Abstract
The EGF-CFC factor Oep/Cripto1/Frl1 has been implicated in embryogenesis and several human cancers. During vertebrate development, Oep/Cripto1/Frl1 has been shown to act as an essential coreceptor in the TGFβ/Nodal pathway, which is crucial for germ layer formation. Although studies in cell cultures suggest that Oep/Cripto1/Frl1 is also implicated in other pathways, in vivo it is solely regarded as a Nodal coreceptor. We have found that Rasl11b, a small GTPase belonging to a Ras subfamily of putative tumor suppressor genes, modulates Oep function in zebrafish independently of the Nodal pathway. rasl11b down regulation partially rescues endodermal and prechordal plate defects of zygotic oep−/− mutants (Zoep). Rasl11b inhibitory action was only observed in oep-deficient backgrounds, suggesting that normal oep expression prevents Rasl11b function. Surprisingly, rasl11b down regulation does not rescue mesendodermal defects in other Nodal pathway mutants, nor does it influence the phosphorylation state of the downstream effector Smad2. Thus, Rasl11b modifies the effect of Oep on mesendoderm development independently of the main known Oep output: the Nodal signaling pathway. This data suggests a new branch of Oep signaling that has implications for germ layer development, as well as for studies of Oep/Frl1/Cripto1 dysfunction, such as that found in tumors.
Introduction
Endoderm and mesoderm germ layer formation in vertebrate embryos requires the activity of the conserved TGFβ/Nodal signaling pathway [1], [2], [3], [4], [5], [6]. Extensive studies in mouse, Xenopus and zebrafish have allowed the establishment of the current TGFβ/Nodal pathway: Nodal signaling is activated by the interaction of Nodal ligands with activin (Alk4/ActR-IIB/Taram-A) receptors [7], [8], [9], [10], [11], [12] and is regulated extracellularly by an EGF-CFC coreceptor (see below) and by antagonists belonging to the TGFβ/Lefty family [13], [14], [15], [16]. Nodal signaling is then transmitted intracellularly by phosphorylation of Smad2, and Smad2-P translocation in the nucleus in turn induces the expression of conserved mesendodermal transcription factors (see [17], [18] for review).
Mesendoderm induction by Nodal signaling requires a conserved EGF-CFC factor encoded by the gene Cripto1 in mouse [19], [20], Frl1 (Fgf receptor ligand 1) in Xenopus [21] and one-eyed-pinhead (oep) in zebrafish [22], [23]. The function of Oep/Cripto1/Frl1 as a coreceptor of the Nodal pathway has been clearly demonstrated. In mouse, cripto mutants fail to form embryonic mesendoderm and resemble the mouse nodal mutant [10], [19], [24], [25]. Similarly, in zebrafish, embryos lacking both maternal and zygotic oep function (MZoep, obtained by crossing rescued oep−/− adult mutants [22]) display a phenotype similar to the zebrafish cyclops;squint (cyc;sqt) nodal double mutant [22]. They have an alterated antero-posterior axis and fail to develop endoderm, posterior mesoderm and prechordal plate (PP, a mesendodermal structure). More recently, biochemical analyses have shown that Oep/Cripto1/Frl1 is required for Nodal ligand binding to the Alk4/ActR-IIB/Taram-A receptor complex and subsequent Smad2 phosphorylation [10], [26], [27].
Other signaling pathways may also require the participation of EGF-CFC molecule either as a cofactor or as a ligand. For instance, in Xenopus and zebrafish, Frl1/Oep could function as antagonist of BMP [28] and more recently, in Xenopus, Frl1 has been implicated in the Wnt pathway and also been shown to be a coreceptor for Wnt11 [29]. Moreover, in cell culture, a soluble form of Cripto1 has been shown to bind the receptor Glypican1 and activate both the ras/raf/ERK/MAPK and PI3-K/AKT/GSK-3β intracellular signaling pathways, which are both well known for their involvement in oncogenic processes [30], [31], [32], [33]. Interestingly, Cripto1 has been found in the conditioned medium of several human carcinoma cell lines suggesting that a naturally soluble isoform of Cripto1 could act as a diffusible ligand [34], [35], [36]. Thus, several reports suggest a multifunctional capability, both as ligand and as coreceptor of Oep/Cripto1/Frl1. The role of Oep/Cripto1/Frl1 in pathways other than Nodal in vivo is still a matter of debate, certainly due in part to this complexity. The identification of additional elements which respond to Oep signaling stands to elucidate important signaling outputs of this protein.
Here we provide genetic data which supports that oep can influence mesendoderm formation independently of Nodal signaling. We report the analysis of Rasl11b and the first developmental function for a member of the Rerg/Rasl11-12 subfamily [37], [38], [39], [40], [41]. We find that rasl11b down regulation acts as a specific suppressor of the oep phenotype and partially rescues endoderm and prechordal plate formation in oep deficient embryos. Further, Rasl11b activation inhibits mesendoderm formation only when oep is down regulated, and not in wild-type embryos. Interestingly, rasl11b loss of function does not rescue mesendoderm formation in other mutants within the Nodal pathway and does not affect the activation level of Nodal signaling as assayed by phosphorylated Smad2 monitoring. This genetic interaction between oep and rasl11b strongly suggests that Oep can have a Nodal-Smad2 independent influence on mesendoderm formation. This study has implication for endoderm and mesoderm germ layer development but also potentially for analysis of Oep/Frl1/Cripto1 dysfunction, such as that found in tumors.
Results
Rasl11b is a cytosolic small GTPase
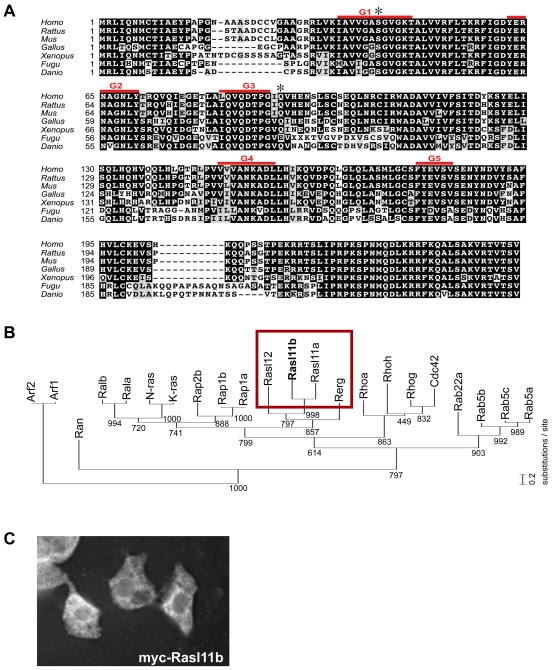
rasl11b cDNA has been isolated from a subtractive library between embryos expressing a constitutively activated Nodal signaling pathway and control embryos [42]. Activation of the Nodal pathway forces most blastomeres to adopt a mesendodermal fate [43], [44], so this strategy was designed to isolate either Nodal pathway downstream components or other genes specifically expressed in mesendodermal territories. This library previously allowed us to isolate the endodermal gene casanova [42] and to integrate it in the Nodal transcriptional cascade [45]. The Rasl11b protein is highly conserved among vertebrates (Fig. 1A), sharing on average 94% homology with its mammalian orthologues. Rasl11b, along with Rerg [39], Rasl11a [38] and Rasl12/Ris [41], constitute a poorly documented group of the Ras small GTPase family [40] (Fig. 1B). Like other members of this particular branch of GTPases, Rasl11b lacks any known carboxy-terminal recognition site (such as a CAAX box, A = aliphatic, X = terminal amino acid) for post-translational lipid modification [39], [40], [46], which is used in most Ras small GTPases to allow anchorage to the plasma membrane (Materials and Methods, and Fig. 1A). Consistent with this, embryos expressing myc-Rasl11b revealed no accumulation at the plasma membrane but rather a distribution in the cell cytoplasm (Fig. 1C).
