Abstract
Objective
To examine schizophrenia patients' visual attention to social contextual information during a novel mental state perception task.
Method
Groups of healthy participants (n = 26) and schizophrenia patients (n = 24) viewed 7 image pairs depicting target characters presented context-free and context-embedded (i.e., within an emotion-congruent social context). Gaze position was recorded with the EyeLink I Gaze Tracker while participants performed a mental state inference task. Mean eye movement variables were calculated for each image series (context-embedded v. context-free) to examine group differences in social context processing.
Results
The schizophrenia patients demonstrated significantly fewer saccadic eye movements when viewing context-free images and significantly longer eye-fixation durations when viewing context-embedded images. Healthy individuals significantly shortened eye-fixation durations when viewing context-embedded images, compared with context-free images, to enable rapid scanning and uptake of social contextual information; however, this pattern of visual attention was not pronounced in schizophrenia patients. In association with limited scanning and reduced visual attention to contextual information, schizophrenia patients' assessment of the mental state of characters embedded in social contexts was less accurate.
Conclusion
In people with schizophrenia, inefficient integration of social contextual information in real-world situations may negatively affect the ability to infer mental and emotional states from facial expressions.
Medical subject headings: social perception, facial expression, attention, visual perception, eye movements, schizophrenia
Abstract
Objectif
Examiner l'attention visuelle que les patients atteints de schizophrénie accordent à l'information contextuelle sociale au cours d'une nouvelle tâche portant sur la perception de l'état mental.
Méthode
Des groupes de participants en bonne santé (n = 26) et de patients atteints de schizophrénie (n = 24) ont visualisé sept paires d'images décrivant des personnages cibles présentés sans contexte et en contexte (c.-à-d. dans un contexte social congruent aux émotions). On a consigné la position du regard au moyen du système de poursuite oculaire EyeLink I pendant que les participants exécutaient une tâche d'inférence liée à l'état mental. On a calculé les variables moyennes du mouvement de l'œil pour chaque série d'images (en contexte c. sans contexte) afin d'examiner les différences entre les groupes au niveau du traitement du contexte social.
Résultats
Les patients atteints de schizophrénie ont eu des mouvements oculaires saccadés beaucoup moins nombreux pendant qu'ils visualisaient des images sans contexte et une fixation beaucoup plus longue lorsqu'ils visualisaient des images en contexte. Les sujets en bonne santé ont raccourci considérablement la durée de la fixation oculaire lorsqu'ils visionnaient des images en contexte comparativement aux images sans contexte, ce qui leur a permis d'analyser et d'absorber rapidement de l'information contextuelle sociale, mais cette tendance de l'attention visuelle n'était pas prononcée chez les patients atteints de schizophrénie. Conjuguée à une limitation du balayage et à une réduction de l'attention visuelle portée à l'information contextuelle, l'évaluation effectuée par les patients atteints de schizophrénie de l'état mental des personnages intégrés dans des contextes sociaux était moins précise.
Conclusion
Chez les personnes atteintes de schizophrénie, l'intégration inefficiente de l'information contextuelle sociale dans des situations réelles peut avoir une incidence négative sur leur capacité de déduire des états mentaux et émotionnels à partir d'expressions faciales.
Introduction
Impairments in social cognition are a pervasive feature of schizophrenia that directly affect social functioning,1 such that there has been increasing focus on these skills in targeted remediation strategies.2–5 Common examples of social cognitive deficits in schizophrenia include poor facial expression perception6 and mental state inference,7 for which there is considerable evidence, as well as more recent findings of difficulty interpreting emotions from vocal cues,8 body posture9 and biological motion.9,10 Given the evidence for abnormal context processing in schizophrenia during the performance of nonsocial cognitive tasks,11,12 recent studies suggest that deficits in emotion perception extend beyond perception of facial emotions, which would be consistent with an interaction between broader deficits in the processing of context and social cognitive skills in schizophrenia.13
Contextual cues are important for discriminating simple emotions and complex mental states from facial expressions,14 and indeed, some facial expressions may simply represent conventions for social communication rather than expressions of emotion per se.15,16 Previous studies examining the direct impact of context processing on social cognitive performance in healthy participants have shown the influence of context on the perception of emotion in faces by presenting visual (e.g., other facial expressions17) or verbal information (e.g., vignettes to establish context) before presenting target face stimuli.18,19 In these studies, healthy participants have judged neutral and expressive faces according to the emotional cues presented in contextual information, even when these cues conflict with the target in emotional content (i.e., a “fearful” face is perceived as “angry” when paired with vignette cueing anger). Other research has demonstrated the impact of simultaneously presented multimodal (e.g., visual and auditory) information,20 and recent studies have sought to delineate the time course, neuroanatomical substrates and role of attention and awareness in this integrative social cognitive process.21–26
Accumulating evidence supports a link between poor context processing and social cognitive performance in people with schizophrenia.13,27 For example, a recent series of studies reports poor integration of emotional cues from simultaneously presented auditory (vocal) and visual (facial) information in schizophrenia, evident in the reduced effects of an emotional voice on the categorization of a facial expression and the exaggerated effect of a facial expression on the categorization of an emotional voice,8 even though no generalized impairment in audiovisual integration was evident.28 With regard to the ability to process concurrent visual contexts, recent studies report an association between early visual grouping processes and poor theory-of-mind in schizophrenia29 as well as impairments in the ability to use visual contextual information to modify judgments of emotional intensity.30 Finally, research using the face-vignette task described above has also observed a reduced influence of verbal contextual information on facial expression perception in schizophrenia.31
Inefficient use of contextual information available in daily social situations may thus contribute to difficulties with emotion and mental state inference in this population. Indeed, a recent study of healthy individuals demonstrated that the deployment of visual attention to salient contextual cues within complex scenes is important for determining where another person is directing his or her attention.32 Studies of eye movements provide an index of where visual attention is directed; most commonly, these use measures of foveal gaze position that is associated with detailed visual inspection of a stimulus.33 Under certain experimental conditions, it is possible for attention to be allocated away from the point of fixation; however, in cases of complex scene viewing, the evidence weighs heavily in support of a functional relation between the allocation of attention and overt eye movement.34
Early studies of visual attention in schizophrenia employed eye movement recordings to measure attention directed to complex visual stimuli under free viewing conditions (see Green and colleagues35 for a comprehensive review). In general, these studies converge in demonstrating limited (or restricted) visual scanning in schizophrenia when subjects viewed geometric stimuli,36–38 faces39–42 and social scenes.43,44 More recent studies demonstrate a pattern of visual avoidance of the salient features of emotional faces in association with deficits in the recognition of particular emotions,45,46 in the context of generally limited scanning in schizophrenia. These studies concur with the results of neuropsychological investigations implicating early visual (i.e., preattentive) processing in social perception deficits in schizophrenia.47,48 To further examine the relation between visual context processing and social perception in schizophrenia, the present study sought to examine whether schizophrenia patients direct adequate visual attention to concurrent contextual cues when judging the meaning of facial expressions presented in the context of a social scene.
