978;253:4224–4229. [PubMed] [Google Scholar]
- 6.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Escherichia coli as a catalytically active, full-length form that binds to bacterial membranes. European Journal of Biochemistry. 1991;202:161–165. doi: 10.1111/j.1432-1033.1991.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 8.Kelley RW, Reed JR, Backes WL. Effects of ionic strength on the functional interactions between CYP2B4 and CYP1A2. Biochemistry. 2005;44:2632–2641. doi: 10.1021/bi0477900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. Journal of Biological Chemistry. 1981;256:3956–3962. [PubMed] [Google Scholar]
- 10.Yoshida T, Kikuchi G. Purification and properties of heme oxygenase from rat liver microsomes. Journal of Biological Chemistry. 1979;254:4487–4491. [PubMed] [Google Scholar]
- 11.Brown NA, King RF, Shillcock ME, Brown SB. Haemoglobin catabolism: the role of ferrihaems in studies of the degradation pathway. Biochemical Journal. 1974;137:135–137. doi: 10.1042/bj1370135. [DOI] [PMC free article] [PubMed] [Google Scholar]