Figure 1. Rasl11b, an atypical cytoplasmic Ras small GTPase, is strongly conserved in Vertebrates.
(A) Zebrafish rasl11b encodes a Ras-related small GTPase of 244 amino acids (accession number DQ983377) containing the 5 highly conserved domains (G1–G5, overlined in red) responsible for the guanine nucleotide-dependent molecular switches. Rasl11b has no obvious orthologues in Drosophila melanogaster or Caenorhabditis elegans, but is highly conserved among vertebrates. Note that, in contrast to most of the Ras small GTPases, Rasl11b lacks a COOH-terminal CAAX motif and any known recognition signal for C-terminal lipidation found in Ras proteins such as farnesylation or palmitoylation allowing membrane anchorage. The amino acid positions mutated to create the activated forms Rasl11bS42V and Rasl11bQ82L are indicated with stars. (B) Phylogenic analysis of zebrafish small GTPase proteins. The degree of relatedness is indicated by the length of the vertical lines. Numbers indicate bootstrap support for nodes. Red box: Rasl11b, Rasl11a, Rasl12 and Rerg constitute an uncharacterized branch of Ras proteins devoid of lipid modification signals. (C) Epifluorescent microscopy of zebrafish embryonic cells expressing myc-Rasl11b revealed by immunostaining.
Rasl11b has a maternal and a zygotic component
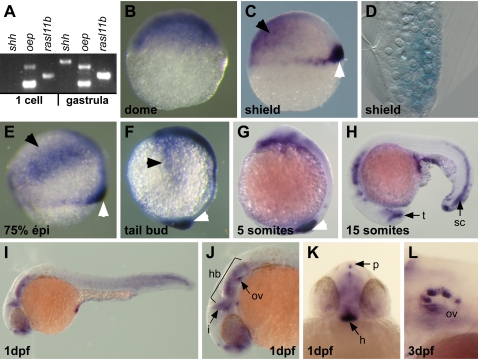
In zebrafish embryos, we found that rasl11b was first maternally expressed and transcripts were homogenously distributed in the blastoderm during the segmentation stage, as detected using RT–PCR and ISH (Figs. 2A–B). Then, throughout gastrulation rasl11b displayed a more restricted zygotic expression at the dorsal margin (Figs. 2C–F, white arrowheads) and in a bilateral domain at the animal pole corresponding to the border of the neural plate (Figs. 2C–F, black arrowheads). During the entire gastrulation period, rasl11b mRNA was detected in a dorso-ventral gradient at the dorsal margin and sagittal section revealed expression in both the hypoblast and epiblast layers at the margin (Fig. 2D). The rasl11b expression pattern at the margin coincided with presumptive mesendodermal territories including the dorsal organizer [47], [48]. Later, during somitogenesis and organogenesis, rasl11b mRNA was detected in the tailbud and tailtip, the posterior spinal chord and several head structures including forebrain, isthmic organizer, hindbrain, hypophysis and pineal gland (Figs. 2G–K). Finally, at 3 day post-fertilization (dpf) rasl11b expression was only observed in the otic vesicle (Fig. 2L). Thus, this dynamic expression pattern could allow rasl11b to function in very early development events as well as in late organogenesis processes. Most importantly, for our studies, the dorso-marginal expression suggests a putative function in endoderm and/or mesoderm germ layer formation.
Figure 2. rasl11b expression pattern during zebrafish embryogenesis.
(A) RT-PCR analysis showing that rasl11b has a maternal and a zygotic component. RNA extractions have been done before (1 cell stage) and after (gastrula) the midblastula transition, the time of activation of the zygotic transcription. The maternally and zygotically expressed oep gene and the strictly zygotic sonic hedgehog (shh) gene have been used as controls. (B) During the cleavage period (dome stage), rasl11b is ubiquitously expressed. (C) At the onset of gastrulation (shield stage), rasl11b is still detected at the animal pole (Black arrowhead) but is also expressed in a dorso-ventral gradient at the dorsal margin (white arrowhead). This marginal expression overlaps with the mesendodermal territory in zebrafish embryos. (D) Sagittal section. rasl11b transcript accumulates in both hypoblastic and epiblastic dorsal blastomeres. (E, F) rasl11b expression is maintained at the margin throughout gastrulation (white arrowheads). Gastrulae also expressed rasl11b mRNA in ectodermal precursors located at the lateral borders of the blastoderm (black arrowheads). (G–K) During somitogenesis and organogenesis, rasl11b is expressed in the tail tip and the spinal cord (sc), and in several head structures such as the hindbrain (hb), the isthmic organizer (i), the otic vesicle (ov), the pineal gland (p), the ventral hypothalamus (h) and the posterior boundary of the telencephalon (t). (J) lateral close up and (K) frontal view. (L) At 3 dpf, rasl11b is no longer expressed except in the otic vesicle (lateral close up).
rasl11b is expressed in mesendodermal cells and controlled by the Nodal pathway
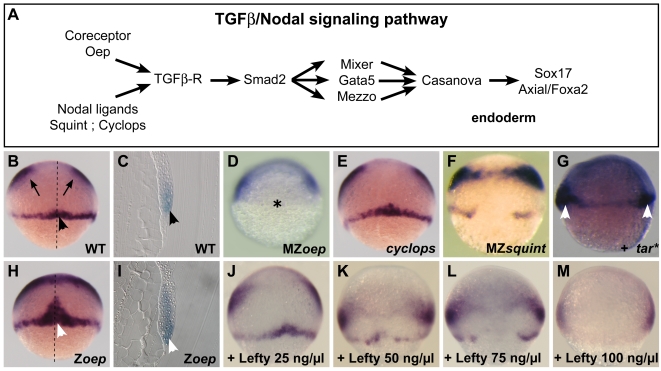
We then tested whether rasl11b putative mesendodermal expression could be modulated by Nodal pathway members (see pathway Fig. 3A). As expected, the rasl11b marginal expression domain (Figs. 3B,C) was completely lost in the Nodal signaling-deficient MZoep mutant (lacks both maternal and zygotic components of oep, Fig. 3D), which fails to form mesendoderm [22]. We further examined this dorsal expression domain in the nodal mutants cyc and MZsqt (lacks both maternal and zygotic components of sqt) that have intermediate Nodal signaling levels [5], [6], [49], [50]. rasl11b marginal expression was unaffected in cyc gastrulae (Fig. 3E) but was reduced in MZsqt gastrulae (Fig. 3F). Besides, no change in rasl11b expression was observed in the Nodal downstream transcriptional effector mutants or morphants, bonnie and clyde/mixer (bon) [51], faust/gata5 [52], mezzo [53], casanova/sox32 (cas) [42] and monorail/foxa2 [54] (data not shown). Finally, we tested whether activation of Nodal signaling could induce rasl11b expression in vivo. As expected, wild-type embryos injected with mRNA encoding the activated form of the Nodal receptor Tarama (Tar* [43]) displayed ectopic expression of rasl11b (Fig. 3G). Altogether these results show that rasl11b is expressed in mesendodermal dorsal territories and its expression requires the Nodal signaling pathway.