This study therefore employed eye movement recordings to provide an objective measure of attention directed to contextual cues when participants judged the meaning of facial expressions in scene stimuli. We employed visual stimuli consisting of picture pairs that presented target characters in relative isolation and in the context of a social scene. These stimuli allowed the simultaneous recording of voluntary visual scan paths while participants completed an emotion–mental state perception task. The ecologic validity of this approach is supported by evidence from a recent study showing “extended” scanning of scene images (i.e., an increased number of eye-fixations of short duration with longer scan path) under conditions of mental inference as oppposed to simple description of the scene.32 On the basis of previous findings of restricted visual scanning in schizophrenia and pervasive impairments in the perception of emotion, we hypothesized that limited visual attention to social contextual cues in scene images would be associated with aberrant perception of facial expressions.
Methods
All procedures were approved by the Macquarie University Human Research Ethics Committee (approval no. 22FEB2002-R008) and the Northern Sydney Area Health Service (approval no. 0309–185M).
Participants
Exclusion criteria were use of diazepam, methadone and barbiturates, owing to the effects of these medications on the ocular motor system49; regular use of illicit drugs or alcohol; significant ocular pathology; epilepsy or other neurologic disorder; mental retardation; or head injury. For control participants, a history of psychotic illness or such history in first-degree relatives were part of the exclusion criteria. Inclusion criteria were age 18–60 years and normal Snellen visual acuity.
Clinical participants
We recruited 11 female and 13 male (n = 24) participants with a diagnosis of schizophrenia, representing a heterogeneous group of outpatients. Nine participants were outpatients recruited from the Macquarie Centre for Cognitive Science Belief Formation Participant Register, 3 individuals were recruited from the outpatient cottages at Macquarie Hospital, and the remaining 12 were outpatients recruited through the Schizophrenia Research Institute research register. The diagnosis of schizophrenia was confirmed with the use of the Diagnostic Interview for Psychosis.50 Schizophrenia symptoms were rated on the Scale for the Assessment of Positive Symptoms51 and the Scale for the Assessment of Negative Symptoms.52 Of the patients, 15 were taking atypical antipsychotics, 5 were taking conventional antipsychotics (D2 blockers), 3 were taking a combination of typical and atypical medications, and 1 patient was not medicated at the time of testing. The level of medication was converted to a chlorpromazine equivalent dosage according to the formula provided by Humberstone and colleagues.53
Nonclinical participants
We recruited a control group of 26 healthy participants (10 male and 16 female) from the general community.
Materials
Apparatus and stimuli
Eye movements were recorded binocularly with the use of the EyeLink I eye-tracking system (SR Research Ltd., Mississauga, Ont.) sampling at a temporal resolution of 250 Hz (every 4 ms) and a spatial resolution of 0.1°. An eye movement was classified as a saccade when its distance exceeded 0.2° and velocity reached 30°/s or when its length exceeded 0.2° and its acceleration had reached 8000°/s2. All other eye movements (i.e., those falling outside the limits of the saccade definition) were recorded as eye-fixation periods unless determined by the recording software as blinks. Blinks were excluded from the data used for analysis with dedicated software.
Face and scene images
In this study, we employed 14 colour photographs from the Social Context Appreciation Task (SCAT) (Green and colleagues, unpublished). The SCAT comprises 7 picture pairs: 1 image in each pair depicts a person eliciting a facial expression embedded within an emotion-congruent social context (context-embedded [CE]); the counterpart image depicts the same person against a standard background that contains no contextual cues (context-free [CF]). Figure 1 presents an example of paired SCAT stimuli. Stimuli were displayed across a full screen set at a resolution of 800 × 600 pixels (31.5 × 23 cm) at a viewing distance of 60 cm and thus forming a horizontal visual angle of 30° and a vertical visual angle of 14°. In a pilot study conducted with healthy participants, use of this task showed that the perception of mental state was influenced by information contained in the social context.

Fig. 1: Example of context-free (CF) (left) and context-embedded (CE) (right) stimuli.
IQ test
The Quick Test,54 which relies on verbal semantic knowledge and perceptual ability, was employed as a measure of current IQ. The National Adult Reading Test (NART),55 which relies on word reading skills only, was employed as an estimate of premorbid IQ. Three clinical participants and 1 control participant did not complete the NART because they were familiar with the task.