Figure 3. Opposite effects of Nodal and Oep on rasl11b expression.
(A) Scheme of the Nodal cascade. The Oep coreceptor is necessary for the binding of the Nodal ligand Cyc and Sqt to the TGFβ receptors (containing the type I receptor Taram-A, the zebrafish Alk4 orthologue) that in turn phosphorylate Smad2. Smad2-P is translocated to the nucleus and triggers the transcription of a first set of genes encoding the transcription factors, Mixer, Gata5 and Mezzo. This set is required for the expression of the Sox factor Casanova that in turn initiates the transcription of the endodermal markers sox17 and axial/foxa2. (B–M, Dorsal view of gastrulae save C, I, sagittal sections and G, lateral view) (B, C) In wild-type (WT) embryos rasl11b is expressed at the animal pole (arrows) and at the dorsal margin (black arrowheads). (D) This marginal expression is lost in MZoep mutants (star), devoid of maternal and zygotic oep, consequently devoid of Nodal signaling, and so, unable to form most of the mesendoderm. (E, F) rasl11b dorso-marginal expression is normal in the cyclops Nodal mutant and largely reduced in the MZsquint Nodal mutant. (G) Activation of the Nodal signal by injection of a constitutively activated form of the Nodal type I receptor Taram-A (tar*) leads to a duplication of the rasl11b marginal expression domain (likely by inducing a second organizer, white arrowheads) (H, I) In Zoep mutants, devoid of zygotic oep, this mesendodermal expression domain is extended (white arrowheads). (J–M) A large series of embryos expressing different levels of Nodal signal was generated by injecting between blastula cells increasing doses of the recombinant Lefty protein, a Nodal pathway extracellular inhibitor. Here, only four representative doses are displayed. A progressive decrease of rasl11b marginal expression but no expansion was observed, even with concentrations of Lefty able to mimic a Zoep-like phenotype.
oep has the opposite influence from nodal on rasl11b dorsal expression
During epistatic analyses, we noticed that zygotic oep (Zoep) mutant embryos (oep−/− embryos only containing maternally-expressed Oep) displayed a dramatic increase of rasl11b expression in the dorsal domain (Fig. 3H, white arrowheads). This increase corresponds to an expansion in the mesodermal layer in the antero-posterior (A-P) axis (compare sagittal sections, Figs. 3C,I). This result was surprising since Zoep phenotype resembles to cyc and MZsqt phenotype's and is supposed to be due to a reduction of Nodal signaling. This suggested either rasl11b expression in mesoderm could be restricted by Oep in a manner different from Nodals, or Zoep embryos could have a particular Nodal signal level, different than in Nodal mutants, that had the opposite impact on rasl11b expression. To discriminate between these two possibilities and precisely investigate how Nodal signaling might affect rasl11b expression, we generated a large panel of embryos expressing different levels of Nodal signal by injecting different concentrations of the Nodal extracellular inhibitor Lefty [13], [14], [15], [16]. Interestingly, we observed that the inhibition of Nodal signaling with increasing doses of Lefty leads to a progressive reduction of rasl11b marginal expression (Figs. 3J–M, here only four representative doses are displayed). Even with Lefty doses able to mimic the oep phenotype at 24 hour post-fertilization (hpf), we never observed any overexpression of rasl11b. This data indicates that the expansion of rasl11b dorsal expression is a specificity of the Zoep mutant and is not due to a reduction of Nodal signaling but to the loss of function of oep and suggests that oep may influence mesendoderm formation independently of Nodal signaling.
Rasl11b zygotic component has a subtle influence on antero-posterior axis formation
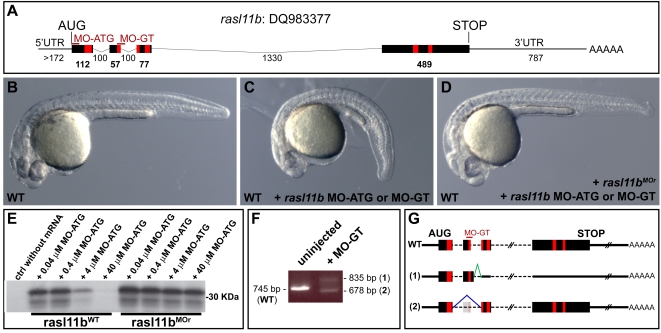
Rasl11b function was next investigated using morpholino (MO) knock down and mRNA misexpression experiments. MOs directed against a gene's first codon (MO-ATG) blocks translation of both maternal and zygotic expression, while MOs directed against a splice donor site (MO-GT) blocks pre-mRNA maturation, thus blocking only zygotic expression (rasl11b maternal mRNAs are not targeted, Fig. 4A) [55]. Wild-type embryos injected with either type of rasl11b morpholino displayed a curly down phenotype by 24 hpf but no missing structures (Figs. 4B,C). This phenotype was specific, as it was rescued by injecting rasl11b mRNA resistant to MO knock down (Materials and Methods, Fig. 4D), while rasl11b MO potency was confirmed using translational and RT-PCR assays and the two spliced variants produced after MO-GT injection were shown to be null as they both were unable to rescue this curly down phenotype (Figs. 4E–G and data not shown). Zygotic rasl11b is continuously expressed in the dorsal margin, the tail bud and tail tip throughout gastrulation and somitogenesis (Figs. 2C–H, white arrowheads), and therefore, could actually be involved in the proper organization of the trunk and tail. However, the absence of loss of structures in the rasl11b morphant embryos rather suggests a subtle role in A-P axis formation.
Figure 4. rasl11b knock down induces a specific curly down phenotype in zebrafish.
(A) Scheme of the rasl11b transcript. The Morpholino targeted sequences are represented by thin red lines. The red boxes correspond to the G1 to G5 domains described in Fig. 1. (B–D) Lateral view of 1 dpf embryos. Both rasl11b MO-ATG and rasl11b MO-GT induce a curly tail down phenotype when injected in WT. This phenotype can be rescued by the coinjection of rasl11b mRNA resistant to MO knock down, rasl11bMOr (D). (E) In vitro translational assay showing the efficient knock down effect of the rasl11b MO-ATG on wild-type rasl11b mRNA compared to the MO resistant rasl11bMOr mRNA. (F,G) rasl11b splice-blocking morpholino (rasl11b MO-GT) knocks down endogenous rasl11b mRNA. RT-PCR analysis detected two splice variants that were cloned and sequenced. Sequence comparison revealed that the splice variant (1) resulted from aberrant splicing to an upstream cryptic slice donor site that is present in intron 2, resulting in an early frame shift. The splice variant (2) only missed exon 2 but is not functional (data not shown).
rasl11b knock down partially suppresses oep phenotype
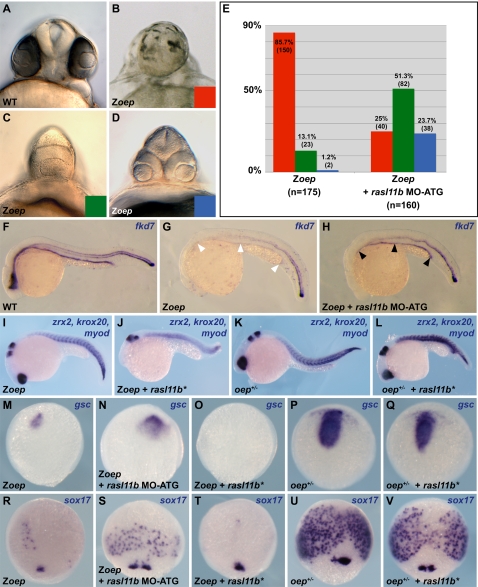
rasl11b overexpression in the Zoep mutant revealed a particular genetic interaction between oep and rasl11b. We next analyzed this interaction by blocking Rasl11b function in Zoep mutant (As for the epistatic analysis described above, we used the oeptz57 null allele [23]). Zoep mutants display strong gut and forebrain defects at 24 hpf [56], [57]. However, as mentioned above oep is maternally expressed, thus depending on the oocyte's accumulation of maternal oep mRNA and protein, Zoep larvae have variable head phenotypes. These phenotypes can be classified in three categories: 1) absence of forebrain and lens structures, 2) only one lens and 3) two fused lenses (Fig 5B–D). Strikingly, eliminating maternal and zygotic components of rasl11b using MO-ATG partially rescued anterior defects found in Zoep mutants. Indeed, whereas most of the control embryos did not develop any lens nor forebrain (85.7%), most of Zoep;rasl11b MO-ATG larvae displayed a better forebrain development and had one (51.3%; Fig. 5E) or two lens (23.7%; Fig. 5E). In addition to the head structure improvement, the gut phenotype in Zoep mutants was also partially rescued by knock down of rasl11b (Figs. 5F–H). This suppression of phenotype was also observed in oep morphants injected with rasl11b MO-ATG, dismissing any allele-specific interaction between rasl11b and oep (data not shown).
Figure 5. Rasl11b inhibits endoderm and PP formation in an Oep deficient background.
(A–D) Frontal views of 1 dpf wild-type embryo and Zoep embryos. Depending on the quantity of oep mRNA accumulated during oogenesis, Zoep embryos display different levels of anterior structure development: from absence of lens and forebrain (red class, B), via a cyclopia phenotype (green class, C) to two separate retinae (blue class, D, very rare). In each clutch, these categories can have slightly different proportions depending on the female, but a large majority of Zoep embryos do not even develop one retina. Whatever females (n>10) were used for the rasl11b knock down experiments, a drastic rescue (>70%) of the forebrain formation was always observed, and a large category of embryos with two retinae appeared. (E) Cumulated numbers for each class from 5 independent rasl11b MO-ATG knock down experiments. (F–H) forkhead7 (fkd7) expression pattern in 1 dpf embryos. Gut defects (white arrowheads) are also rescued (black arrowheads) in Zoep embryos injected by rasl11b MO-ATG. (I–L) 1 dpf embryos lateral views. Rasl11b constitutively activated forms (Rasl11b*) prevent head formation and disturb the antero-posterior axis formation in Zoep and oep +/− but not in wild-type embryos (not shown). zrx2 is expressed in the retina, krox20 in rhombomeres 3 and 5, myod is expressed in the somitic mesoderm. (M–V) dorsal view of late gastrulae. rasl11b knock down rescues expression of the prechordal plate marker goosecoid (gsc) and the endodermal marker sox17 in Zoep whereas Rasl11b* reduces their expression in Zoep and oep +/− embryos.
The Zoep phenotype observed at 24 hpf has been shown to be due to a dramatic reduction of the prechordal plate (PP) and endoderm formation during gastrulation [56], [57]. We thus analyzed PP formation and endodermal cell number in rasl11b MO-ATG injected Zoep gastrulae. In all these gastrulae, the PP was extended (Figs. 5M,N), while the endodermal cell number was up to 10 times as high as in the controls (Figs. 5R,S). Moreover, this rescue was inhibited by the co-injection of the MO-resistant mRNA of rasl11b confirming the specificity of our MO (Fig. 6A and data not shown). Thus, rasl11b MO-ATG mediated knock down suppresses a large fraction of Zoep early defects and hence demonstrates that Rasl11b function is partially responsible for Zoep phenotype. Interestingly, in contrast to the rescue observed with the rasl11b MO-ATG, we never observed any rescue in rasl11b MO-GT injected Zoep embryos. These results point out an important role of rasl11b maternal expression and suggest that the increase of rasl11b expression observed in Zoep mutants (Fig. 3H,I) is unlikely a cause of Zoep endoderm and PP defects. Besides, rasl11b inhibition did not rescue any defects of MZoep mutant, showing that the oep maternal expression is required for rasl11b knock down to be effective. It is most likely that the total absence of Nodal signaling pathway in MZoep mutant prevents any formation of dorsal mesendoderm and consequently impedes rasl11b knock down compensatory action. Finally, these results show that rasl11b is a negative modifier that can modulate mesendoderm formation in Zoep mutant.
Figure 6. rasl11b interacts specifically with oep but does not affect the Nodal/Smad2 transduction pathway.
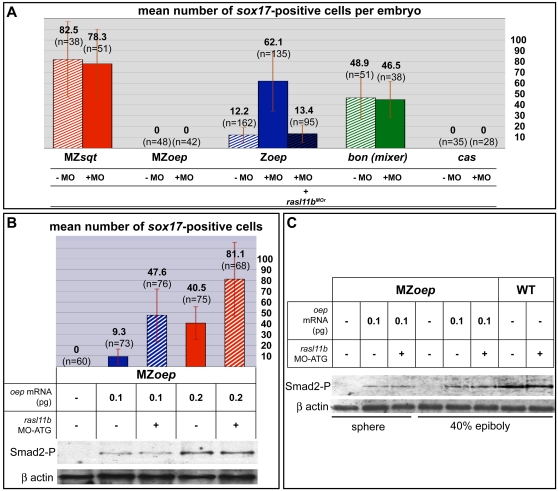
(A) The MZsqt, MZoep, Zoep, bon and cas mutants have a clear reduction of endodermal cells and so were used to quantify the putative impact of rasl11b knock down at different steps/levels of the nodal pathway. The Zoep mutant was the only one rescued by the rasl11b MO-ATG injection. This rescue was abolished by co-injection of rasl11b MO-resistant mRNA (rasl11bMOr). Error bars indicate standard deviation. (B, C) It is impossible to generate clutches of 100% Zoep embryos, and because one embryo cannot give enough material for both immunoblot and genotyping experiments, 100% Zoep-like mutant clutches were produced by injecting clutches of 100% MZoep eggs with low doses of wild-type oep mRNA. Half of them were then co-injected with rasl11b MO-ATG. Each batch was split in two, one used for phosphorylated Smad2 (Smad2-P) level analysis, the second for endodermal cell number count (assayed by sox17 in situ hybridization). Proteins were detected by western blotting using a Smad2-P antibody. Note that increasing doses of wild-type oep mRNA were correlated with an increase of Smad2-P and sox17 endodermal cell number, whereas co-injection with rasl11b MO-ATG increased endodermal cell number without generating more Smad2-P (Error bars indicate standard deviation). The same experiment was done at three pregastrula stages: dome (B), sphere and 40% epiboly (C).