Procedures
Eye movement recording
We calibrated eye-fixation position before the experiment to ensure that a robust fixation recording could be achieved at all points on the computer screen; experimental recording did not proceed until adequate calibration was achieved. The initial point of retinal attention was controlled by the presentation of a centrally positioned fixation cross for 1000 milliseconds before each experimental stimulus. Participants viewed the sequence of 14 stimuli in 2 blocks of 7, with the CF (Series 1) images always presented before the CE (Series 2). The images were presented in a pseudorandom order within each block, and each stimulus was presented for 10 seconds on a 17-inch colour monitor. Subjects viewed each image with instructions to “decide what the person in the photo is feeling or thinking.” Participants were asked to respond verbally in their own time and were not restricted in their use of mental state terms. Responses were recorded by the experimenter. There were no practice trials.
Results
Group characteristics
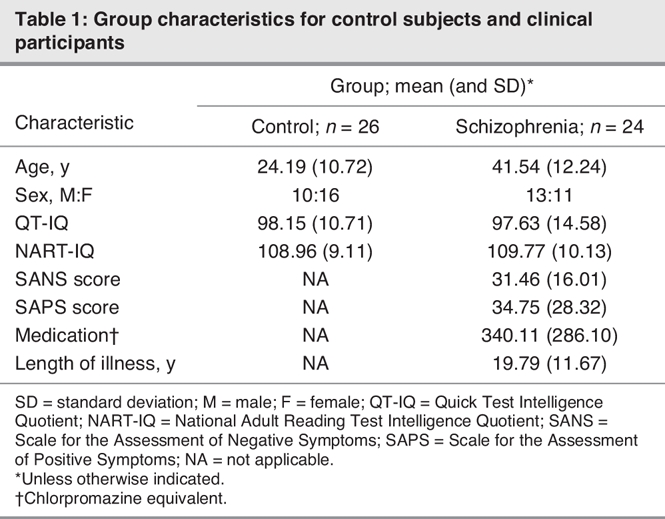
Descriptive characteristics for each group are presented in Table 1. There were no significant differences between the clinical and control groups on measures of premorbid and current IQ (Quick Test: t1,48 = 0.147, p = 0.88; NART: t1,45 = –0.29, p = 0.77) or in the ratio of male to female subjects (t1,48 = 1.10, p = 0.28). However, the control group was significantly younger than the schizophrenia group (t1,48 = –5.34, p < 0.01). Age was thus entered as a covariate in all focal analyses.
Table 1
Eye movement analysis
Eye movement analyses were undertaken in 2 phases. Phase 1 was concerned with the general effects of contextual information on eye movement parameters and compared data from combined data sets of CF and CE images; eye movement data falling in any region of these images were used. Phase 2 was concerned with the analysis of eye-fixations on particular interest areas (IAs) within CF and CE images. More specifically, this involved the examination of eye-fixations to the target face as opposed to the surrounding context.
Effects of context on eye movements
We investigated general patterns of viewing CE versus CF images within the following eye movement parameters: total number of fixations, total and median fixation duration and total number of saccades. All eye movement variables were normally distributed. Mean eye movement parameters calculated for each group are presented in Table 2 according to image series.
Table 2
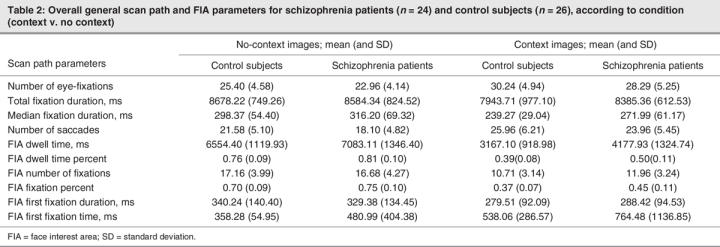
To examine the effects of context on viewing strategies within each group, we employed a mixed-design 2 × 4 × 2 repeated-measures multiple analysis of covariance (MANCOVA) controlling for age. Context (2 levels: CE and CF) and eye parameter (4 levels: number of fixations, number of saccades, total duration of fixations and median duration of fixations) were the 2 within-group factors. Group (2 levels: control and schizophrenia) was the between-subjects factor. Dependent variables were the mean eye parameters for the CE and CF conditions (Table 1). There was no significant effect of age (F1,47 = 1.29, p = 0.26) and no significant interactions between age and any other variables. Age was therefore removed from the model. Subsequent analyses revealed a significant main effect of context (F1,48 = 36.16, p < 0.01) and eye parameter (F3,46 = 3855.67, p < 0.01) but no main effect of group. There were significant interactions between context × group (F1,48 = 10.68, p < 0.01) and context × eye parameter (F3,46 = 46.04, p < 0.01) as well as a significant 3-way interaction between eye parameter × context × group (F3,46 = 3.81, p < 0.01).
To further examine the interaction between eye parameter, context and group, we conducted a series of mixed-design 2 × 2 multivariate analyses of variance (MANOVAs) for each eye movement parameter, with group (2 levels) as the between-subjects factor and context (2 levels) as the within-subjects factor. There was a main effect of context on the number of fixations, median duration of fixations and number of saccades, such that both groups exhibited more fixations (F1,48 = 119.05, p < 0.01) of shorter median duration (F1,48 = 99.51, p < 0.01), along with more saccadic eye movements (F1,48 = 106.57, p < 0.01) and shorter total duration of fixation (F1,48 = 31.77, p < 0.01) for CE images, compared with CF images. In addition, there was a significant context × group interaction for the total duration of fixation (F1,48 = 10.45, p < 0.01). Paired t tests conducted for each group revealed that this interaction was due to the control subjects demonstrating significantly shorter total fixation duration on CE images, compared with CF images (t25 = 5.32, p < 0.01), while the schizophrenia patients exhibited no such difference in the total duration of fixation across the 2 conditions.