Rasl11b activation inhibits endodermal and prechordal plate development in Oep deficient embryos
The ability of rasl11b knock down to ameliorate Zoep phenotypes suggested that rasl11b acts as an inhibitor of mesendoderm formation. To test this hypothesis we created two constitutively active Rasl11b molecules by introducing activating mutations based on the tumor-derived RAS mutations G12V and Q61L [58] (Materials and Methods, and Fig. 1A), thereafter both named Rasl11b* because of their similar influence. Rasl11b* mRNAs were injected in MZoep, Zoep, wild-type and in oep+/− embryos (which are phenotypically wild-type). Injection of Rasl11b* had minor effects on wild-type embryos, only occasionally reducing the tail tip (data not shown). It had no effects on MZoep embryos (data not shown), which do not form any endodermal precursors nor PP. However, Rasl11b* strongly exacerbated head/forebrain formation defects in over 80% of the injected Zoep (n = 65) and oep+/− (n = 88) embryos (Figs. 5I–L). While analysis of early markers showed Rasl11b* had no effect in wild-type gastrulae (data not shown), Rasl11b* inhibited the remaining expression of PP and endodermal cell markers in Zoep gastrulae (Figs. 5O,T), and reduced the PP size and endodermal cell number in oep +/− gastrulae (Figs. 5P,Q,U,V). Hence, it appears that Rasl11b activity inhibits endoderm and PP formation, but only when oep expression is decreased. This data suggests that Rasl11b is an inhibitor of mesendoderm formation antagonized or counteracted by Oep.
Rasl11b knock down does not rescue other Nodal pathway mutants
The oep phenotype is generally believed to be only a consequence of a Nodal signaling decrease, so a simple hypothesis was that Rasl11b acts as an inhibitor of the Nodal pathway. If this were the case, one might predict that rasl11b knock down would rescue mesendodermal defects in other Nodal pathway mutants. To investigate this, the effect of rasl11b knock down was examined in cyc, MZsqt, bon and cas mutants, which are characterized by a reduction in Nodal signaling and display severe or total absence of PP or endoderm [6], [42], [49], [51]. However, in contrast to Zoep mutant, rasl11b MO-ATG injection had no effect on any of these mutants. We examined cyclopia, cardia bifida, endodermal cell number and prechordal plate by morphological studies and in situ hybridization, yet no rescue was observed (Fig. 6A and data not shown). Similarly, Rasl11b* mRNA injections did not exacerbate defects in cyc and MZsqt mutants (data not shown). These results suggest that Rasl11b does not act as an inhibitor of Nodal pathway and that the rescue of Zoep mutants by rasl11b knock down is not due to a simple compensation for a decrease in Nodal signaling but highlight the specificity of rasl11b knock down suppressor effect on the Zoep phenotype .
Rasl11b does not act on Smad2 phosphorylation level
It was still possible that Rasl11b modulates Nodal signaling by acting specifically via Oep. To investigate this, we monitored phosphorylation of the Nodal intracellular signal transducer Smad2 in oep mutants with a normal or down regulated expression of rasl11b. To detect subtle variations of Smad2 phosphorylation (Smad2-P) level, we wanted to monitor it in each Zoep or rasl11b MO-ATG; Zoep embryo. However, a zebrafish embryo at blastula or gastrula stage does not contain enough material for both a Smad2 phosphorylation assay and DNA extraction to determine its Zoep (oep −/−) or oep −/+ or oep+/+ genotype.
To bypass this limitation we performed our experiments on groups of 100% Zoep-like embryos. To generate these 100% homogenous Zoep-like groups, we produced clutches of 100% MZoep embryos (by crossing oep −/− fish, see [22] for generation of viable and fertile oep −/− adults) in which we artificially reintroduced an oep maternal component by injecting at one cell stage a small quantity of wild-type oep mRNA (0.1pg) mimicking perfectly the Zoep phenotype (see Materials and Methods). We then compared the number of endodermal cells and Smad2-P level in Zoep-like embryos and Zoep-like embryos injected with rasl11b MO-ATG. Whereas rasl11b knock down improved the Zoep-like embryo phenotype (similarly to what was observed for Zoep embryos), as assayed by sox17 positive cell number, this had no effect on Smad2 phosphorylation level (Fig. 6B, compare 2nd and 3rd lanes). To account for possible transient or stage-specific effects on Smad2-P, we repeated the Smad2-P detection at different pre-gastrulation stages (Fig. 6C and data not shown) but we never observed any variation of Smad2-P level. It was still possible that the rescue was due to a subtle effect on Smad2, too weak to be detected. To address this possibility, we injected MZoep eggs with a slightly higher dose of wild-type oep mRNA (0.2 pg) compared to the dose used to generate Zoep-like embryos (0.1 pg). Whereas the number of sox17 positive cell was smaller in these embryos than in rasl11b MO-ATG; Zoep-like embryos (mean of 40 vs 47 cells, Fig. 6B), we observed a clear increase of the corresponding Smad2-P level (Fig. 6B, compare 3rd and 4th lanes). Moreover, consistently with this observation, the inhibition of rasl11b in MZoep embryos injected with this higher dose of oep mRNA further improved their endodermal phenotype but without affecting the Smad2-P level (Fig. 6B, see last lane).
Finally, confirming this data, we never observed during all our experiments any transcript level increase of the intermediate Nodal pathway members downstream of Smad2, i.e. mixer, gata5 and mezzo, in Zoep or Zoep-like embryos injected with rasl11b MO-ATG. Altogether, these results demonstrate that rasl11b does not act upstream of Smad2 and confirm that Rasl11b does not influence the activation level of the Nodal pathway but rather acts on mesendoderm formation in a Nodal-independent way.
Discussion
In conclusion, we report here the identification of Rasl11b, a new small GTPase belonging to a poorly studied subgroup of atypical cytoplasmic Ras-related GTPases. In vivo functional analyses of Rasl11b revealed the first developmental role for a member of this Ras subgroup. rasl11b loss-of-function acts as a specific suppressor of oep phenotype showing that Rasl11b can function as a negative modulator of endoderm and prechordal plate formation, and suggesting a Nodal-independent role of Oep on mesendoderm formation.
Rasl11b belongs to a poorly understood group of cytosolic Ras small GTPase
The Ras family of small GTPases is subdivided into Rho, Rab, Arf and Ras subfamilies. In humans, the Ras subfamily is composed of 35 members classified into 12 structural or functional subgroups (for review see [40]). Rasl11b, together with Rasl11a, Ris/Rasl12 and Rerg, form one of these subgroups. Most Ras subfamily proteins are membrane-localized, due to sequence-specific lipid modifications. These lipid modifications are in turn thought to be responsible for distinct membrane localization characteristics [59], [60] and signaling properties [61] of Ras GTPases. Interestingly, the Rasl11b subgroup is devoid of all standard lipid membrane localization signals. Consistent with this, we have shown that zebrafish Rasl11b localization, like Rerg, is cytosolic. It is possible that this difference in subcellular localization reflects a new type of function for the Rasl11b class of small GTPases. This report is the first to describe a role for a member of this cytosolic Ras GTPase subgroup, Rasl11b, in development. Other studies have implicated a role for Rasl11b subgroup members in oncogenesis. For instance, rasl11a mRNA is less abundant in some prostate cancers [38], ris/rasl12 tumor suppressor function in breast cancer is controversial [37], [62], and rerg expression inhibits tumor formation in nude mice and is decreased or lost in primary human breast tumors [39]. Thus, clarifying the biological role of this subgroup of small GTPase necessitates further studies.