We also undertook further examination of the context × group interaction in a series of 1-way ANOVAs carried out to investigate group differences on particular eye movement parameters for each condition. These analyses revealed that on CF images, the schizophrenia patients tended to exhibit fewer fixations (trend only: F1,48 = 3.87, p < 0.06) and fewer saccades (F1,48 = 6.14, p < 0.02), compared with healthy control participants. When viewing CE images, schizophrenia patients exhibited longer median fixation duration (F1,48 = 5.99, p < 0.02) and total fixation duration (trend only: F1,48 = 3.60, p < 0.06), compared with control subjects. These findings for CE images concur with the finding reported above (i.e., a significantly shorter total fixation duration for control subjects when viewing CE images that is not paralleled in the schizophrenia patients): the group differences in fixation duration are borne out only in comparisons of viewing styles for complex scenes (i.e., CE images).
Interest area analyses: attention to faces in CE versus CF images
Interest area analyses examined the characteristics of eye-fixations to target faces, compared across image sets. Image-specific interest areas (target faces) were manually traced and colour coded with Photoshop 7.0 (Adobe Systems, Inc., 2002) to enable processing with the EyeLink II Data Viewer Package (SR Research Ltd. Mississauga, Ont.). Analyses of visual attention to face interest areas within CE and CF images were undertaken on fixation parameters derived from the Data Viewer software. These parameters included dwell time, percentage dwell time, number of fixations, percentage fixations, first fixation duration and first fixation time in relation to each interest area. All eye movement parameters were normally distributed except for the first fixation time variable, which demonstrated an extreme positive kurtosis (> 33) in all participants across both CE and CF conditions. We therefore conducted between-group and within-group comparisons of this variable separately, using nonparametric statistics.
Mean data on these parameters are presented in Table 2 for each group according to condition. We employed a mixed design 2 × 5 × 2 repeated-measures MANCOVA (controlling for age), with context (2 levels: CE v. CF) and eye parameter (5 levels: dwell time, percentage dwell time, number of fixations, percentage fixations and first fixation time) as within-subjects variables and group (2 levels: control v. schizophrenia) as the between-subjects variable.
Again, there was no significant effect of age (F1,47 = 0.82, p = 0.37) and no significant interactions between age and any other variable. Age was therefore removed from the model. A subsequent analysis with age removed revealed significant main effects of group (F1,48 = 5.98, p < 0.02), context (F1,48 = 559.76, p < 0.01) and eye parameter (F5,44 = 519.32, p < 0.01), as well as significant 2-way interactions between eye parameter × group (F4,45 = 2.83, p < 0.05) and context × eye parameter (F4,45 = 146.42, p < 0.01), with the context × group interaction approaching significance (F1,48 = 4.29, p < 0.07). There was a significant 3-way interaction between eye parameter × context × group (F4,45 = 3.13, p < 0.05).
Given our a priori hypotheses regarding the interaction of group and context, we undertook further analyses by means of a series of 2 × 2 repeated-measures MANOVAs for each eye parameter, with context (CE or CF) as the within-subjects factor and group (schizophrenia or control) as the between-subjects factor. A significant main effect of context was revealed for all fixation parameters (first fixation duration, F1,48 = 7.40, p < 0.01; number of fixations, F1,48 = 201.53, p < 0.01; percentage fixations, F1,48 = 743.34, p < 0.01; percentage dwell time, F1,48 = 8.34, p < 0.01; dwell time, F1,48 = 575.37, p < 0.01). Significant group × context interactions were revealed for the number of fixations (F1,48 = 4.81, p < 0.05), the percentage of total fixations (F1,48 = 4.06, p < 0.05) and the percentage of total dwell time to faces (F1,48 = 8.34, p < 0.01). A subsequent series of 1-way between-group ANOVAs conducted for each condition on these fixation parameters revealed that the schizophrenia patients directed a larger percentage of total dwell time to faces in both the CE (F1,48 = 17.97, p < 0.01) and CF (F1,48 = 4.21, p < 0.05) conditions, compared with control subjects. An additional consistent finding was that the schizophrenia patients directed a higher percentage of eye-fixations to faces in the CE condition (F1,48 = 13.49, p < 0.01) and thus spent less time fixating on contextual information, compared with control subjects (see Fig. 2).

Fig. 2: Representative scan path of a schizophrenia patient (left) and control subject (right) to the same context (C1) photograph. Note: These images represent plots of raw, or unprocessed, gaze position data that reflect a visual scan path. The scan path of the schizophrenia patient is intended to illustrate increased attention to the target face stimulus, with little visual attention directed to the social context, in contrast to the control subject's eye-fixation distribution that is extended to facilitate context processing. The upward eye movements of this particular schizophrenia patient reflect voluntary and repeated attention directed to nonsignificant parts of the stimulus (including off-screen gazes); these may not be undertaken by all schizophrenia patients and do not represent blink artifacts.
Wilcoxon signed-rank tests were used to examine within-group differences across image type in the time of the first fixation to interest area regions. Both the schizophrenia patients (Z = –3.32, p < 0.01) and the control subejcts (Z = –3.73, p < 0.01) showed a significant delay in first fixation time to the target face in the CE, compared with the CF, images. When we used Mann–Whitney U tests for between-group contrasts of first fixation time for each viewing condition, the schizophrenia patients demonstrated a significant delay in first fixation time to the target face in the CF images, compared with control subjects (U = 190, p < 0.02). However, there were no group differences in first fixation time to the target face for CE images.