Rasl11b is an antagonist of Oep function
We show here that inhibition of rasl11b efficiently rescues formation of endoderm, PP and their derivatives in Zoep embryos. This rescue is rather dramatic and demonstrates a strong genetic interaction between rasl11b and oep but it is also remarkable because mutants or loss-of-functions with suppressor abilities have not often been described in zebrafish [63], [64]. One notable feature of rasl11b is that, on its own, rasl11b loss-of-function appears to have little effect on zebrafish normal development. We found that knock down of rasl11b in wild-type embryos caused no major defects save a curly down phenotype. Similarly, rasl11b knock down does not have a general ability to rescue mesendodermal defects, as cyc, sqt, bon and cas mutant phenotypes were not affected by rasl11b knock down. In fact, the effects of Rasl11b knock down and activation appear to be highly specific to animals which have a reduced level or function of Oep. rasl11b loss-of-function only suppresses Zoep and Rasl11b* enhances Zoep phenotype and aggravates oep +/− embryo phenotype (normally wild-type). It is therefore likely that oep (but not Nodal) acts to prevent the mesendoderm-inhibiting function of rasl11b, whether directly or in parallel. In this model, rasl11b role would already be inhibited in a wild-type context, which is consistent with our finding that rasl11b knock down has weak phenotype in wild-type embryos. In conclusion, perhaps Rasl11b modifies the output of Oep influence, but only in a context where oep expression is decreased. Alternatively, Rasl11b could act in a parallel pathway whose activity is only revealed when oep expression is compromised.
Rasl11b does not act on the currently known Nodal pathway
It is well established that Oep/Frl1/Cripto1 is crucial for Nodal ligand–receptor association and the cascade leading to mesendoderm formation in vertebrates. Thus, our hypothesis was that Rasl11b acts as an inhibitor of the TGFβ/Nodal pathway and hence we explored very carefully the different possibilities. First, if Rasl11b acted upstream or at the level of the Oep/Cyc/Sqt/TGFβ receptor complex, rasl11b knock down would have led to an upregulation of Nodal signaling and so to an increase of the Smad2-P level and mixer, mezzo and gata5 mRNAs expression. Second, if Rasl11b acted downstream of the Oep/Cyc/Sqt/TGFβ receptor complex, we would have expected to observe a rescue not only of Zoep but also at least of the cyc and/or sqt phenotypes. As no variation of Smad2-P and its downstream targets was observed, and no other Nodal pathway mutant phenotype (cyc, sqt, mixer, gata5, cas, foxa2) is suppressed by rasl11b loss-of-function, it is likely that Rasl11b does not act on the Nodal pathway in its currently known organization and composition.
Rasl11b suggests the existence of a Nodal-independent Oep influence on mesendoderm development
So far, oep/cripto1 has been regarded solely as a component of the Nodal pathway during development. However, Oep/Frl1/Cripto1 and Nodals may have independent functional roles: Nodal inhibits Bone Morphogenetic Protein signaling independently of Cripto1, whereas Oep can affect cell motility independently of Nodals [10], [65]. Here we describe two sets of evidence suggesting that Oep can have a Nodal-independent function in mesendoderm development. First, the large rasl11b expression domain in Zoep gastrulae, potentially a consequence of the expansion of dorsal mesodermal region previously described in this mutant [56], [57], is not due to a weakening of the Nodal pathway since a reduction in the Nodal ligands (Cyc and Sqt) or an interference with Lefty does not lead to such an expansion. Second, Rasl11b knock down and activation dramatically affect oep−/− and oep+/− phenotypes but have no effect at all on the Nodal pathway, strongly suggesting for Oep a role of its own in endoderm and PP development. This data yields the first molecular cue for a Nodal-independent role for Oep on mesendoderm development. Such an oep-rasl11b interaction could be used, for example, to modulate the mesendodermal specification at a very precise cellular level depending on the local concentration of Oep.
In addition to its role in the Nodal pathway, Oep could influence mesendoderm formation through other signaling pathways. For example, in Xenopus, its orthologue Frl1 has been implicated in the Wnt pathway [29]. Moreover, its mammalian orthologue Cripto1 has also been implicated in oncogenic signaling pathways. Cripto1 is well known to produce hyperplasias and adenocarcinomas in the mammary gland [66]. cripto1 is also dysregulated in a majority of human carcinomas [66]. In cell culture, Cripto1 binds Glypican 1 and activates the ras/raf/ERK/MAPK and PI3-K/AKT/GSK-3b intracellular signaling pathways [30], [31], [32], both of which are involved in regulating cell proliferation and survival. The precise mechanism by which Cripto1 activity drives oncogenesis remains unclear. However, Rasl11b, a member of a putative tumor suppressor subgroup and only potent when Oep function is compromised, may represent an undescribed link between Cripto1 and the oncogenic process. This study prompts an investigation of a putative role for Rasl11b in oncogenic processes.
Finally, how Oep and Rasl11b interact at the molecular level remain to be established. Oep is an extracellular factor and Rasl11b is cytosolic, making a direct interaction in mesendoderm formation unlikely, except during Oep synthesis and trafficking toward the cytoplasmic membrane. Further studies will be required to identify the intermediate actors and signaling pathways that relate the role of Oep and Rasl11b in the embryo. Here we provide strong evidence that this interaction does not involve Nodal, the main inductive pathway involved in mesendoderm formation, suggesting that other pathways, yet to be identified, are at stake.
Materials and Methods
Zebrafish strains
The following mutant alleles were used: oeptz57, sqtcz35, cycm294, bonm425, faus26, casta56, moltv53a. MZoep fertile adult fish were generated as previously described [22]. Crossed together, these fish laid clutches containing 100% of MZoep embryos. Clutches containing 25% of Zoep (oep −/−) embryos were obtained by crossing oeptz57 heterozygous. Zoep-like embryos were generated by microinjection of wild-type oep mRNA (0.1pg or 0.2pg) into MZoep eggs.
Isolation of rasl11b, plasmid construction and directed mutagenesis
rasl11b cDNA in pSport was isolated from the same substracted library (embryos with over-activated Nodal signaling versus controls) that previously allowed us to clone the casanova gene [42], [67]. The rasl11b sequence is deposited at GenBank under the accession number DQ983377. The coding sequence of rasl11b was subcloned in the pCS2+ vector for mRNA synthesis. The Morpholino resistant form rasl11bMOr and the two constitutively activated forms rasl11bS32V, rasl11bQ82L were generated as described in the exsite mutagenese kit (Stratagen, La Jolla, CA).
Sequence analyses
Rasl11b protein analysis was performed with Prosite database http://www.expasy.org and Conserved Domain Database http://www.ncbi.nlm.nih.gov .