Mental state perception
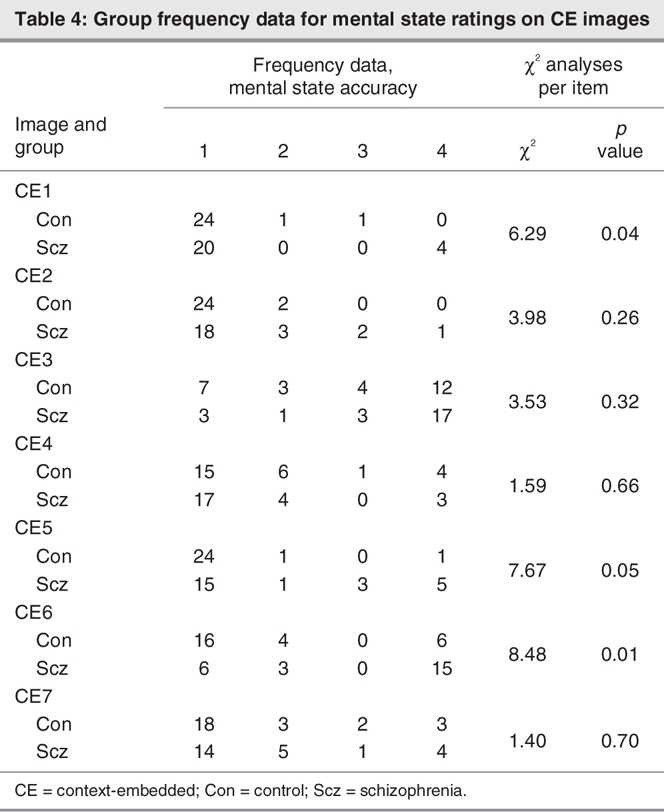
Free responses on the mental state perception component of the task were coded as 1 of 20 mutually exclusive subcategories and 4 major mental state categories (affective, cognitive, volitional and dispositional), as presented in Box 1. Data generated from the 20-item classification system were rank-ordered according to accuracy (1–3), with remaining responses coded 4; items coded 1 were regarded as accurate. Group frequency data for these rank-ordered mental state ratings are presented in Table 3 (CF images) and Table 4 (CE images) for each image. Chi-square analyses conducted on these data revealed group differences in the frequency of accurate responses for 2 CE images only, in which the target character was perceived as happy (CE1) or curious–inquisitive (CE5) when embedded in a situational context (Table 3 and Table 4). There were no group differences in the frequency of accurate responses for CF images.
Box 1.
Table 3
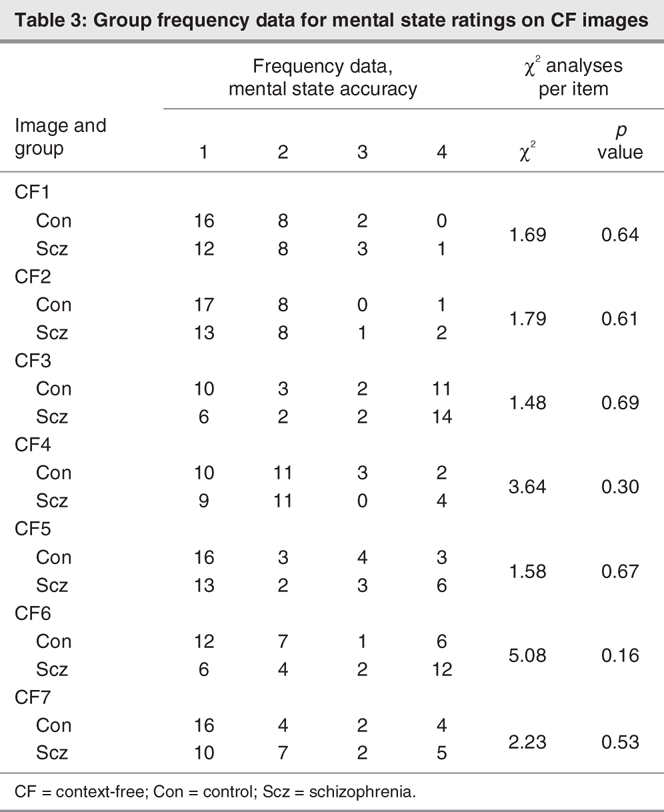
Table 4
We calculated a measure of total accuracy for CE and CF images by summing the number of responses coded 1 from the above procedure. The mean (and standard deviation [SD]) accuracies of mental state ratings on CF and CE images were 4.23 (SD 1.55) and 5.58 (SD 1.21) for the control subjects, respectively, and 3.33 (SD 1.71) and 4.5 (SD 1.58) for the schizophrenia patients. Independent sample t tests revealed that the schizophrenia patients were significantly impaired in their perception of mental states within CE images, with fewer accurate responses, compared with control subjects (t(48) = 2.71, p < 0.01). Despite the existence of a trend toward a similar pattern of reduced accuracy for CF images in schizophrenia patients, this difference failed to reach significance (t(48) = 1.92, p < 0.06). There was no association between accuracy on the mental state perception task and IQ.
Association between visual attention and mental state perception
We examined associations between mental state perception and visual attention by means of partial correlations between scan path parameters and mean accuracy scores derived from the CE condition, with controlling for medication effects (chlorpromazine equivalent dosage). These analyses revealed that accurate judgments of mental state within social contexts was associated with an increased number of eye-fixations (r16 = 0.50, p < 0.05) of shorter median duration (r16 = –0.51, p < 0.05) as well as a nonsignificant trend in the direction of a reduced percentage of dwell time on faces (r16 = –0.42, p < 0.08) in association with increased accuracy. This pattern of eye movements reflects the pattern associated with the appreciation of broader aspects of scene stimuli, wherein visual attention to social contextual information may facilitate accurate mental state attribution in social contexts.
Discussion
We examined visual processing of social contextual information in schizophrenia patients and healthy participants, during a mental state perception task in which target characters were depicted under 2 conditions. All participants exhibited a differential pattern of extended visual scanning when viewing scene images containing social contextual information, compared with images containing limited situational cues This pattern of viewing complex (CE) scenes consisted of increased eye-fixations of shorter duration along with increased saccadic activity that has been previously demonstrated in studies of complex scene-viewing in healthy individuals56 and is consistent with a recent study showing “extended” scanning of scene images under conditions of mental inference as opposed to simple description of the scene.32 In addition, both the schizophrenia patients and control subjects showed a significant delay in the first eye-fixation to the target faces when they were presented in social contexts, consistent with extended scanning of these images.
Group differences in general viewing strategies were consistent with previous findings of limited scanning (fewer eye-fixations and saccades) in schizophrenia patients, compared with control subjects, for scenes containing no contextual information. Additional group differences in scanning particular image types were borne out most consistently in within-group contrasts between image type; whereas the control subjects spent a significantly shorter time fixating on CE (v. CF) images, the schizophrenia patients demonstrated no such difference in their pattern of fixations across the 2 image types and, instead, exhibited significantly longer fixations (staring behaviour) for complex scenes (CE images) than did control subjects. These findings of generally limited scanning alongside increased fixation durations in schizophrenia patients are therefore consistent with considerable evidence for restricted viewing of geometric stimuli,36–38 faces39–42 and social scenes.43,44
With regard to where schizophrenia participants directed their (albeit limited) visual attention during the viewing of CE and CF stimuli, the schizophrenia patients spent a significantly larger percentage of total dwell time viewing faces under both conditions, compared with control subjects. This represents a tendency toward local viewing bias rather than the employment of a global viewing strategy.57 When considered along with recent findings of visual avoidance of the salient features of emotional faces in association with deficits in the recognition of particular emotions,45,46 the present pattern of avoiding significant information from the social context may indicate an increased likelihood of misinterpreting facial displays in real-world situations. In addition, compared with control subjects, the schizophrenia patients demonstrated a significant delay in the first eye-fixation directed at the target face of CF images, which may reflect distraction of attention from salient features of the stimuli even when no contextual information was present.
With respect to the interpretation of mental states in the present task, the schizophrenia patients showed a significant impairment in their perception of mental states within contextual scenes, compared with control subjects. This finding reflects more idiosyncratic responses in the schizophrenia patients' mental state attributions in the CE condition. In addition, when we considered mental state responses for each image separately, the schizophrenia patients demonstrated reduced frequency of accurate responses for 2 CE images in particular, compared with consensus in the healthy participant group; these were CE1 (target characters perceived as happy) and CE5 (target characters perceived as curious or inquisitive). However, there were no group differences in the frequency of accurate responses for individual CF images. Overall, these findings are consistent with recent studies that suggest a role for poor context processing in the social cognitive impairments demonstrated in schizophrenia.8,27,29–31
Finally, when we examined the role of visual attention on mental state inferences, we found significant associations between mean accuracy of mental state judgments within social contexts and an increased number of fixations of short duration, as well as a trend toward reduced fixations directed at target faces, in the entire sample. These findings suggest that accurate mental state attributions for characters presented in social contexts are associated with more active scanning (more frequent and shorter fixations) of social scenes to facilitate the uptake of contextual information. Because our analyses of visual attention revealed that schizophrenia patients demonstrate both reduced attention to social context and limited scanning behaviour, the reduced accuracy of mental state attributions in this group may be attributable to these visual scanning aberrations.
Potential limitations of the present study include the significant difference between experimental groups in terms of age; however, we have attempted to rule out the possibility of age-related factors accounting for significant group differences in visual attention to contextual information by including age as a covariate in the relevant statistical analyses. Second, we acknowledge that the present set of visual stimuli used to assess attention to contextual information during mental state inference had only 7 paired items (i.e., 14 images in total). The set did not, therefore, assess an exhaustive range of mental or emotional states, compared with standardized tasks such as those used to assess the ability to recognize basic emotions from facial expressions.58 However, given the present investigation's focus on examining attention to simultaneously presented visual contextual information during mental state perception in schizophrenia, we employed a limited set of social scene stimuli appropriate to this aim. In schizophrenia patients, these stimuli have demonstrated utility in revealing preliminary evidence for limited attention to social contextual information in association with reduced accuracy on a mental state inference task; however, future investigations should employ similar tasks that encompass a wider range of stimuli to examine patterns of visual attention and response biases that may arise for particular emotional and mental states. Indeed, a concurrent study that uses a modified version of this task to measure forced-choice emotional intensity ratings has revealed impaired use of contextual information to moderate the perception of emotional intensity. As well, assessments of emotional intensity in regard to expressions of anger and disgust are exaggerated when contextual cues are absent.30 Finally, since the clinical group in this study comprised a heterogeneous sample of schizophrenia outpatients, we have not examined potential relations between reduced social context processing and subgroups of patients that may be defined on the basis of symptom clusters.59 This may be important in further investigations, given previous evidence for aberrant context processing in association with disorganized syndromes specifically.48
In sum, the present findings converge with recent evidence that suggests simple emotion recognition and social cognitive deficits in schizophrenia may be underpinned by pervasive deficits in context processing in both auditory and visual domains (for a comprehensive discussion of neuropsychological contributions to social cognition in schizophrenia, see Russell and Green60). To our knowledge this is the first study to provide objective evidence of marked attentional avoidance of social contextual information in schizophrenia by monitoring gaze position during a novel mental state perception task. Given the recent trend toward targeted remediation of social cognitive deficits in schizophrenia, the present findings suggest that training individuals to orient attention to broader contextual elements in everyday social interactions might facilitate more accurate perception of others' mental and emotional states, forming a building block on which to improve social functioning more generally.