Sequence Accession numbers, alignments and phylogenic tree
Peptide alignments were performed using the ClustalX interface. The phylogenic tree was constructed using the neighbor-joining method. The following peptide sequences were used: Dr Rasl11b, ABI97979; Gg Rasl11b, ENSGALP00000022564; Hs RASL11B, NP_076429; Mm RASL11B, AAH83068; Rn Rasl11b, AAH83755; Tr Rasl11b, NEWSINFRUP00000130569; Xl Rasl11b, DAA02255; Dr Rasl11a, ABK96901; Dr Arf1, AAS92646; Dr Arf2, AAH66632; Dr Cdc42, AAQ97755; Dr K-ras, ABF46832; Dr N-ras, AAB40625; Dr Rab5a, AAH49057; Dr Rab5b, AAH66634; Dr Rab5c, NP_958909; Dr Rab22a, NP_991282; Dr RalA, AAH53216; Dr RalB, AAH78184; Dr Ran, AAB97093; Dr Rap1a, AAH71360; Dr Rap1b, AAQ97994; Dr Rap2b, AAH54999; Dr Rasl12, ENSDARP00000022937; Dr Rerg, XP_701315; Dr Rhoa, NP_997914; Dr Rhog, NP_956334; Dr Rhoh, AAX20135. Dr, Danio rerio; Gg, Gallus gallus; Hs, Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus; Tr, Takifugu rubripes; Xl, Xenopus laevis.
Whole-mount in situ hybridization and immunostaining
In situ hybridizations and immunohistochemistry were performed as previously described [68]. For sectioning, embryos were embedded in resin (JB4, Polysciences). For immunohistochemistry, polyclonal antibody against Myc-tag (Anti-Myc Tag, Upstate Biotechnology) was used at 1:500.
RT-PCR
Total RNA was obtained from staged embryos using TRIzol Reagent (Invitrogen). 1 µg of total RNA was then used to produce cDNA with the StrataScript® First Strand cDNA Synthesis Kit (Stratagene, La Jolla , CA). PCR was performed using the following primers:
rasl11b-Forward 5′-ATGcgtctgatccagaacatg-3′;
rasl11b-Reverse 5′-gtcacactgaagtgacggtgc-3′;
oep-Forward 5′-gtgaaagttggggtttctgg-3′;
oep-reverse 5′-ggacattcgactagcgagaact-3′;
shh-forward 5′-gactgggtctattacgagtccaaa-3′;
shh-reverse 5′-gcctgagttcacgcgagaataaat-3′.
Morpholino, mRNA and lefty protein microinjection
pCS2+ plasmids containing rasl11b, rasl11bQ82L, rasl11bS32V, rasl11bMOr, myc-rasl11b, oep, Tar* were linearized with NotI and sense RNA transcribed with SP6 RNA polymerase using the mMESSAGE mMACHINE kit (Ambion, Austin, TX). The sequences of the morpholino oligos (Gene Tools, Philomath, OR) used in this paper are: rasl11b-MO-ATG 5′-TTGACATGTTCTGGATCAGACGCAT-3′ and rasl11b-MO-GT 5′-AAACTTACCAACGTTTCTCTCGTAG-3′. oep-MO was previously described [69], [70]. 5 silent mismatches were introduced in the MO-ATG targeted sequence to create rasl11bMOr: 5′-ATGCG(T>A)CT(G>A)AT(C>A)CA(G>A)AA(C>T)ATGTCAA-3′. Lefty injections were performed by injecting Lefty protein solutions at 12.5; 25; 50; 75; 100 and 200 ng/μl in PBS+BSA 0.1% (Recombinant-mLefty1, R&D systems, Minneapolis, MN) between blastomeres at hight, sphere or dome stages.
Smad2 phosphorylation analysis
Analyses were performed following the standard method with a chemiluminescent detection kit (Amersham Biosciences). Embryos devoid of vitellus were resuspended in a Laemmli/RIPA solution (RIPA: 20 mM Tris pH 8 buffer containing 50 mM NaCl, 50 mM EDTA, 100 mM NaF, 1 mM orthovanadate (NaVO4), 25 mM b-glycerophosphate, 1% NP-40 and a protease inhibitor cocktail (Roche, Nutley, NJ)). Total proteins were separated by 10% polyacrylamide gels and transferred to Hybond ECL membranes (Amesham Biosciences) by electroblotting. Polyclonal antibody against Smad2P (anti-phospho-smad2 antibody, Chemicon International) was used at 1∶500.
Morpholino efficiency and specificity assay
The rasl11b MO-ATG and rasl11b MO-GT efficiencies were assayed by in vitro translation and RT-PCR analyses respectively. For in vitro translation, capped rasl11b and rasl11bMOr RNA (20 ng/µl) were translated in vitro in the presence of increasing concentration of MO-ATG (0.04 to 40 µM) using [35S] methionine in Rabbit reticulocyte lysate (Promega). RT-PCR was performed as described above on control embryos and MO-GT injected embryos. PCR products were analyzed by electrophoresis and purified for sequencing.
Acknowledgments
We are very grateful to Jennifer Berman, Marnie Halpern, Wilfredo Marin and Nicolas David for critical reading of the manuscript and fruitful discussion. We thank Firas Bouallague for excellent fish care.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: GP was supported by a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche and La ligue Nationale Contre le Cancer. FR was supported by grants ZF-MODELS (EU FP6 program) and Association pour le Recherche contre le Cancer.
References
- 1.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- 3.Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- 4.Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev Biol. 1998;199:261–272. doi: 10.1006/dbio.1998.8935. [DOI] [PubMed] [Google Scholar]
- 5.Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, et al. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 6.Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 7.Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- 8.Willis SA, Zimmerman CM, Li LI, Mathews LS. Formation and activation by phosphorylation of activin receptor complexes. Mol Endocrinol. 1996;10:367–379. doi: 10.1210/mend.10.4.8721982. [DOI] [PubMed] [Google Scholar]
- 9.Gray PC, Greenwald J, Blount AL, Kunitake KS, Donaldson CJ, et al. Identification of a binding site on the type II activin receptor for activin and inhibin. J Biol Chem. 2000;275:3206–3212. doi: 10.1074/jbc.275.5.3206. [DOI] [PubMed] [Google Scholar]
- 10.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 11.Renucci A, Lemarchandel V, Rosa F. An activated form of type I serine/threonine kinase receptor TARAM-A reveals a specific signalling pathway involved in fish head organiser formation. Development. 1996;122:3735–3743. doi: 10.1242/dev.122.12.3735. [DOI] [PubMed] [Google Scholar]
- 12.Aoki TO, Mathieu J, Saint-Etienne L, Rebagliati MR, Peyrieras N, et al. Regulation of nodal signalling and mesendoderm formation by TARAM-A, a TGFbeta-related type I receptor. Dev Biol. 2002;241:273–288. doi: 10.1006/dbio.2001.0510. [DOI] [PubMed] [Google Scholar]
- 13.Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- 14.Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- 15.Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, et al. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AM, Thisse B, Thisse C, Wright CV. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in xenopus. Development. 2000;127:1049–1061. doi: 10.1242/dev.127.5.1049. [DOI] [PubMed] [Google Scholar]
- 17.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 18.Tian T, Meng AM. Nodal signals pattern vertebrate embryos. Cell Mol Life Sci. 2006;63:672–685. doi: 10.1007/s00018-005-5503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Yang L, Yan YT, Chen A, Desai N, et al. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 20.Shen MM, Wang H, Leder P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development. 1997;124:429–442. doi: 10.1242/dev.124.2.429. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita N, Minshull J, Kirschner MW. The identification of two novel ligands of the FGF receptor by a yeast screening method and their activity in Xenopus development. Cell. 1995;83:621–630. doi: 10.1016/0092-8674(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 22.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 25.Rosa FM. Cripto, a multifunctional partner in signaling: molecular forms and activities. Sci STKE. 2002;2002:PE47. doi: 10.1126/stke.2002.158.pe47. [DOI] [PubMed] [Google Scholar]