Acknowledgments
The authors acknowledge the support of the Schizophrenia Research Institute's Research Register for assistance with the recruitment of volunteers participating in this research. Salary and research support was provided from the NHMRC (Australian Clinical Training Fellowship ID 192111 & Program Grant ID 222708); the Macquarie Centre for Cognitive Science, Macquarie University; and the Schizophrenia Research Institute, Australia, with the use of infrastructure funding from NSW Health.
Footnotes
Contributors: Drs. Green and Coltheart designed the study. Dr. Green, Ms. Waldron and Mr. Simpson acquired and analyzed the data. Dr. Green wrote the article, and Ms. Waldron, Mr. Simpson and Dr. Coltheart revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Melissa J. Green, School of Psychiatry, University of New South Wales, Black Dog Institute Building, Hospital Road, Prince of Wales Hospital, Randwick, NSW 2031, Australia; fax 0061(2) 9382 8151; melissa.green@unsw.edu.au
References
- 1.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull 2006;32(1 Suppl):S44-63. [DOI] [PMC free article] [PubMed]
- 2.Frommann N, Streit M, Wolwer W. Remediation of facial affect recognition impairments in patients with schizophrenia: a new training program. Psychiatry Res 2003;117:281-4. [DOI] [PubMed]
- 3.Penn DL, Combs D. Modification of affect perception deficits in schizophrenia. Schizophr Res 2000;46:217-29. [DOI] [PubMed]
- 4.Silver M, Oakes P. Evaluation of a new computer intervention to teach people with autism or asperger syndrome to recognize and predict emotions in others. Autism 2001;5:299-316. [DOI] [PubMed]
- 5.Wolwer W, Frommann N, Halfmann S, et al. Remediation of impairments in facial affect recognition in schizophrenia: Efficacy and specificity of a new training program. Schizophr Res 2005;80: 295-303. [DOI] [PubMed]
- 6.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin Psychol Rev 2002;22:789-832. [DOI] [PubMed]
- 7.Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: A critical review. Cognit Neuropsychiatry 2005;10:249-86. [DOI] [PubMed]
- 8.de Gelder B, Vroomen J, de Jong SJ, et al. Multisensory integration of emotional faces and voices in schizophrenics. Schizophr Res 2005;72:195-203. [DOI] [PubMed]
- 9.Bigelow NO, Paradiso S, Adolphs R, et al. Perception of socially relevant stimuli in schizophrenia. Schizophr Res 2006;83:257-67. [DOI] [PubMed]
- 10.Kim J, Doop ML, Blake R, et al. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res 2005;77:299-307. [DOI] [PubMed]
- 11.Cohen JD, Barch DM, Carter C, et al. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol 1999;108:120-33. [DOI] [PubMed]
- 12.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci 2003;26:65-137. [DOI] [PubMed]
- 13.Green MJ, Uhlhaas PJ, Coltheart M. Context processing and social cognition in schizophrenia. Current Psychiatry Reviews 2005;1:11-21.
- 14.Ellis HD, Young AW. Faces in their social and biological context. In: Young AW, editor. Face and mind. New York: Oxford University Press; 1998. p. 67-95.
- 15.Fernandez-Dols J, Ruiz-Belda M. Are smiles a sign of happiness? Gold medal winners at the olympic games. J Pers Soc Psychol 1995;69:1113-9.
- 16.Fernandez-Dols JM, Carrera P, Russell JA. Are facial displays social? Situational influences in the attribution of emotion to facial expressions. Span J Psychol 2002;5:119-24. [DOI] [PubMed]
- 17.Tanaka-Matsumi J, Attivissimo D, Nelson S, et al. Context effects on the judgment of basic emotions in the face. Motivation and Emotion 1995;19:139-55.
- 18.Carroll JM, Russell JA. Do facial expressions signal specific emotions? Judging emotion from the face in context. J Pers Soc Psychol 1996;70:205-18. [DOI] [PubMed]
- 19.Carrera-Levillain P, Fernandez-Dols J-M. Neutral faces in context: Their emotional meaning and their function. J Nonverb Behav 1994;18:281-99.
- 20.de Gelder B, Vroomen J, Teunisse JP. Perceiving emotion by ear and by eye. Bull Psychon Soc 1995;30:50.
- 21.de Gelder B, Bocker KB, Tuomainen J, et al. The combined perception of emotion from voice and face: Early interaction revealed by human electric brain responses. Neurosci Lett 1999;260:133-6. [DOI] [PubMed]
- 22.Dolan RJ, Morris JS, de Gelder B. Crossmodal binding of fear in voice and face. Proc Natl Acad Sci U S A 2001;98:10006-10. [DOI] [PMC free article] [PubMed]
- 23.Pourtois G, de Gelder B, Vroomen J, et al. The time-course of intermodal binding between seeing and hearing affective information. Neuroreport 2000;11:1329-33. [DOI] [PubMed]
- 24.Pourtois G, Debatisse D, Despland PA, et al. Facial expressions modulate the time course of long latency auditory brain potentials. Brain Res Cogn Brain Res 2002;14:99-105. [DOI] [PubMed]
- 25.Righart R, de Gelder B. Context influences early perceptual analysis of faces–an electrophysiological study. Cereb Cortex 2006;16:1249-57. [DOI] [PubMed]
- 26.Vroomen J, Driver J, de Gelder B. Is cross-modal integration of emotional expressions independent of attentional resources? Cogn Affect Behav Neurosci 2001;1:382-7. [DOI] [PubMed]
- 27.Penn DL, Ritchie M, Francis J, et al. Social perception in schizophrenia: The role of context. Psychiatry Res 2002;109:149-59. [DOI] [PubMed]
- 28.de Gelder B, Vroomen J, Annen L, et al. Audio-visual integration in schizophrenia. Schizophr Res 2003;59:211-8. [DOI] [PubMed]
- 29.Uhlhaas PJ, Phillips WA, Schenkel LS, et al. Theory of mind and perceptual context-processing in schizophrenia. Cognit Neuropsychiatry 2006;11:416-36. [DOI] [PubMed]
- 30.Monkul SE, Green MJ, Barrett JA, et al. A social cognitive approach to emotion recognition deficits in schizophrenia. Schizophr Res 2007;94:245-52. [DOI] [PubMed]
- 31.Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cognit Neuropsychiatry 2007;12:259-80. [DOI] [PubMed]
- 32.Smilek D, Birmingham E, Cameron D, et al. Cognitive ethology and exploring attention in real-world scenes. Brain Res 2006;1080:101-19. [DOI] [PubMed]
- 33.Noton D, Stark I. Eye movements and visual perception. Sci Am 1971;224:35-43. [PubMed]
- 34.Henderson JM. Visual attention and eye movement control during reading and picture viewing. In: Rayner K, editor. Eye movements and visual cognition: scene perception and reading. New York: Springer-Verlag; 1992. p. 260-283.
- 35.Green MJ, Williams LM, Hemsley DR. Cognitive theories of delusion formation: The contribution of visual scan path research. Cognit Neuropsychiatry 2000;5:63-74.
- 36.Kojima T, Matsushima E, Ando K, et al. Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophr Bull 1992;18:85-94. [DOI] [PubMed]
- 37.Kojima T, Matsushima E, Nakajima K, et al. Eye movements in acute, chronic, and remitted schizophrenics. Biol Psychiatry 1990; 27:975-89. [DOI] [PubMed]
- 38.Kojima T, Potkin SG, Kharazmi M, et al. Limited eye movement patterns in chronic schizophrenic patients. Psychiatry Res 1989;28:307-14. [DOI] [PubMed]
- 39.Gordon E, Coyle S, Anderson J, et al. Eye movement response to a facial stimulus in schizophrenia. Biol Psychiatry 1992;31:626-9. [DOI] [PubMed]
- 40.Phillips ML, David AS. Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia 1997;35:99-105. [DOI] [PubMed]
- 41.Streit M, Woelwer W, Gaebel W. Facial-affect recognition and visual scanning behaviour in the course of schizophrenia. Schizophr Res 1997;24:311-7. [DOI] [PubMed]
- 42.Williams LM, Loughland CM, Gordon E, et al. Visual scan paths in schizophrenia: Is there a deficit in face recognition? Schizophr Res 1999;40:189-99. [DOI] [PubMed]
- 43.Gaebel W, Ulrich G, Frick K. Visuomotor performance of schizophrenic patients and normal controls in a picture viewing task. Biol Psychiatry 1987;22:1227-37. [DOI] [PubMed]
- 44.Phillips ML, Senior C, David AS. Perception of threat in schizophrenics with persecutory delusions: An investigation using visual scan paths. Psychol Med 2000;30:157-67. [DOI] [PubMed]
- 45.Green MJ, Williams LM, Davidson D. Visual scan paths to threat-related faces in deluded schizophrenia. Psychiatry Res 2003;119:271-85. [DOI] [PubMed]
- 46.Loughland CM, Williams LM, Gordon E. Visual scan paths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res 2002;55:159-70. [DOI] [PubMed]
- 47.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res 2003;59:233-41. [DOI] [PubMed]
- 48.Uhlhaas PJ, Phillips WA, Mitchell G, et al. Perceptual grouping in disorganized schizophrenia. Psychiatry Res 2006;145:105-17. [DOI] [PubMed]
- 49.Griffiths AN, Marshall RW, Richens A. Saccadic eye movement analysis as a measure of drug effects on human psychomotor performance. Br J Clin Pharmacol 1984;18(Suppl 1):73S-82S. [DOI] [PMC free article] [PubMed]
- 50.Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med 2006;36:69-80. [DOI] [PubMed]
- 51.Andreasen NC. The scale for the assessment of positive symptoms (SAPS). Iowa City (IA): University of Iowa; 1984.
- 52.Andreasen NC. The scale for the assessment of negative symptoms (SANS). Iowa City (IA): University of Iowa; 1983.
- 53.Humberstone V, Wheeler A, Lambert T. An audit of outpatient antipsychotic usage in three health sectors of Auckland, New Zealand. Aust N Z J Psychiatry 2004;38:240-5. [DOI] [PubMed]
- 54.Ammons RB, Ammons CH. The Quick test. Missoula (MT): Psychological Test Specialists; 1962.
- 55.Nelson HE, Willison J. National adult reading test (NART). 2nd ed. Windsor (UK): NFER-Nelson; 1991
- 56.Henderson JM, Hollingworth A. High-level scene perception. Annu Rev Psychol 1999;50:243-71. [DOI] [PubMed]
- 57.Zangemeister WH, Sherman K, Stark L. Evidence for a global scan path strategy in viewing abstract compared with realistic images. Neuropsychologia 1995;33:1009-25. [DOI] [PubMed]
- 58.Ekman P, Friesen WV. Pictures of facial affect. Palo Alto (CA): Consulting Psychologists Press; 1976.
- 59.Liddle PF. The symptoms of chronic schizophrenia: A re-examination of the positive and negative dichotomy. Br J Psychiatry 1987; 151:145-51. [DOI] [PubMed]
- 60.Russell TA, Green MJ. The neuropsychology of social cognition: implications for psychiatric disorders. In: Wood SJ, Allen NB, Pantelis C, editors. The Neuropsychology of Mental Illness. Cambridge (UK): Cambridge University Press. Forthcoming.