- 26.Yan YT, Liu JJ, Luo Y, E C, Haltiwanger RS, et al. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22:4439–4449. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, et al. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiecker C, Muller F, Wu W, Glinka A, Strahle U, et al. Phenotypic effects in Xenopus and zebrafish suggest that one-eyed pinhead functions as antagonist of BMP signalling. Mech Dev. 2000;94:37–46. doi: 10.1016/s0925-4773(00)00329-4. [DOI] [PubMed] [Google Scholar]
- 29.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.De Santis ML, Kannan S, Smith GH, Seno M, Bianco C, et al. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21ras-and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–1266. [PubMed] [Google Scholar]
- 31.Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, et al. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- 32.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, et al. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannan S, De Santis M, Lohmeyer M, Riese DJ, 2nd, Smith GH, et al. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J Biol Chem. 1997;272:3330–3335. doi: 10.1074/jbc.272.6.3330. [DOI] [PubMed] [Google Scholar]
- 34.Brandt R, Normanno N, Gullick WJ, Lin JH, Harkins R, et al. Identification and biological characterization of an epidermal growth factor-related protein: cripto-1. J Biol Chem. 1994;269:17320–17328. [PubMed] [Google Scholar]
- 35.Minchiotti G, Parisi S, Liguori G, Signore M, Lania G, et al. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech Dev. 2000;90:133–142. doi: 10.1016/s0925-4773(99)00235-x. [DOI] [PubMed] [Google Scholar]
- 36.Normanno N, De Luca A, Bianco C, Maiello MR, Carriero MV, et al. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J Cell Physiol. 2004;198:31–39. doi: 10.1002/jcp.10375. [DOI] [PubMed] [Google Scholar]
- 37.Silva J, Silva JM, Barradas M, Garcia JM, Dominguez G, et al. Analysis of the candidate tumor suppressor Ris-1 in primary human breast carcinomas. Mutat Res. 2006;594:78–85. doi: 10.1016/j.mrfmmm.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Louro R, Nakaya HI, Paquola AC, Martins EA, da Silva AM, et al. RASL11A, member of a novel small monomeric GTPase gene family, is down-regulated in prostate tumors. Biochem Biophys Res Commun. 2004;316:618–627. doi: 10.1016/j.bbrc.2004.02.091. [DOI] [PubMed] [Google Scholar]
- 39.Finlin BS, Gau CL, Murphy GA, Shao H, Kimel T, et al. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J Biol Chem. 2001;276:42259–42267. doi: 10.1074/jbc.M105888200. [DOI] [PubMed] [Google Scholar]
- 40.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barradas M, Gonos ES, Zebedee Z, Kolettas E, Petropoulou C, et al. Identification of a candidate tumor-suppressor gene specifically activated during Ras-induced senescence. Exp Cell Res. 2002;273:127–137. doi: 10.1006/excr.2001.5434. [DOI] [PubMed] [Google Scholar]
- 42.Dickmeis T, Aanstad P, Clark M, Fischer N, Herwig R, et al. Identification of nodal signaling targets by array analysis of induced complex probes. Dev Dyn. 2001;222:571–580. doi: 10.1002/dvdy.1220. [DOI] [PubMed] [Google Scholar]
- 43.Peyrieras N, Strahle U, Rosa F. Conversion of zebrafish blastomeres to an endodermal fate by TGF-beta-related signaling. Curr Biol. 1998;8:783–786. doi: 10.1016/s0960-9822(98)70303-3. [DOI] [PubMed] [Google Scholar]
- 44.David NB, Rosa FM. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development. 2001;128:3937–3947. doi: 10.1242/dev.128.20.3937. [DOI] [PubMed] [Google Scholar]
- 45.Aoki TO, David NB, Minchiotti G, Saint-Etienne L, Dickmeis T, et al. Molecular integration of casanova in the Nodal signalling pathway controlling endoderm formation. Development. 2002;129:275–286. doi: 10.1242/dev.129.2.275. [DOI] [PubMed] [Google Scholar]
- 46.Key MD, Andres DA, Der CJ, Repasky GA. Characterization of RERG: An Estrogen-Regulated Tumor Suppressor Gene. Methods Enzymol. 2005;407:513–527. doi: 10.1016/S0076-6879(05)07041-2. [DOI] [PubMed] [Google Scholar]
- 47.Warga RM, Nusslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- 48.Melby AE, Warga RM, Kimmel CB. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development. 1996;122:2225–2237. doi: 10.1242/dev.122.7.2225. [DOI] [PubMed] [Google Scholar]
- 49.Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci U S A. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gritsman K, Talbot WS, Schier AF. Nodal signaling patterns the organizer. Development. 2000;127:921–932. doi: 10.1242/dev.127.5.921. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, et al. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- 52.Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poulain M, Lepage T. Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development. 2002;129:4901–4914. doi: 10.1242/dev.129.21.4901. [DOI] [PubMed] [Google Scholar]
- 54.Norton WH, Mangoli M, Lele Z, Pogoda HM, Diamond B, et al. Monorail/Foxa2 regulates floorplate differentiation and specification of oligodendrocytes, serotonergic raphe neurones and cranial motoneurones. Development. 2005;132:645–658. doi: 10.1242/dev.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- 56.Strahle U, Jesuthasan S, Blader P, Garcia-Villalba P, Hatta K, et al. one-eyed pinhead is required for development of the ventral midline of the zebrafish (Danio rerio) neural tube. Genes Funct. 1997;1:131–148. doi: 10.1046/j.1365-4624.1997.00010.x. [DOI] [PubMed] [Google Scholar]
- 57.Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- 58.Sekiya T, Fushimi M, Hori H, Hirohashi S, Nishimura S, et al. Molecular cloning and the total nucleotide sequence of the human c-Ha-ras-1 gene activated in a melanoma from a Japanese patient. Proc Natl Acad Sci U S A. 1984;81:4771–4775. doi: 10.1073/pnas.81.15.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, et al. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 60.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 62.Nieto M, Barradas M, Criado LM, Flores JM, Serrano M, et al. Normal cellular senescence and cancer susceptibility in mice genetically deficient in Ras-induced senescence-1 (Ris1). Oncogene. 2007;26:1673–1680. doi: 10.1038/sj.onc.1209978. [DOI] [PubMed] [Google Scholar]
- 63.Halpern ME, Hatta K, Amacher SL, Talbot WS, Yan YL, et al. Genetic interactions in zebrafish midline development. Dev Biol. 1997;187:154–170. doi: 10.1006/dbio.1997.8605. [DOI] [PubMed] [Google Scholar]
- 64.Etard C, Behra M, Ertzer R, Fischer N, Jesuthasan S, et al. Mutation in the delta-subunit of the nAChR suppresses the muscle defects caused by lack of Dystrophin. Dev Dyn. 2005;234:1016–1025. doi: 10.1002/dvdy.20592. [DOI] [PubMed] [Google Scholar]
- 65.Warga RM, Kane DA. One-eyed pinhead regulates cell motility independent of Squint/Cyclops signaling. Dev Biol. 2003;261:391–411. doi: 10.1016/s0012-1606(03)00328-2. [DOI] [PubMed] [Google Scholar]
- 66.Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731–5741. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- 67.Dickmeis T, Mourrain P, Saint-Etienne L, Fischer N, Aanstad P, et al. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 2001;15:1487–1492. doi: 10.1101/gad.196901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- 69.Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, et al. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- 70.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]